Abstract
Preserved immune cell function has been reported in mice that achieve extreme longevity. Since cytokines are major modulators of immune responses, we aimed to determine the levels of 21 cytokines secreted ex vivo by peritoneal leukocytes cultured under basal- and mitogen- (conconavalin A (ConA) and LPS) stimulated conditions in middle-aged (44 ± 4 weeks), old (69 ± 4 weeks), very old (92 ± 4 weeks), and extreme long-lived (125 ± 4 weeks) ICR (CD1) female mice. The secretion of cytokines was measured by multiplex luminometry. Increased basal levels of proinflammatory IL-1β, IL-6, IL-12 (p70), IFN-γ, and TNF-α were seen in the old and very old animals, accompanied by decreased IL-10. In contrast, the extreme long-lived mice maintained the overall cytokine profile of middle-aged mice, though the basal secretion of IL-2, IL-9, IL-10, IL-13, and IL-12 (p40) was raised. Under LPS- and/or ConA-stimulated conditions, leukocytes from old and very old animals showed a significantly impaired response with respect to secretion of Th1 cytokines IL-3, IL-12p70, IFN-γ, and TNF-α; Th2 cytokines IL-6, IL-4, IL-10, and IL-13; and the regulatory cytokines IL-2, IL-5, and IL-17. Extreme long-lived mice preserved the middle-aged-like cytokine profile, with the most striking effect seen for the IL-2 response to ConA, which was minimal in the old and very old mice but increased with respect to the middle-aged level in extreme long-lived mice. Chemokine responses in regard to KC, MCP-1, MIP1β, and RANTES were more variable, though similar secretion of LPS-induced KC and MCP-1 and ConA-induced MCP-1, MIP-1β, and RANTES was found in long-lived and middle-aged mice. Thus, extreme long-lived animals showed only a minimal inflammatory profile, much lower than the old and very old groups and also lower than the middle-aged, which is likely mediated by the increase of anti-inflammatory cytokines such as IL-10. This was coupled to a robust response to immune stimuli across an appropriated Th1/Th2 and regulatory cytokine secretion, which seems to be a factor contributing to the preserved immune response reported in very long-lived animals and thus to their extended longevity.
Keywords: Aging, Cytokines, Longevity, Peritoneal leukocytes
Introduction
The disruption and overall impairment in host immunity that occurs with aging (immune senescence) are evidenced by the higher incidence and severity of infections and the increased susceptibility to cancer among aged subjects (Miller 1996; Castle 2000). Indeed, several age-related changes in immune functions have been shown to correlate with increased mortality (Wayne et al. 1990), such as low lymphoproliferative response to mitogens and NK cytotoxicity, defining the immune risk phenotype in man (Wikby et al. 2005; De la Rosa et al. 2006). These age-related changes reported in human peripheral blood leukocytes have also been found in mouse peritoneal immune cells and related to higher morbidity and mortality in aged animals (Guayerbas and De la Fuente 2003; De la Fuente and Miquel 2009). However, centenarians and exceptionally long-lived mice seem to exhibit a high degree of preservation of those immune functions, which may be essential to reach very advanced age in a healthy condition (Franceschi et al. 1995; Puerto et al. 2005; Alonso-Fernández et al. 2008; De la Fuente and Miquel 2009) and suggests maintenance of adequate immune function with aging as a positive health and longevity marker (Wayne et al. 1990; De la Fuente and Miquel 2009).
Cytokines are major mediators of the complex interactions among immune cells, being responsible for development and resolution of immune responses. However, aging is characterized by a chronic low-grade inflammatory status, so-called “inflamm-aging” (Franceschi and Bonafè 2003; De Martinis et al. 2006), suggesting a loss of homeostasis in cytokine networks with aging which contributes significantly to health loss in old age (Salvioli et al. 2006). For instance, increased production of proinflammatory cytokines such as TNF-α in resting cells leading to elevations of its circulating levels is associated with frailty and morbidity in older adults, including dementia, functional disability, and high mortality risk (Mooradian et al. 1991; De Martinis et al. 2006). High plasma IL-6 levels also correlate with greater disability, morbidity, and mortality in the elderly (Ferrucci et al. 1999). Additionally, high levels of IL-6 and IL-1β are significantly associated with poor physical performance and muscle strength in older people (Cesari et al. 2004), and IL-1β levels are predictors of high diastolic pressure in the elderly (Barbieri et al. 2003) and correlate with congestive heart failure and angina (Di Iorio et al. 2003).
In contrast, when an immune stimulus such as LPS is added in vitro, it has been shown that human subjects who at age 85 years produce low ex vivo TNF-α levels have a more than twofold increased overall mortality risk compared to peers with a higher production (van den Biggelaar et al. 2004). Data on mice have shown that macrophages from old animals produce less TNF-α and IL-6 following LPS stimulation than the young (Renshaw et al. 2002; Boehmer et al. 2005). Furthermore, skewing of CD4 T cell responses during infection from a Th1 (which activate macrophages and are responsible for cell-mediated immunity and phagocyte-dependent protective responses) towards a Th2 profile (which are responsible for strong antibody production, eosinophil activation, and inhibition of several macrophage functions) has been well established in old individuals (Lord et al. 2001). Thus, coincident with the decreased age-related lymphoproliferative responsiveness to antigens or mitogens, decline of the T cell growth factor IL-2 has been described in both humans and mice (Guayerbas et al. 2002b; Pawelec et al. 2002).
Importantly, studies on human centenarians have focused on only a few cytokine parameters, losing the overall picture of the cytokine network in those individuals and its relation to longevity. Moreover, research on long-lived mice has been very scarce in this regard to date. Thus, the aim of the present work was to study the ex vivo levels of a broad range of proinflammatory and anti-inflammatory cytokines, regulatory cytokines, and growth factors as well as chemokines (including Th1 and Th2 mediators) in both resting and stimulated peritoneal leukocytes, throughout the aging process, including extreme long-lived mice. Our data show that for the majority of cytokines studied, both the basal and stimulated responses were disrupted in aged animals but maintained in those mice that had reached extreme old age. The data thus support the proposal that maintaining the cytokine network is a valid marker for attaining longevity.
Materials and methods
Animals
We used 38 female ICR/CD-1 mice purchased from Harlan Ibérica (Barcelona, Spain) of different ages, namely middle-aged (44 ± 4 weeks, n = 14), old (69 ± 4 weeks, n = 7), very old (92 ± 4 weeks, n = 7), and extreme long-lived (125 ± 4 weeks, n = 10). The latter had achieved healthy aging and longevity since the average life span for females of the ICR/CD1 mice strain in our animal facility is 91.9 ± 5.6 weeks (Guayerbas et al. 2002a). The mice were specifically pathogen free as tested by Harlan and according to the Federation of European Laboratory Science Associations recommendations. They were housed 6 ± 1 per cage (polyurethane boxes) and maintained at a constant temperature (22 ± 2°C) in sterile conditions inside an aseptic air negative-pressure environmental cabinet (Flufrance, Cachan, France), on a 12/12 h reversed light/dark cycle (lights on at 2000 hours). Mice were fed tap water and standard Sander Mus pellets (A04 diet from Panlab L.S., Barcelona, Spain) ad libitum. Diet was in accordance with the recommendations of the American Institute of Nutrition for laboratory animals. Mice were treated according to the guidelines of the European Community Council Directives (86/6091 EEC).
Collection of peritoneal leukocytes
The peritoneal compartment offers the potential to study unfractionated leukocytes, which better preserve the physiological environment surrounding the immune cells in vivo. This is a fundamental issue in studies aiming to reproduce immune cell responses ex vivo, including cytokine secretion, considering the observation that these responses may vary or be lost in purified isolates (Salvioli et al. 2006). Peritoneal suspensions were collected between 0800 and 1000 hours to minimize circadian variations in the immune system, without sacrificing the animals which allowed monitoring the life spans of the mice. None of the middle-aged and only one animal from the old and very old age groups reached longevity (138 and 132 weeks, respectively). Thus, all the age groups used in the present work, with the exception of the exceptionally long-lived mice, were representative of normal aging and not of longevity. It should be noted that although peritoneal sampling is a low-invasive method to access the immune system in mice, it could be stressful for animals and might have had a negative impact on the mice life spans. However, none of the mice used in the present work died shortly after the study and the range of life spans reached did not differ significantly from that observed for other mice in the colony not exposed to peritoneal sampling.
Mice were held by the cervical skin, the abdomen was cleansed with 70% ethanol and 3 mL of sterile Hank’s solution, previously tempered at 37°C, was injected intraperitoneally. After massaging the abdomen, 80% of the injected volume was recovered. Leukocytes from peritoneal suspensions were identified by their morphology and quantified, in Neubauer chambers using optical microscopy (40×) and flow cytometry. Briefly, aliquots of the cellular suspensions were washed at 400 g for 10 min, and adjusted to 3 × 105 leukocytes/mL in PBS with BSA 1% (Sigma, St Louis, USA), which was used to reduce nonspecific binding. Cells were then centrifuged at 560 g for 10 min, supernatants were discarded, and 30 μL of single antibodies (CD11b, CALTAG Laboratories, Burlingame, USA; CD11c and CD19, BD Pharmingen, San Diego, USA) or 30 μL of a mixture of antibodies conjugated to different fluorochromes (CD3/CD4/CD8, BD Pharmingen) were added to each tube. Isotype control antibodies were also used to establish and substract nonspecific staining. PBS-BSA (30 μL) was added to blank tubes. Cells were incubated in the presence of the antibodies for 30 min at 4°C in the dark. Tubes were washed twice at 560 g for 5 min in PBS-BSA to remove excess antibody. Immunostaining was measured using a flow cytometer (FACSCalibur Flow Cytometer, Becton Dickinson, Franklin Lakes, USA) immediately after staining. Cells were gated according to their forward- and side-scatter characteristics. For CD11b and CD11c, only the high expression of the leukocyte differentiation antigen was taken as positive (FL-H>101). Results were analyzed with Cell Quest Pro software (BD Biosciences, San Jose, USA) and expressed as percentage of CD11b (macrophages), CD11c (dendritic cells), CD19 (B lymphocytes), CD3CD4 (T helper), and CD3CD8 (T cytotoxic) cells with regard to the total number of cells present in the samples.
Cytokine measurement
A previously described method (Alvarado et al. 2006) was used with minor modifications. Cell suspensions were adjusted to 5 × 105 lymphocytes/mL in complete medium containing RPMI-1640 (PAA, Pasching, Austria), 10% fetal bovine serum (Gibco), and 1% gentamicin (PAA). Cell viability was checked by Trypan Blue (Sigma) exclusion and only suspensions with cell viability of 99% or higher were used. Briefly, 1 × 105 lymphocytes were cultured in 96-well plates in 200 µL complete medium alone or medium containing LPS (Escherichia coli, 055:B5, 1 µg/mL per well; Sigma) or concanavalin A (ConA, 1 µg/mL per well; Sigma) as control and stimulated cells, respectively. LPS is known to directly activate innate immune cells, such as macrophages which produce proinflammatory cytokines and several regulatory cytokines, chemokines and growth factors that promote inflammation, and adaptive immune B cells. The activation of T cells by LPS requires direct cell-to-cell contact with viable antigen presenting cells, mainly macrophages and dendritic cells in the peritoneum, which present the antigen to the CD4 T cells, causing them to differentiate into Th1 or Th2 cells, proliferate and produce Th1 or Th2 cytokines, respectively. ConA is a T cell mitogenic lectin. After 48 h of incubation at 37°C in a sterile and humidified atmosphere of 5% CO2, 100 µL of culture supernatants were collected for cytokine measurements. Supernatant samples were collected 48 h after stimulation with LPS or ConA, since we had previously observed that differences in lymphocyte proliferative responses between the mice in different age groups were seen within that time frame (De la Fuente and Miquel 2009). Thus, we were interested in studying the immediate cytokine environment surrounding immune cells that would be influencing the lymphoproliferative responses in the mice of different ages. Ex vivo levels of cytokines, including regulatory cytokines and growth factors (IL-2, IL-5, IL-9, IL-17, GM-CSF, VEGF), proinflammatory (IL1-α/IL-1β, IL-3, IL-6, IL-12 (p40)/IL-12 (p70), IFN-γ, TNF-α) and anti-inflammatory cytokines (IL-4, IL-10, IL-13), and chemokines (KC, MCP-1, MIP-1β, RANTES), were measured simultaneously by multiplex luminometry (Beadlyte mouse 21-plex cytokine detection system, Upstate, Millipore). From those, IL-1, IL-2, IL-3, IL-12, IL-17, IFN-γ, TNF-α, GM-CSF, and VEGF are Th1-related cytokines, whereas IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13 are Th2 mediators.
Statistical analysis
The results were expressed as mean ± standard error. The normality of the samples and the homogeneity of variances were checked by the Kolmogorov–Smirnov and Levene analyses, respectively. Age-related differences were detected by ANOVA. The Tukey test with a level of significance set at p < 0.05 was used for post hoc comparisons when variances were homogeneous, whereas its counterpart analysis, Games–Howell set at the same significance level, was used in case of unequal variances.
Results
Peritoneal leukocyte populations
Regarding peritoneal leukocyte populations, the most significant changes were found in old animals, which showed extremely low percentages of macrophages (CD11b) and dendritic cells (CD11c), with respect to the long-lived mice (Table 1, p < 0.05). In contrast, although not statistically significant, the long-lived animals showed a trend towards increased percentages of both innate immune cell types when compared to the middle-aged group (Table 1). No significant age-related differences were observed in regard to the composition of the lymphocyte populations CD19, CD3CD4, and CD3CD8 in the peritoneal sample (data not shown).
Table 1.
CD11b and CD11c cells as a percentage of total cells in the peritoneal suspensions of middle-aged (n = 10), old (n = 7), very old (n = 7), and long-lived (n = 10) ICR/CD1 female mice
| Middle-aged | Old | Very old | Long-lived | |
|---|---|---|---|---|
| CD11b (%) | 14.11 ± 3.71 | 8.27 ± 2.30* | 37.44 ± 16.46 | 42.17 ± 9.60 |
| CD11c (%) | 2.32 ± 0.76 | 0.59 ± 0.15* | 1.30 ± 0.48 | 7.20 ± 1.94 |
The mean ± standard error of 7–10 values corresponding to that number of animals is shown. Samples from each animal were assessed in duplicate. *p < 0.05 versus long-lived
Proinflammatory cytokine levels
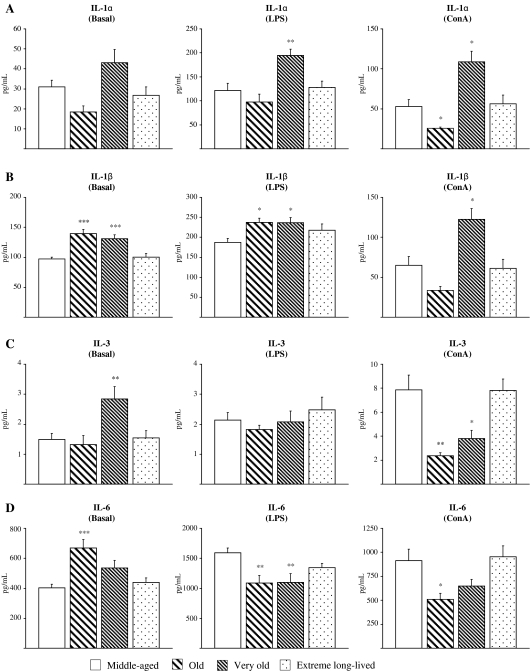
Ex vivo levels of proinflammatory cytokines (IL-1 α/β, IL-3, IL-6, IL-12 p40/p70, IFN-γ, TNF-α) in basal, LPS-, and ConA-stimulated peritoneal leukocyte culture supernatants from middle-aged, old, very old, and extreme long-lived animals are shown in Fig. 1. In general, basal levels of proinflammatory cytokines seen in the extreme long-lived mice were close to the levels observed in the middle-aged animals, while significant increases were exhibited by old and very old animals. In particular, old and very old mice showed increased levels of IL-1β (Fig. 1b, p < 0.001) and TNF-α (Fig. 1h; p < 0.05 and p < 0.001, respectively) compared to the middle-aged mice. The old mice also had raised levels of IL-6 (Fig. 1d, p < 0.001) and the very old animals showed higher basal IL-3 (Fig. 1c; p < 0.01) and IFN-γ levels (Fig. 1g; p < 0.05). In contrast, values for IFN-γ, TNF-α, IL-1β, IL-3, and IL-6 did not differ significantly between middle-aged and extreme long-lived mice (Fig. 1b–d, g, h). Moreover, though basal IL-12 p40 subunit concentration (Fig. 1e) was found to be moderately increased in extreme long-lived mice as compared to the middle-aged (p < 0.05), levels of the active IL-12 p70 heterodimer (Fig. 1f) were similar for both age groups but were higher in the old animals (Fig. 1f; p < 0.05). Thus the phenomenon of increased proinflammatory cytokine production in resting leukocytes with age, termed “inflamm-aging,” was not seen in those animals that attained extreme old age.
Fig. 1.
Proinflammatory cytokines. Levels (pg/mL) of IL-1α (a), IL-1β (b), IL-3 (c), IL-6 (d), IL-12 (p40; e), IL-12 (p70; f), IFN-γ (g), and TNF-α (h) in supernatants of peritoneal leukocytes cultured under resting, LPS-, and ConA-stimulated conditions, from middle-aged, old, very old, and extreme long-lived female mice. Each column represents the mean ± standard error of 7–14 values, and each value is the mean of duplicate assays. ***p < 0.001, **p < 0.01, and *p < 0.05 with respect to the value in middle-aged animals
In LPS-stimulated samples, the old and very old animals had higher values of IL-1β (Fig. 1b) with respect to the middle-aged mice (p < 0.05), and the very old animals also exhibited higher IL-1α (Fig. 1a; p < 0.01), but both age groups showed decreased production of IL-6 (Fig. 1d; p < 0.01). Moreover, LPS-stimulated IFN-γ (Fig. 1g) and TNF-α (Fig. 1h) levels were also significantly lower in old compared to middle-aged animals (p < 0.01 and p < 0.001, respectively). No differences were observed for LPS-stimulated production of IL-3 and IL-12 p70 between any age group (Fig. 1c, f), though extreme long-lived mice showed higher IL-12 p40 than the middle-aged mice (p < 0.05; Fig.1e). Therefore, the response of leukocytes to an immune stimulus was decreased in the old and very old animals, with the exception of IL-1 production, but was preserved in the extreme long-lived mice (Fig. 1a–d, f–h).
A similar pattern of cytokine responses was seen for ConA-stimulated leukocytes with only minor differences. IL-1α (Fig. 1a) was found to be lower in old animals when compared to the middle-aged group (p < 0.05), but was increased in the very old group (p < 0.05), which also showed increased IL-1β (Fig. 1b; p < 0.05). IL-3 (p < 0.01), IL-6, IL-12 p70 (p < 0.05), IFN-γ, and TNF-α (p < 0.01) levels were all lower in old animals when compared to the middle-aged (Fig. 1c, d, f–h), but only ConA-stimulated IL-3 values were decreased in very old mice (Fig. 1c; p < 0.05). Extreme long-lived animals showed similar levels to the middle-aged mice for all proinflammatory cytokines measured under ConA-stimulated conditions, with the exception of higher values for IL-12 p40 (p < 0.01).
Anti-inflammatory cytokine levels
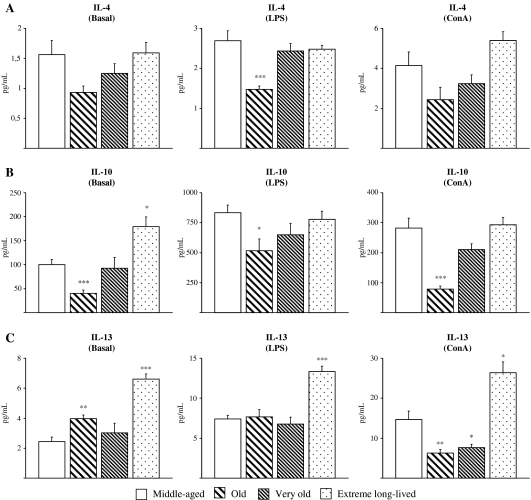
In general, extreme long-lived animals showed levels of anti-inflammatory cytokines more similar to the middle-aged than old and very old mice, which exhibited lower values (Fig. 2). Interestingly, in basal conditions, IL-10 levels (Fig. 2b) were lower in old mice as compared to the middle-aged (p < 0.001) and extreme long-lived animals showed increased levels of this anti-inflammatory cytokine compared to the middle-aged group (p < 0.05). However, resting levels of IL-13 (Fig. 2c) were higher in both old (p < 0.01) and extreme long-lived animals (p < 0.001) compared to the middle-aged mice. No differences were found for IL-4 (Fig. 2a).
Fig. 2.
Anti-inflammatory cytokines. Levels (pg/mL) of IL-4 (a), IL-10 (b), and IL-13 (c) in supernatants of peritoneal leukocytes cultured under resting, LPS-, and ConA-stimulated conditions, from middle-aged, old, very old, and extreme long-lived female mice. Each column represents the mean ± standard error of 7–14 values, and each value is the mean of duplicate assays. ***p < 0.001, **p < 0.01, and *p < 0.05 with respect to the value in middle-aged animals
IL-4 and IL-10 responses to LPS (Fig. 2a, b) were seen in all mice but were lower in old animals as compared to the middle-aged (p < 0.001 and p < 0.05, respectively). However, IL-4 and IL-10 responses were maintained in the extreme long-lived animals in comparison to the middle-aged mice, whereas LPS-induced IL-13 levels were higher in extreme long-lived mice (p < 0.001; Fig. 2c).
ConA-induced levels of IL-10 (Fig. 2b) were also diminished in the old group (p < 0.001), whereas IL-13 was lower in old (p < 0.01) and very old (p < 0.05) animals with respect to the middle-aged. Again, IL-4 and IL-10 responses to ConA were preserved in the extreme long-lived as compared to the middle-aged, whereas IL-13 levels were higher (p < 0.05; Fig. 2c).
Regulatory cytokine and growth factor levels
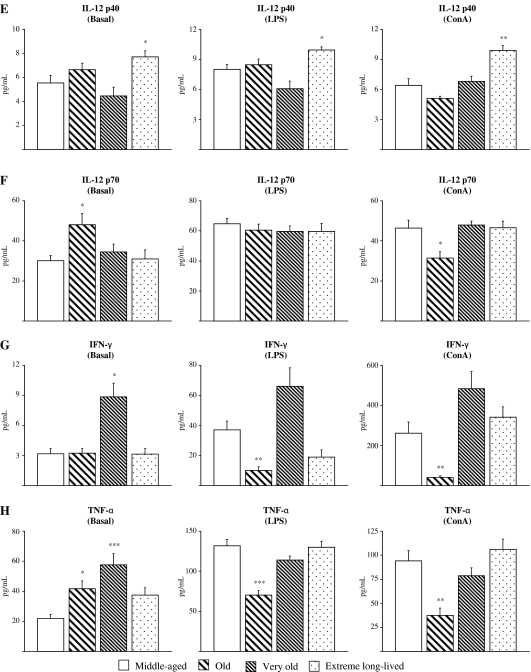
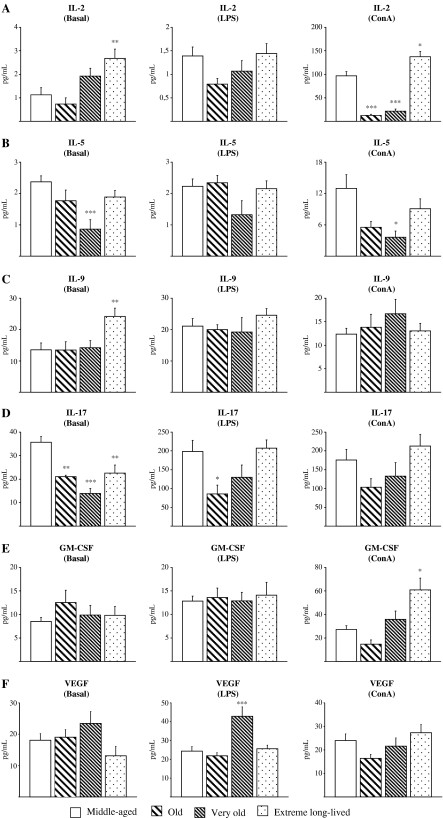
Figure 3 shows the striking impairment of ConA-stimulated IL-2 levels seen in old and very old animals (p < 0.001) compared to the middle-aged, which was not seen in the extreme long-lived mice who actually showed increased values for IL-2 production in both ConA-stimulated (p < 0.05) and resting conditions (p < 0.01; Fig. 3a). Very old animals also showed decreased levels of IL-5 in basal (p < 0.001) and ConA-stimulated conditions (p < 0.05), whereas these levels were preserved in the extreme long-lived mice (Fig. 3b). Conversely, extreme long-lived animals showed increased levels of IL-9 (p < 0.01) in resting cultures (Fig. 3c) and increased GM-CSF following ConA stimulation (p < 0.05; Fig.3e). Some regulatory cytokines did not show any particular response pattern associated with attainment of longevity. Basal IL-17 was decreased in all the older groups (p < 0.001 for very old; p < 0.01 for old and extreme long-lived), whereas IL-17 levels in response to LPS were impaired only in the old group (p < 0.05; Fig. 3d). The LPS-stimulated VEGF response was increased only in very old animals (p < 0.001; Fig. 3f).
Fig. 3.
Regulatory cytokines and growth factors. Levels (pg/mL) of IL-2 (a), IL-5 (b), IL-9 (c), IL-17 (d), GM-CSF (e), and VEGF (f) in supernatants of peritoneal leukocytes cultured under resting, LPS-, and Con A-stimulated conditions, from middle-aged, old, very old, and extreme long-lived female mice. Each column represents the mean ± standard error of 7–14 values, and each value is the mean of duplicate assays. ***p < 0.001, **p < 0.01, and *p < 0.05 with respect to the value in middle-aged animals
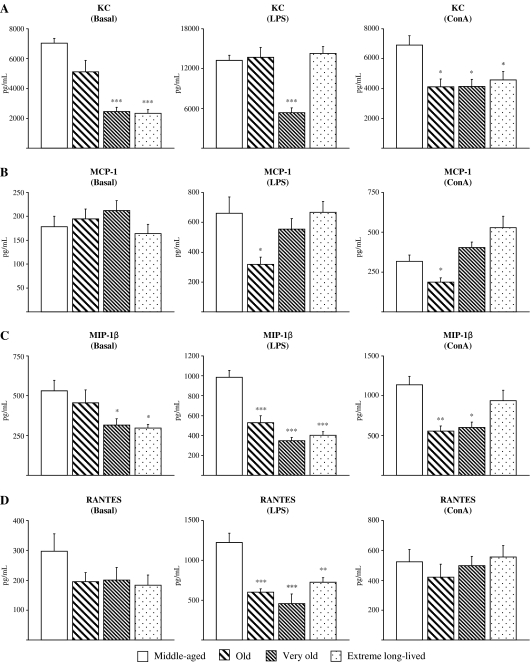
Chemokine levels
In general, chemokine values decreased with age, even in the extreme long-lived mice (Fig. 4). The most remarkable chemokine-related changes seen with the aging process in resting cultures included diminished levels of KC and MIP-1β for both very old (p < 0.001 and p < 0.05, respectively) and extreme long-lived mice (p < 0.001 and p < 0.05, respectively; Fig. 4a, c). In response to LPS, all the older groups showed impaired levels of MIP-1β (p < 0.001) and RANTES (p < 0.001 for old and very old; p < 0.01 for extreme long-lived), as compared to the middle-aged animals (Fig. 4c, d). LPS-induced levels of KC were decreased in very old animals (p < 0.001; Fig. 4a), whereas old animals showed reduced levels of MCP-1 (p < 0.05; Fig. 4b). In contrast, LPS-stimulated levels of these chemokines were found to be preserved in extreme long-lived animals. All the older groups showed lower levels of KC in response to ConA (p < 0.05) as compared to the middle-aged mice (Fig. 4a). ConA-induced levels of MCP-1 (Fig. 4b) were lower in old animals (p < 0.05), whereas both old (p < 0.01) and very old (p < 0.05) mice showed reduced levels of MIP-1β (Fig. 4c). ConA-stimulated levels of these chemokines were found to be preserved in extreme long-lived mice.
Fig. 4.
Chemokines. Levels (pg/mL) of KC (a), MCP-1 (b), MIP-1β (c), and RANTES (d) in supernatants of peritoneal leukocytes cultured under resting, LPS-, and ConA-stimulated conditions, from middle-aged, old, very old, and extreme long-lived female mice. Each column represents the mean ± standard error of 7–14 values, and each value is the mean of duplicate assays. ***p < 0.001, **p < 0.01, and *p < 0.05 with respect to the value in middle-aged animals
Table 2 summarizes the most significant changes in the cytokine network found in the older groups as compared to middle-aged animals under (a) basal, (b) LPS-, or (c) ConA-stimulated conditions.
Table 2.
Summary of the most significant age-related changes found in pro- and anti-inflammatory cytokines, regulatory cytokines, including growth factors, and chemokines, in supernatants of peritoneal leukocytes cultured under (a) basal, (b) LPS-, and (c) ConA-stimulated conditions from old, very old, and extreme long-lived ICR/CD1 female mice as compared to the middle-aged mice
| Old | Very old | Long-lived | |
|---|---|---|---|
| (a) Basal | |||
| Proinflammatory cytokines | |||
| IL-1β | ↑↑↑ | ↑↑↑ | = |
| IL-3 | = | ↑↑ | = |
| IL-6 | ↑↑↑ | = | = |
| IL-12 p70 | ↑ | = | = |
| IFN-γ | = | ↑ | = |
| TNF-α | ↑ | ↑↑↑ | = |
| Anti-inflammatory cytokines | |||
| IL-10 | ↓↓↓ | = | ↑ |
| IL-13 | ↑↑ | = | ↑↑↑ |
| Regulatory cytokines | |||
| IL-2 | = | = | ↑↑ |
| IL-5 | = | ↓↓↓ | = |
| IL-9 | = | = | ↑↑ |
| IL-17 | ↓↓ | ↓↓↓ | ↓↓ |
| Chemokines | |||
| KC | = | ↓↓↓ | ↓↓↓ |
| MIP-1β | = | ↓ | ↓ |
| (b) LPS | |||
| Proinflammatory cytokines | |||
| IL-1α | = | ↑↑ | = |
| IL-1β | ↑ | ↑ | = |
| IL-6 | ↓↓ | ↓↓ | = |
| IFN-γ | ↓↓ | = | = |
| TNF-α | ↓↓↓ | = | = |
| Anti-inflammatory cytokines | |||
| IL-4 | ↓↓↓ | = | = |
| IL-10 | ↓ | = | = |
| IL-13 | = | = | ↑↑↑ |
| Regulatory cytokines | |||
| IL-17 | ↓ | = | = |
| VEGF | = | ↑↑↑ | = |
| Chemokines | |||
| KC | = | ↓↓↓ | = |
| MCP-1 | ↓ | = | = |
| MIP-1β | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| RANTES | ↓↓↓ | ↓↓↓ | ↓↓ |
| (c) ConA | |||
| Proinflammatory cytokines | |||
| IL-1α | ↓ | ↑ | = |
| IL-1β | = | ↑ | = |
| IL-3 | ↓↓ | ↓ | = |
| IL-6 | ↓ | = | = |
| IL-12 p70 | ↓ | = | = |
| IFN-γ | ↓↓ | = | = |
| TNF-α | ↓↓ | = | = |
| Anti-inflammatory cytokines | |||
| IL-10 | ↓↓↓ | = | = |
| IL-13 | ↓↓ | ↓ | ↑ |
| Regulatory cytokines | |||
| IL-2 | ↓↓↓ | ↓↓↓ | ↑ |
| IL-5 | = | ↓ | = |
| GM-CSF | = | = | ↑ |
| Chemokines | |||
| KC | ↓ | ↓ | ↓ |
| MCP-1 | ↓ | = | = |
| MIP-1β | ↓↓ | ↓ | = |
↑↑↑ P<0.001, ↑↑ P<0.01, ↑ P<0.05 increase, and ↓↓↓ P<0.001, ↓↓ P<0.01, ↓ P<0.05 decrese, with respect to the value in middle-aged animals
Discussion
This is the first study which analyses the changes ex vivo in the levels of a whole panel of leukocyte-derived immune mediators both in normal aging and extreme longevity as well as under resting and stimulated conditions. It is well known that immune cells communicate with each other through these soluble mediators and that the complex network of cytokine interactions is crucial for the functioning of the immune system and its fine tuning. However, to date most studies of immune senescence have been focused on isolated subsets of immune cell types and a limited range of soluble mediators (Salvioli et al. 2006). The data described here are thus of special relevance since this study was performed using unfractionated peritoneal leukocytes in order to better reproduce the in vivo cytokine response.
The results obtained confirm published data in humans and rodents showing chronic low-grade inflammation in old age (Franceschi and Bonafè 2003; De Martinis et al. 2006; Salvioli et al. 2006; De la Fuente and Miquel 2009) and extend these data by revealing that a broad range of cytokines are affected, such as IL-1β, IL-3, IL-6, IL-12 (p70), IFN-γ, and TNF-α, and that there was also a concomitant age-related decrease in IL-10. Conversely, extreme long-lived mice in general showed a preservation of the middle-aged-like cytokine profile under resting conditions, and if anything, the secretion of certain cytokines was raised in these animals, notably anti-inflammatory mediators such as IL-10. TNF-α and related cytokines determine the strength, effectiveness, and duration of inflammation, whereas IL-10 and other anti-inflammatory cytokines have the opposite role, limiting and terminating the process (Lio et al. 2003). Thus, the high basal levels of anti-inflammatory mediators in the extreme long-lived mice could have direct beneficial effects for life-span, with high proinflammatory cytokines by contrast shortening life span. In humans, high circulating levels of TNF-α are known to be associated with increased morbidity and mortality in the elderly (Mooradian et al. 1991; De Martinis et al. 2006) and gene polymorphisms determining increased production of anti-inflammatory cytokines or decreased production of proinflammatory cytokines have been found to be associated with successful aging and longevity (Lio et al. 2002; 2003; Cederholm et al. 2007). In particular, the polymorphism of the IL-10 promoter associated with high levels of IL-10 is a specific marker of longevity in centenarians (Lio et al. 2002). Our results support this key role for raised IL-10, among all mediators measured in the present work, in controlling chronic inflammation and in longevity achievement in mice.
Profound alterations in cytokine production were also seen in peritoneal leukocytes under LPS and ConA-stimulated conditions in the old and very old mice. Upon LPS stimulation, we found a reduced secretion of proinflammatory cytokines (IFN-γ, TNF-α, IL-6) as well as anti-inflammatory Th2 cytokines (IL-4, IL-10) and regulatory cytokines such as IL-17, which could contribute to the impaired immune responses described in both aged humans and rodents (Wikby et al. 2005; De la Rosa et al. 2006; De la Fuente and Miquel 2009). Thus, it seems that the chronic low-grade inflammatory state shown by resting leukocytes from old animals in the present work is contrasted by a defective inflammatory response upon stimulation that will ultimately limit the ability of these animals to deal with infection.
Congruent with this idea, 85-year-old humans who produce low ex vivo levels of LPS-induced pro- and/or anti-inflammatory cytokines, such as IL-6, TNF-α, and IL-10, have a more than twofold elevated mortality risk, compared to their age-matched counterparts with a higher cytokine production (van den Biggelaar et al. 2004). Other authors have also reported that macrophages from old rodents produce less TNF-α and IL-6 following LPS stimulation than macrophages from young mice (Renshaw et al. 2002; Boehmer et al. 2005; Chelvarajan et al. 2006). A reduced capacity to produce IFN-γ in the elderly has been demonstrated by Ouyang et al. (2002) in vitro on stimulation with bacterial products (LPS) or viral antigens (influenza vaccine), and these data were reproduced in macrophages from aged mice by Chelvarajan et al. (2006). All these changes were seen in the aged animals in the present study using whole peritoneal samples, but not in the extreme long-lived mice.
Although reduced LPS-induced IL-1 secretion has been reported in isolated macrophages from aged mice (Chelvarajan et al. 2006), in this study, LPS-induced IL-1 levels stand out as an exception to the age-related suppression of proinflammatory responses from peritoneal leukocytes as they were increased in old or very old animals with respect to the middle-aged mice. The elevated levels of IL-1α and IL-1β could be interpreted as an unsuccessful mechanism by which monocyte-macrophages attempt to activate the Th1 response against infection. Moreover, in very old animals, the increase in membrane IL-1α levels was coincident with elevated levels of VEGF, which is an autocrine activator for monocyte-macrophages (Ramos et al. 1998). These results would support the recently suggested oxidation-inflammation theory of aging, which proposes macrophage over-activation and loss of homeostasis as a key factor accelerating aging of the immune system and thus of the organism (De la Fuente and Miquel 2009).
This study is also the first in mice to show that low-resting inflammation seems to correlate with adequate inflammatory responses towards immune stimuli in extreme old age. The extreme long-lived animals exhibited well-conserved levels of LPS-induced proinflammatory cytokines, similar to the middle-aged profile, suggesting maintenance of homeostatic interactions between innate peritoneal cells including macrophages and dendritic cells to T cells. It is possible that the age-related changes in cytokine secretion could be explained in part by differences in the percentages of cell types present in the peritoneal sample. Although not statistically significant, we noted that long-lived mice showed higher relative amounts of macrophages and dendritic cells in their peritoneal samples as compared to old animals, in which we found dramatically low percentages of both innate immune cell types. A similar finding has been described for spleen macrophages from aged mice. These cells are less efficient at presenting antigens to T cells, and thus more macrophages are needed to effectively activate a T cell, but their numbers are decreased (Garg et al. 1996). Thus, in the long-lived mice, the increase in innate immune cells could be interpreted as a critical homeostatic mechanism aimed at preserving innate immune responses and cytokine-related production. Peritoneal sampling offers the potential to study immune cell responses ex vivo, with a high-grade preservation of their environment under physiological conditions. In contrast, from our results it is not possible to conclude whether the age-related changes in the cytokine network are due to these age-related differences in cell populations and/or due to individual cell aging. Future works aiming to elucidate the specific role played by the differences that we have shown in cell populations could be of special relevance. Moreover, to fully characterize the peritoneal compartment and its changes with aging, other major cell types from innate immunity, such as neutrophils and NK cells, should be considered; and these studies are now underway.
It is also important to note that several reports of age-related differences in in vivo responses to intravenous or intraperitoneal LPS injection, showed increased production of several cytokines, such as IL-1β, IL-6, TNF-α, and IL-10, in old compared to young animals (Tateda et al. 1996; Saito et al. 2003). However, the majority of such studies are models of lethal endotoxic shock, in which animals die shortly after the injection of high LPS concentrations. Thus, the apparent discrepancies in results from in vivo studies and our ex vivo analysis are likely to result from dose differences. Furthermore, in aged animals, ex vivo unresponsiveness of immune cells to these lower levels of inflammatory stimulus is more likely to predict a poor response to the infection in vivo, while the over-activation of leukocytes seen in vivo to moderately high stimulus concentration would lead to excess of inflammation. Premature death would likely be the ultimate consequence of both. Hence, these discrepancies might not be contradictory and instead could show a dose-dependent pattern of dysregulated immune system with aging, which highly influences life span and merits the endeavor of future research.
Skewing of the cytokine response of T cells towards Th2 has also been extensively reported to be associated with aging (Lord et al. 2001). In general, this has been observed upon stimulation of isolated T cells from aged subjects with T cell mitogens, such as ConA. Our data did not show a skewing towards Th2 when the peritoneal suspension was studied as a whole in the presence of ConA and instead the key factor associated with aging was an overall decline in Th1 and Th2-related cytokine responses (IL-2, IL-3, IL-12 (p70), IFN-γ, and TNF-α and IL-5, IL-6, IL-10, and IL-13, respectively) with IL-2 responses being severely restricted. This decline in the T cell growth factor IL-2 is a well-described observation in both aging humans and mice (Guayerbas et al. 2002b; Pawelec et al. 2002). Low ConA-induced IL-3 levels with aging have previously been described on lymphocytes isolated from spleen (Cai et al. 1990), and our data confirm these findings in peritoneum. Taken together, these data would suggest that lymphocytes from old donors would be impaired in their capacity to stimulate antigen presenting cells to produce certain cytokines such as IL-12 p70, which could result in low cytokine release, including IL-1, by monocyte-macrophages. Indeed, our data and that of others in splenocytes have found decreased ConA-stimulated levels of IL-12 p70 and IL-1 in old mice (Khansari and Gustad 1991; Park et al. 2008). However, in the present results the very old mice showed increased IL-1α and IL-1β in response to this mitogen. This result suggests a complex remodeling of the immune system along with the aging process, resulting in multiphasic processes attempting to adapt to changes and recover homeostasis.
Of special relevance is the fact that long-lived rodents showed well-preserved ConA-induced levels of the whole panel of proinflammatory cytokines studied, demonstrating the preserved capacity of T cells to induce adequate immune responses. Although we found slightly higher ConA-stimulated levels of Th2-derived IL-13 in long-lived than in middle-aged animals, Th1-derived IL-2 was also increased whereas all the other Th1- and Th2-related mediators measured were similar for both, suggesting a well-conserved Th1/Th2 response in the long-lived. The phenotype of the CD4 T cell populations present in the peritoneum of aging mice including long-lived animals should be considered in future works. Moreover, the increase in the ConA-induced IL-2 levels found in the long-lived could be crucial for the preserved lymphoproliferative responsiveness to this mitogen reported in those animals and addressed as longevity biomarker (De la Fuente and Miquel 2009). Another sign of the high T cell competence in long-living specimens is observed in the elevated ConA-induced levels of GM-CSF. In this context, ConA-stimulated GM-CSF would be part of the inflammatory response elicited by T cells against pathogens, leading to a rapid increase in the number of macrophages, which could be crucial to deal with the infection through pathogen microorganism destruction by these cells.
The majority of rodent studies indicate an age-related decline in the secretion of macrophage-derived proinflammatory chemokines upon stimulation with LPS (Renshaw et al. 2002; Chelvarajan et al. 2006) though controversial results have been obtained for isolated CD4 and CD8 T cells under stimulated conditions (Chen et al. 2003). Our results show decreased levels of LPS-induced MIP-1β and RANTES and ConA-induced KC chemokines along with the aging process and also in long-lived animals. Thus, the contribution of chemokine responses to longevity achievement is less clear. However, similar secretion of LPS-induced KC and MCP-1 and ConA-induced MCP-1, MIP-1β, and RANTES was found in long-lived and middle-aged mice, whereas it was decreased in old mice. These could be key factors contributing to the well-preserved chemotactic function of peritoneal lymphocytes and macrophages from long-lived rodents as compared to middle-aged animals (Puerto et al. 2005; De la Fuente and Miquel 2009).
In summary, this work offers important information on the immediate cytokine environment surrounding peritoneal leukocytes coincident with preserved lymphoproliferative responses and correlated with the attainment of extreme old age (De la Fuente and Miquel 2009). Future time-course studies would certainly be of relevance to unveil the kinetics of the cytokine profile that we report. However, the overall picture shown here pinpoints substantial candidate mediators which should be considered in future works aiming to elucidate the primary mechanisms leading to preservation of the immune system in longevity, basal IL-10 and ConA-stimulated IL-2 stand out among them.
Thus, from our data it can be concluded that extreme long-lived mice showed a minimal basal inflammatory profile, much lower than younger-aged animals and also lower than the middle-aged, which is likely mediated by increased IL-10. This was coupled to a robust response to LPS and ConA stimulation across a broad range of cytokines. Maintenance of an appropriate cytokine profile under stimulated conditions thus seems to be a factor in the preserved immune response reported in longevity (Franceschi et al. 1995; Puerto et al. 2005; Alonso-Fernández et al. 2008) and thus for the achievement of such exceptional longevity in good health.
Acknowledgments
This work was supported by the Ministry of Science and Innovation (BFU2008-04336) and the Ministry of Health and Consumption (RETICEF, RD06/0013/003) of Spain, and Research Group of Madrid Complutense University (910379). We would like to thank John S. Curnow (MRC Centre for Immune Regulation, Birmingham University Medical School) for his technical assistance with the luminometry assay.
References
- Alonso-Fernández P, Puerto M, Maté I, Ribera JM, Fuente M. The neutrophils of centenarians show function levels similar to those of adults. J Am Geriatr Soc. 2008;56:2244–2251. doi: 10.1111/j.1532-5415.2008.02018.x. [DOI] [PubMed] [Google Scholar]
- Alvarado C, Álvarez P, Puerto M, Gausserès N, Jiménez L, Fuente M. Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition. 2006;22:767–777. doi: 10.1016/j.nut.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Ferrucci L, Corsi AM, Macchi C, Lauretani F, Bonafè M, Olivieri F, Giovagnetti S, Franceschi C, Paolisso G. Is chronic inflammation a determinant of blood pressure in the elderly? Am J Hypertens. 2003;16:537–543. doi: 10.1016/S0895-7061(03)00861-6. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Cai NS, Li DD, Cheung HT, Richardson A. The expression of granulocyte/macrophage colony-stimulating factor in activated mouse lymphocytes declines with age. Cell Immunol. 1990;130:311–319. doi: 10.1016/0008-8749(90)90274-U. [DOI] [PubMed] [Google Scholar]
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- Cederholm T, Persson M, Andersson P, Stenvinkel P, Nordfors L, Madden J, Vedin I, Wretlind B, Grimble RF, Palmblad J. Polymorphisms in cytokine genes influence long-term survival differently in elderly male and female patients. J Intern Med. 2007;262:215–223. doi: 10.1111/j.1365-2796.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- Chen J, Mo R, Lescure PA, Misek DE, Hanash S, Rochford R, Hobbs M, Yung RL. Aging is associated with increased T-cell chemokine expression in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2003;58:975–983. doi: 10.1093/gerona/58.11.b975. [DOI] [PubMed] [Google Scholar]
- Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15:3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- Rosa O, Pawelec G, Peralbo E, Wikby A, Mariani E, Mocchegiani E, Tarazona R, Solana R. Immunological biomarkers of ageing in man: changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology. 2006;7:471–481. doi: 10.1007/s10522-006-9062-6. [DOI] [PubMed] [Google Scholar]
- Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Iorio A, Ferrucci L, Sparvieri E, Cherubini A, Volpato S, Corsi A, Bonafè M, Franceschi C, Abate G, Paganelli R. Serum IL-1beta levels in health and disease: a populationbased study. ‘The InCHIANTI study’. Cytokine. 2003;22:198–205. doi: 10.1016/S1043-4666(03)00152-2. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31:457–461. doi: 10.1042/BST0310457. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today. 1995;16:12–16. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- Garg M, Luo W, Kaplan AM, Bondada S. Cellular basis of decreased immune responses to pneumococcal vaccines in aged mice. Infect Immun. 1996;64:4456–4462. doi: 10.1128/iai.64.11.4456-4462.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guayerbas N, Fuente M. An impairment of phagocytic function is linked to a shorter life span in two strains of prematurely aging mice. Dev Comp Immunol. 2003;27:339–350. doi: 10.1016/S0145-305X(02)00103-9. [DOI] [PubMed] [Google Scholar]
- Guayerbas N, Catalán M, Víctor VM, Miquel J, Fuente M. Relation of behaviour and macrophage function to life span in a murine model of premature immunosenescence. Behav Brain Res. 2002;134:41–48. doi: 10.1016/S0166-4328(01)00449-1. [DOI] [PubMed] [Google Scholar]
- Guayerbas N, Puerto M, Víctor VM, Miquel J, Fuente M. Leukocyte function and life span in a murine model of premature immunosenescence. Exp Gerontol. 2002;37:249–256. doi: 10.1016/S0531-5565(01)00190-5. [DOI] [PubMed] [Google Scholar]
- Khansari DN, Gustad T. Effects of long-term, low-dose growth hormone therapy on immune function and life expectancy of mice. Mech Ageing Dev. 1991;57:87–100. doi: 10.1016/0047-6374(91)90026-V. [DOI] [PubMed] [Google Scholar]
- Lio D, Scola L, Crivello A, Colonna-Romano G, Candore G, Bonafè M, Cavallone L, Franceschi C, Caruso C. Gender-specific association between -1082 IL-10 promoter polymorphism and longevity. Genes Immun. 2002;3:30–33. doi: 10.1038/sj.gene.6363827. [DOI] [PubMed] [Google Scholar]
- Lio D, Scola L, Crivello A, Colonna-Romano G, Candore G, Bonafè M, Cavallone L, Marchegiani F, Olivieri F, Franceschi C, Caruso C. Inflammation, genetics, and longevity: further studies on the protective effects in men of IL-10–1082 promoter SNP and its interaction with TNF-alpha -308 promoter SNP. J Med Genet. 2003;40:296–299. doi: 10.1136/jmg.40.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunosenescence. Mech Ageing Dev. 2001;122:1521–1535. doi: 10.1016/S0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Miller RA. The ageing immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Reed RL, Osterweil D, Scuderi P. Detectable serum levels of tumor necrosis factor alpha may predict early mortality in elderly institutionalized patients. J Am Geriatr Soc. 1991;39:891–894. doi: 10.1111/j.1532-5415.1991.tb04456.x. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Wagner WM, Wikby A, Remarque E, Pawelec G. Compromised interferon gamma (IFN-gamma) production in the elderly to both acute and latent viral antigen stimulation: contribution to the immune risk phenotype? Eur Cytokine Netw. 2002;13:392–394. [PubMed] [Google Scholar]
- Park HR, Jo SK, Jung U, Yee ST. Restoration of the immune functions in aged mice by supplementation with a new herbal composition, HemoHIM. Phytother Res. 2008;22:36–42. doi: 10.1002/ptr.2255. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Barnett Y, Forsey R, Frasca D, Globerson A, McLeod J, Caruso C, Franceschi C, Fülop T, Gupta S, Mariani E, Mocchegiani E, Solana R. T-cells and aging. Front Biosci. 2002;7:d1056–d1183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- Puerto M, Guayerbas G, Álvarez P, Fuente M. Modulation of neuropeptide Y and norepinephrine on several leucocyte functions in adult, old and very old mice. J Neuroimmunol. 2005;165:33–40. doi: 10.1016/j.jneuroim.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Ramos MA, Kuzuya M, Esaki T, Miura S, Satake S, Asai T, Kanda S, Hayashi T, Iguchi A. Induction of macrophage VEGF in response to oxidized LDL and VEGF accumulation in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1998;18:1188–1196. doi: 10.1161/01.atv.18.7.1188. [DOI] [PubMed] [Google Scholar]
- Renshaw W, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Salvioli S, Capri S, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging, cytokines and aging: state of the art, new hypothesis on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:3161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggelaar AH, Huizinga TW, Craen AJ, Gussekloo J, Heijmans BT, Frölich M, Westendorp RG. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 1990;114:80–88. doi: 10.1093/geronj/45.2.m45. [DOI] [PubMed] [Google Scholar]
- Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]