Abstract
Aging is characterized by decline in metabolic function and insulin resistance, and both seem to be in the basis of neurodegenerative diseases and cognitive dysfunction. Estrogens prevent age-related changes, and phytoestrogens influence learning and memory. Our hypothesis was that estradiol and genistein, using rapid-action mechanisms, are able to modify insulin sensitivity, process of learning, and spatial memory. Young and aged ovariectomized rats received acute treatment with estradiol or genistein. Aged animals were more insulin-resistant than young. In each age, estradiol and genistein-treated animals were less insulin-resistant than the others, except in the case of young animals treated with high doses of genistein. In aged rats, no differences between groups were found in spatial memory test, showing a poor performance in the water maze task. However, young females treated with estradiol or high doses of genistein performed well in spatial memory task like the control group. Only rats treated with high doses of genistein showed an optimal spatial memory similar to the control group. Conversely, acute treatment with high doses of phytoestrogens improved spatial memory consolidation only in young rats, supporting the critical period hypothesis for the beneficial effects of estrogens on memory. Therefore, genistein treatment seems to be suitable treatment in aged rats in order to prevent insulin resistance but not memory decline associated with aging. Acute genistein treatment is not effective to restore insulin resistance associated to the early loss of ovarian function, although it can be useful to improve memory deficits in this condition.
Keywords: 17β-estradiol, Genistein, Insulin sensitivity, Spatial memory, Aging, Rat
Introduction
Aging is characterized by a decline in metabolic function, which may have particularly significant consequences not only on average life span, but also on the quality of life of the elderly. In this regard, the association of aging with the development of insulin resistance in human and rodents has been reported (Larkin et al. 2001; Reaven and Reaven 1985). However, the mechanism underlying the increase in insulin resistance with advanced age remains unclear, although it seems to be related in females to a decrease in estrogen plasma levels. In spite of this, previous studies (Gonzalez et al. 2000, 2002, 2003) and several clinical observations (Kumagai et al. 1993; Polderman et al. 1994) not only suggest an interaction between insulin and sex hormones, but also demonstrate that estrogen replacement therapy improves insulin sensitivity in postmenopausal women (Colacurci et al. 1998; Karjalainen et al. 2001) and rodents (Alonso et al. 2006a, 2008).
Estrogen deficiency caused by loss of ovarian function has been related with the development of metabolic alterations (dyslipidemia, insulin resistance, and diabetes type II) together with behavioral disorders (changes in mood and memory impairment; Kaaja 2008; Rasgon and Jarvik 2004; Weber and Mapstone 2009). Therefore, these findings suggest that neurodegenerative diseases or memory impairment should be considered as a result of metabolic syndrome and that postmenopausal women are more vulnerable to these diseases than are young women. These conditions suggest that the loss of gonadal function is determinant for the deleterious consequences of aging on brain function. In this way, some clinical data have shown a beneficial effect on memory and cognitive skills in postmenopausal women using estrogen replacement therapy (Simpkins et al. 1994). However, rodent studies have shown that protection against ischemic brain injury disappears following ovariectomy (Simpkins et al. 1997) and that it can be restored by estrogen replacement (Pelligrino et al. 1998). Accordingly, our previous study demonstrated that estradiol treatment did not prevent memory impairment associated with aging (Alonso et al. 2006a) even though insulin sensitivity was improved. We also showed that estradiol treatment was efficient enough to improve some aspects of neuronal homeostasis, which is affected by aging (Alonso et al. 2008).
The beneficial effects of estrogen in the prevention and treatment of age-related physiological changes and neurodegenerative diseases may be a result of neuroprotective effects and benefits of estrogen on cognitive function, mostly due to the antioxidant properties of estrogens (Garcia-Segura et al. 2001; Oge et al. 2003). Estrogens can exert complex effects on learning and memory in rats, which show a clear decline with aging similar to humans, thus representing an appropriate animal model to study the effects of estrogens and phytoestrogens on aging.
Genistein has been identified in many plants, including those commonly consumed by humans and animals, such as soybean, alfalfa, and clover (Cornwell et al. 2004; Reinli and Block 1996). Phytoestrogens bind estrogen receptors (Kuiper et al. 1998; Kurzer and Xu 1997; Patisaul et al. 2002), although weakly in comparison to estrogens. For this reason, it seems that estrogens from plants have some estrogenic activity. The biological activity of phytoestrogens is ubiquitous, and their effects are not always comparable to animal estrogens. Currently, it seems enough to prove the benefits of phytoestrogens on human health (Adlercreutz 1998; Altavilla et al. 2004; Cornwell et al. 2004; Cotter and Cashman 2003; Setchell 1998) because their positive role is related to its ability to modify the metabolic pathways of an organism (Nogowski et al. 2002). However, not all studies have found evidences for these positive effects (Gallagher et al. 2004; Huntley and Ernst 2004; Krebs et al. 2004). Due to its estrogenic potential, genistein has been proposed to play a role in the maintenance of health status by acting on several organs and to prevent cardiovascular risk by regulating lipid and carbohydrate homeostasis (Cruz et al. 2006; Kreijkamp-Kaspers et al. 2004; Park et al. 2005). The estrogenic activity of genistein is reported to depend on its concentration (Wilson et al. 2004) and gender differences (Faughnan et al. 2004).
In the nervous system, phytoestrogens have a potential to act as estrogen receptor agonists (Pan et al. 1999; Schreihofer 2005) or antagonists (Patisaul et al. 2002), affect learning and memory (Lund et al. 2001), and show neuroprotective effects (Azcoitia et al. 2006; Bang et al. 2004; Ho et al. 2003; Linford and Dorsa 2002; Sonee et al. 2004; Zeng et al. 2004; Zhao et al. 2002). Previous studies suggest that the neuroprotective effects of genistein seem to be mediated by estrogen receptors (Bang et al. 2004; Linford and Dorsa 2002; Zeng et al. 2004). However, it is also possible that these effects are independent of estrogen receptor activation (Simpkins et al. 2004). In this regard, genistein has estrogen receptor-independent effects, influencing the activity of enzymes such as protein tyrosine kinases (Akiyama et al. 1987; Foti et al. 2005; Markovits et al. 1989), and therefore, it may alter phosphorylation events associated with the activation of neurotransmitter receptors (Sweatt 2001).
Since it has been previously demonstrated that age-induced insulin resistance in rats is detectable at 4 months of age and insulin resistance has been recently associated with cognitive deficits in rats (affecting spatial memory in particular; Stranahan et al. 2008) and that rats aged between 6 and 24 months represent a suitable animal model to study the “aging” phenomenon (Barzilai and Rossetti 1996; Iossa et al. 1999), in this paper, we have worked on the hypothesis that estradiol and genistein using rapid or non-genomic action mechanisms were able to control two parameters affected by aging process: insulin sensitivity and spatial memory.
Methods
Animals
Virgin female Wistar rats (supplied by the Central Biotery of the University of Oviedo) weighing 130–150 g (age 6–8 weeks, young animals, Y) and 500–600 g (age 90–96 weeks, aged animals, A) were kept under standard conditions of temperature (23 ± 3°C) and humidity (65 ± 1%), and on a regular 12 h light/dark cycle (08:00–20.00). The animals were fed a standard diet (Panlab A04, Barcelona, Spain), and all of them had free access to water. Experimental handling was performed between 09:30 and 12:30 h. All experimental procedures carried out with animals were approved by a local veterinary committee from the University of Oviedo biotery and subsequent handling strictly followed the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Experimental design
Rats were ovariectomized through a midline incision under light anesthesia by inhalation of halothane. Ovariectomy was performed immediately after sexual maturity of young rats (Y) and after reproductive period of aged rats (A). Rats were randomly divided into four groups: sham surgery animals (intact; YC and AC), ovariectomized animals treated with vehicle (YV and AV), ovariectomized animals treated with 17β-estradiol (YE and AE), and ovariectomized animals treated with two different doses of genistein (YG1, YG2, AG1, and AG2) and housed individually throughout the experiment. All experimental treatments began exactly 2 weeks after ovariectomy to ensure a uniform period of estrogen depletion before replacement and to recover from surgical stress. In order to perform glucose and insulin determinations, 1 h prior to killing of the animals, they were injected with vehicle (olive oil/ethanol 3:2 v/v); 17β-estradiol (1.4 μg/kg body weight; Sigma Chemical Co., St Louis, MO, USA) in olive oil/ethanol (3:2 v/v) or genistein (LC Laboratories, Woburn, MA, USA; G1 = 10 mg/kg body weight; G2 = 40 mg/kg body weight) in olive oil/ethanol (3:2 v/v).
Euglycemic insulin clamp
Clamp experiments were performed in anesthetized rats using a previously described procedure (Alonso et al. 2006a, 2008; Gonzalez et al. 2000). After 12 h of fasting, animals were anesthetized with sodium pentobarbital (50 mg/kg), and the left saphenous vein was catheterized for insulin and glucose infusion.
Approximately 30 min after surgery and as soon as anesthesia was assured by loss of pedal and corneal reflexes, blood samples (2 ml) were drawn from the jugular vein and placed into heparinized tubes, centrifuged at 3,000 rpm during 20 min at 4°C, and the plasma was immediately collected and stored frozen at −20°C until assayed in order to determine basal insulin concentration. Another blood sample was collected from the tail in order to determine basal blood glucose. Plasma glucose was measured using an Accutrend System (Accutrend Alpha®. Roche Diagnostic S.L., Barcelona, Spain).
After the clamp study, additional blood samples (4 ml) were collected for the determination of final insulin concentration and 17β-estradiol plasma concentrations as described above. The total blood volume extracted was 5.5–6.5 ml from each animal. Plasma insulin was measured by radio immunoassay (RIA) using a DGR Instruments GmbH (Germany) kit for determination of rat insulin. The sensitivity of the assay was 0.1 ng/ml, and the intra-assay coefficient of variation was 9.32%. The sample was assayed in duplicate. Plasma 17β-estradiol was measured by RIA using Immuchem kits of cover tubes (ICN Biomedicals Inc.). The assay sensitivity was 10 pg/ml, and the intra-assay coefficient of variation was 12.26%. All samples were measured on the same day. Finally, samples of different tissues were collected and immediately frozen in liquid nitrogen for future experiments, and animals were killed by bleeding.
Spatial learning test
The remaining animals in each experimental group were trained in a water maze to test spatial learning and memory (Morris 1984). The maze consisted of a circular pool made of black fiberglass, 1.5 m in diameter and 75 cm high. The pool was filled with tap water to a height of 32 cm, and a black escape platform was placed 2 cm beneath the water surface. The water temperature was kept at 23 ± 1°C during the entire test period. The experimental room had numerous visual cues such as colour maps, posters, and plastic dishes fixed on the walls, a shelf, covered windows, and a table. Lighting was provided by two halogen spotlights (500 W) placed on the floor and facing the walls. Animal cages were kept outside the experimental room to avoid odor cues, and the bucket where the rats remained between consecutive trials was placed randomly around the pool during each trial. Maze performance was recorded live by a video camera mounted in the ceiling and connected to a computerized video tracking system (Ethovision Pro, Noldus Information Technology, Wageningen, The Netherlands).
The pool was conceptually divided into four quadrants, according to the cardinal points (N, S, E, W). Rats were released facing the pool wall from the central border of each quadrant following a pseudorandom sequence, four times each session. We used a 2-day water maze testing procedure based on similar studies previously published (Packard and Teather 1997; Rhodes and Frye 2006). Subjects received three four-trial sessions of training on day 1, with each block spaced 1 h apart. Rats were returned to their home cages between sessions. The escape platform used on the first trial session was painted white and stood up to 2 cm above the water surface. Rats were allowed to swim to locate the escape platform, where they remained for 15 s before they were placed in a black plastic bucket for 30 s. When rats failed to reach the platform within 60 s, they were gently guided to the platform. Since the platform is visible during the first trial session, the task can be considered as a test for visual acuity and sensory-motor coordination. Spatial learning took place during the following three sessions. Testing was identical to the habituation day, but, in this case, the escape platform was hidden beneath the water surface. The platform was located in the same position across training days. Escape latencies and swimming paths were recorded using the video tracking system for each rat. Immediately after training on the first day, the different experimental groups received posttraining injections of vehicle (olive oil/ethanol 3:2 v/v), 17β-estradiol (0.2 mg/kg body weight; Sigma Chemical Co., St Louis, MO, USA) in olive oil/ethanol (3:2 v/v) or genistein (LC Laboratories, Woburn, MA, USA; G1 = 10 mg/kg body weight; G2 = 40 mg/kg body weight) in olive oil/ethanol (3:2 v/v). On day 2, animals received an additional four-trial session to assess spatial memory. Five minutes after finishing the last trial, a probe test was performed. During the probe test, the escape platform was removed, and rats were required to swim for 30 s. The percent of total time spent in the quadrant was recorded for each animal.
Statistical analysis
Data are expressed as mean ±SEM. We evaluated the Gaussian distribution of each variable previously. After this, for each age, data were statistically analyzed using an analysis of variance (ANOVA) design followed by between-group comparisons using the Tukey honestly significant difference test. The comparisons between young and aged were analyzed by unpaired Student's t testing.
Average daily escape latencies in the water maze within a group were analyzed using one-way repeated measures ANOVA, followed by Student–Newman–Keuls post hoc tests to determine significant differences across training days. One-way ANOVAs were used to compare the performance of the experimental groups in the probe tests. Differences between vehicle, estradiol, and genistein-treated rats of each age group were assessed by comparing the mean escape latencies of the last training day using Student's t tests. A p value ≤ 0.05 was considered significant. Statistical analysis was performed using SPSS for Windows v. 6.01.
Results
Table 1 shows fasting blood glucose, fasting serum insulin, and serum insulin corresponding to the clamp experiment. Fasting blood glucose levels were observed to be significantly higher in aged groups than in young groups. Young groups treated with genistein (YG1 and YG2) had significantly higher fasting blood glucose levels than groups YC and YE. However, the highest fasting blood glucose level of aged groups was observed in group AC, and the lowest level was observed in group AE.
Table 1.
Fasting blood glucose, fasting serum insulin, and serum insulin after clamp experiments in control (C), vehicle (V), estradiol (E), and genistein (G1 and G2)-treated rats
| Young | Aged | |||
|---|---|---|---|---|
| Fasting blood glucose (mg/dl) | C | 51.40 ± 1.80 | 119.80 ± 3.46a | |
| V | 56.60 ± 1.50 | 140.80 ± 4.55a | ||
| E | 48.80 ± 1.68 | 105.60 ± 2.11a | ||
| G1 | 62.60 ± 2.06 | 126.20 ± 2.22a | ||
| G2 | 60.60 ± 2.65 | 114.40 ± 2.95a | ||
| Comparisons | C vs G1,G2 | V vs C,E,G1,G2 | ||
| E vs G1,G2 | E vs V,G1 | |||
| Fasting serum insulin (ng/ml) |
C | 6.51 ± 0.24 | 7.05 ± 0.32a | |
| V | 2.34 ± 0.16 | 3.59 ± 0.12a | ||
| E | 1.71 ± 0.33 | 1.95 ± 0.1a | ||
| G1 | 2.66 ± 0.17 | 1.54 ± 0.07a | ||
| G2 | 4.26 ± 0.15 | 1.43 ± 0.10a | ||
| Comparisons | C vs V,E,G1,G2 | C vs V,E,G1,G2 | ||
| G2 vs V,E,G1 | V vs E,G1,G2 | |||
| Serum insulin after clamp experiments (ng/ml) | C | 7.06 ± 0.23 | 11.83 ± 0.06a | |
| V | 2.78 ± 0.26 | 16.34 ± 0.40a | ||
| E | 3.94 ± 0.14 | 9.45 ± 0.30a | ||
| G1 | 3.43 ± 0.37 | 12.56 ± 0.46a | ||
| G2 | 6.62 ± 0.35 | 8.32 ± 0.11a | ||
| Comparisons | C vs V,E,G1 | C vs V,E,G1,G2 | ||
| G2 vs V,E,G1 | V vs E,G1,G2 | |||
| G1 vs E,G2 |
Mean ± standard error of the mean for seven animals
Significant differences are shown
aYoung versus aged
As regards to fasting serum insulin, we found significant higher values in YG1 and YG2 than in AG1 and AG2. However, fasting serum insulin was higher in aged rats than in young rats in groups C, V, and E. On the other hand, fasting serum insulin was significantly higher in groups YC and YG2 than in the remaining groups of young animals. However, fasting serum insulin in aged rats was significantly higher in groups AC and AV as compared with the rest of groups.
Finally, serum insulin levels after clamp experiments were significantly higher in aged groups than in young groups. Moreover, in young groups, this parameter was significantly higher in groups YC and YG2 than in the remaining young groups, whereas group AV had significantly higher serum insulin levels after clamp experiments than the remaining aged groups. Group AG2 had significantly lower values as compared with the rest of groups.
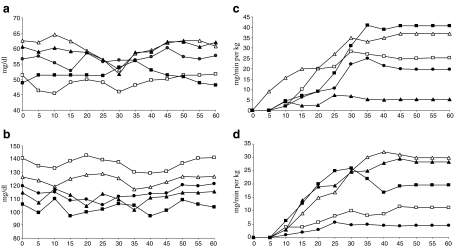
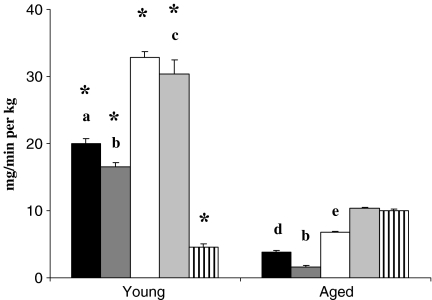
In order to investigate insulin resistance, glucose clamp experiments were carried out under euglycemic and hyperinsulinemic conditions. Figure 1 shows the results of clamp experiments, and Fig. 2 shows the comparison of glucose infusion rates as mean values from 40 to 60 min during euglycemic hyperinsulinemic clamp experiments in young and aged rats. We used this parameter as an in vivo measure of insulin sensitivity. Aged animals were significantly more resistant to the insulin action than young animals, except in group AG2, where aged animals were significantly less resistant than YG2. In young animals, groups treated with estradiol (YE) and low doses of genistein (YG1) were significantly less resistant to insulin action than the rest of young groups, while the most resistant one was group YG2. In aged rats, groups treated with estradiol (AE) and genistein (AG1 and AG2) were significantly less resistant to insulin action than AC and AV groups. Moreover, the genistein-treated groups (AG1 and AG2) were significantly less resistant to insulin action than estradiol treated group (AE).
Fig. 1.
Blood glucose concentrations (a, b) and glucose infusion rate (c, d) during euglycemic hyperinsulinemic clamp experiments. Data shown for experimental groups: young rats (a, c) and aged rats (b, d). Values shown for control (C, open square), vehicle (V, closed circle) and estradiol- (E, closed square) and genistein-treated (G1, open triangle and G2, closed triangle) rats. Mean ± SEM for seven animals
Fig. 2.
Comparison of glucose infusion rates of control (C,  ), vehicle (V,
), vehicle (V,  ) and estradiol-treated (E,
) and estradiol-treated (E,  ) and genistein-treated (G1,
) and genistein-treated (G1,  and G2,
and G2,  ) rats. Mean ± SEM for seven animals. Glucose infusion rate was assessed as the mean values from 40 to 60 min during euglycemic hyperinsulinemic clamp experiments. Mean ± SEM for seven animals. *Young vs aged. a C vs E,G1,G2; b V vs E,G1,G2; c G2 vs E,G1; d C vs V, E,G1,G2; e E vs G1,G2
) rats. Mean ± SEM for seven animals. Glucose infusion rate was assessed as the mean values from 40 to 60 min during euglycemic hyperinsulinemic clamp experiments. Mean ± SEM for seven animals. *Young vs aged. a C vs E,G1,G2; b V vs E,G1,G2; c G2 vs E,G1; d C vs V, E,G1,G2; e E vs G1,G2
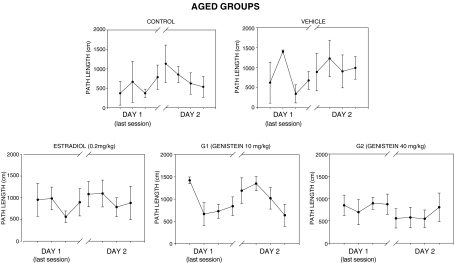
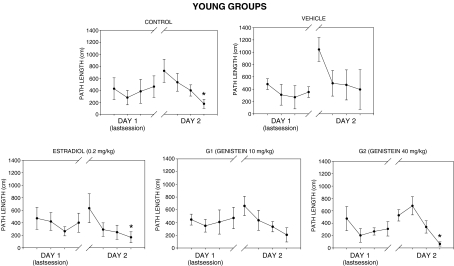
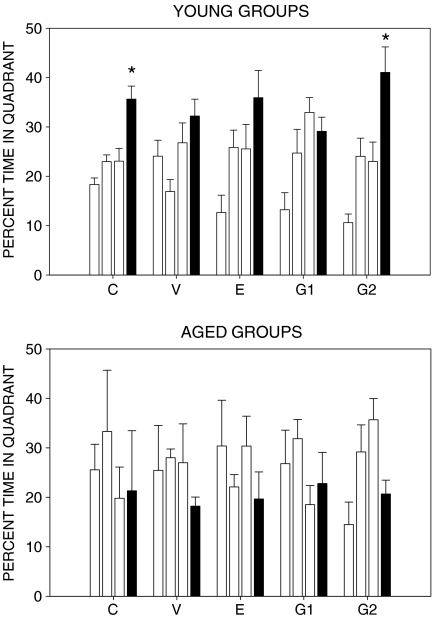
Figures 3, 4, and 5 show the spatial reference memory test results. No significant differences in path lengths to find the hidden escape platform were found across trials during the 2 days of testing in all aged groups (Fig. 3). There was a high inter-individual variability in swim paths as shown by the high error bars calculated in all aged groups. However, young females of groups C, E, and G2 performed significantly better than aged groups as shown by significant decreases in mean path length on day 2 (Fig. 4). It is noteworthy that animals of group G2 reached the shortest mean path length to find the platform in the last trial as compared with groups C and E. Finally, all aged groups did not perform above chance level in the probe test without escape platform available (Fig. 5). Conversely, only young animals in groups C and G2 performed well in the probe test (Fig. 5) because they spent significantly more time searching in the target quadrant in comparison to the other quadrants.
Fig. 3.
Water maze test. Mean path lengths to locate the escape platform across training day 1 (last session block) and day 2 in the aged experimental groups. No significant decreases in path length across learning days were observed for all aged groups
Fig. 4.
Water maze test. Mean path lengths to locate the escape platform across training day 1 (last session block) and day 2 in the young experimental groups. *p < 0.05, significant decrease as compared with the first daily trial
Fig. 5.
Effects of posttraining treatments on spatial reference memory evaluated in the water maze. Results of the probe test. Groups C and G2 of young rats spent significantly more time searching in the target quadrant (black bars). *p < 0.01 as compared with the rest of quadrants. Average percent time in correct quadrant was not higher than chance level (25%) in aged experimental groups. C control, V vehicle, E estradiol, G1 low genistein doses, and G2 high genistein doses
Discussion
Most studies trying to demonstrate that phytoestrogens in general and genistein in particular can attenuate insulin resistance associated with loss of ovarian function have been successful in women (Bhathena and Velasquez 2002; Jayagopal et al. 2002) and female rats (Choi and Song 2009; Jayagopal et al. 2002). In all cases, treatment with phytoestrogens ranged from several days to several weeks or months. However, in the present study we have hypothesized that genistein, like estradiol, is able to perform rapid actions on some steps of the intracellular insulin signaling pathways and thus improve the insulin sensitivity associated with the loss of ovarian function (Fig. 2).
In experimental models of acute cerebral ischemia, the acute administration of estradiol immediately before causing brain damage (between 30 and 120 min earlier) demonstrated the neuroprotective role of estrogen (Bryant et al. 2005; Chiappetta et al. 2007; Corasaniti et al. 2005). In addition, 7 h of treatment with estradiol or genistein appear sufficient to determine changes in uterine weight (Diel et al. 2004). On the other hand, previously conducted pilot experiments showed that 1 h after administration of estradiol produced significant changes in key points of the cascade of intracellular insulin signaling, and these changes had some similarity to those observed 1 h after insulin administration (data not shown and not published). Taken together, we believe that acute administration of estradiol or genistein at a short time had an impact on the sensitivity to insulin action, or learning processes and memory.
We have found that young animals treated with both estradiol (YE) or low doses of genistein (YG1) administered 1 h before clamp experiments was sufficient to increase significantly insulin sensitivity compared with intact (YC) or ovariectomized animals treated with vehicle (YV). However, high doses of genistein (YG2) seem to have the opposite effect in young animals (Fig. 2). On the other hand, insulin sensitivity in aged animals was significantly lower than in young animals as expected, conversely to group AG2. This result is very interesting, especially when the aged group of animals treated with genistein at low or high doses (AG1 and AG2) was more sensitive to insulin action than the rest of aged groups. These results suggest two issues that should be taken into consideration when extrapolating experimental data to the clinical practice: first, insulin sensitivity can be improved in patients who have lost their ovarian function prematurely after surgery, although high doses of genistein did not seem to be an appropriate treatment. Secondly, genistein dosage in elderly patients did not seem to be decisive; but taking into account the side effects of estradiol treatments, genistein seems to be more appropriate to improve insulin sensitivity.
Insulin resistance developing in obese diabetic patients is related with cerebral atrophy (observed mainly in the hippocampus) and alterations in memory and mood (Reagan 2007), and insulin resistance has also been linked to deficits in spatial memory in obese rats (Jurdak et al. 2008). The prevalence of impaired glucose tolerance and type 2 diabetes mellitus increases with age (Meigs et al. 2003) not only in humans, but also in models of aging in rats (Barzilai and Rossetti 1995; Reaven and Reaven 1985). The mechanism underlying this phenomenon remains unclear. It may be possible that decreased insulin sensitivity and/or impairment of β-cell function would be involved (Basu et al. 2003; Roder et al. 2000). Alternatively, it has been shown that the drop in estrogens levels related to menopause is the main factor implicated in the decreased insulin sensitivity and decreased in insulin-mediated glucose uptake (Stoney et al. 2001). This circumstance has profound consequences in the life of postmenopausal women because insulin resistance and related hyperinsulinemia are both the basis of the metabolic syndrome, which includes diabetes, hypertension, hyperuricemia, lipid abnormalities, and alterations in the thrombotic potential. Our results confirm that aging results in a significant increase in the basal levels of glucose, a fact that is exacerbated by ovariectomy. Moreover, as we expected, 1 h of estradiol treatment was enough to improve this parameter, a good evidence suggesting rapid or non-genomic actions of estrogen, as we previously showed (Alonso et al. 2006a,b, 2008; Gonzalez et al. 2000, 2002, 2003). However, one of the most interesting findings in this study was that genistein was also able to control the basal glucose plasma levels (Table 1). We believe, although further experiments will be necessary, that genistein is able to induce similar effects as estradiol in relation to the control of basal glucose plasma levels in aged rats, due to its high binding affinity for estrogen receptors (Kostelac et al. 2003) and/or its influence on the activity of protein tyrosine kinases (Akiyama et al. 1987; Foti et al. 2005; Markovits et al. 1989). Although it is known that genistein acts as an inhibitor of tyrosine kinase at low micromolar concentrations, the effects of genistein observed in this study appear to indicate that genistein may activate intracellular signaling pathways of insulin or stimulate transporter translocation glucose, or both processes. However, genistein impairs glucose control in young animals causing an increase in fasting blood glucose. At the present time, we do not have a plausible explanation for these results.
Likewise, it has been previously demonstrated that the increase in fasting serum insulin is due to impaired suppression of hepatic glucose production, which requires significant portal hyperinsulinemia (Gupta et al. 2000).
On the other hand, insulin is able to cross the blood–brain barrier and act on insulin receptors of brain tissue linked to changes in learning and memory (Zhao and Alkon 2001), and the effect of insulin on memory formation may be mediated by modulation of synaptic activity at short-term and by exerting effects on neural plasticity. Interestingly, in the present study, estradiol and genistein treatment prevented the increase in fasting serum insulin related to aging and lost ovarian function observed in groups AV and AC. Our results reveal that the reduced insulin action commonly described during aging is usually associated with a compensatory increase in plasma insulin and secondly, that estradiol is an essential factor in the modulation of glucose homeostasis during the aging process. Moreover, fasting blood glucose and fasting serum insulin found in AV, AE, AG1, and AG2 aged groups suggest a loss of sensitivity to insulin action in peripheral tissues rather than a primary impaired insulin secretion and that the role of estradiol and genistein seems to improve peripheral insulin sensitivity, therefore preventing hyperinsulinemia. This finding may have a particular relevance since hyperinsulinemia is a common factor in age-related diseases such as hypertension, stroke, type 2 diabetes mellitus, coronary heart disease, cancer, or neurodegenerative diseases.
The differences between fasting serum insulin and serum insulin levels following clamp experiments represent the insulin clearance rate, and the most important organ involved in this process is the liver. We have found significant increases in this parameter in both young and aged animals, although it was more evident in aged animals; therefore, aging impairs insulin clearance (Table 1). Taking these results together, in agreement with other authors (Barzilai and Rossetti 1996), hepatic insulin resistance is suggested because it causes a decrease in the ability of insulin to modulate glycogen stores during aging (Gupta et al. 2000). In this case, estradiol (AE) and high dose of genistein (AG2) also appeared to improve the insulin clearance rate, since serum insulin following clamp experiments was significantly lower in groups AE and AG2 than in groups AC, AV, and AG1 (Table 1). On the other hand, the high dose of genistein seems to impair the insulin clearance rate in young animals; therefore, in relation to this parameter, the high dose of genistein again does not seem to be a very good option in order to preserve the organism against hyperinsulinemia related to the loss of ovarian function.
Regarding the acute effects of phytoestrogens and estradiol on spatial learning and memory, only young animals slightly tended to be benefited from their actions as opposed to aged animals. As previously reported, spatial memory is clearly impaired in female aged animals due in part to the loss of ovarian function (Markowska 1999). Accordingly, both control (AC) and ovariectomized aged (AV) rats were similarly impaired in the spatial reference memory task. In addition, acute treatment with estradiol or genistein did not improve spatial memory consolidation in aged rats. We have previously reported that replacement treatment of aged ovariectomized rats with estradiol had no effect on spatial memory performance even with chronic treatment (Alonso et al. 2006a). The effects of estrogens on memory remains a controversial and complex issue, since estrogen dosage, duration of treatment, timing of treatment as related to the memory task, age at treatment, and type of memory task are important factors influencing the results of studies on hormones and aging (Frick 2009). Probably, the extremely deteriorated memory and associated neural function in aged rats may not be sensitive to the short-term estrogen treatments. Actually, recent clinical trials with aged postmenopausal women suggest that estrogen replacement therapy even increases the risk of cognitive decline (Espeland et al. 2004). It is known that beta estrogen receptors mediating the non-genomic action of estrogens at short-term are decreased in specific brain regions like the hippocampus in aged animals (Mehra et al. 2005; Yamaguchi-Shima and Yuri 2007). A decrease in the density of beta estrogen receptors in the hippocampus of aged animals would contribute to explain the absence of positive effects of estrogens and phytoestrogens that require these receptors for their immediate or short-term action on neurons. However, both estrogen receptor isoforms are involved in the neuroprotective effects of estrogen, therefore the relationship between the two should be the subject of further study. Now, we are working on this possibility in our laboratory in order to demonstrate this hypothesis. Probably, age-related impairment of intracellular signaling cascades that mediate responses to estrogens (Fan et al. 2010) or to several neutrophic factors (Williams et al. 2007) together with the above-mentioned decrease in estrogen receptors would be directly related with the absence of memory enhancement effects of genistein or estradiol in aged animals.
Conversely, estrogen and phytoestrogen treatments actually improved significantly spatial memory in ovariectomized young rats. However, there are numerous studies reporting beneficial effects on memory of acute posttraining administration of 17β-estradiol or several phytoestrogens in adult rodents (Gresack and Frick 2006; Packard 1998; Packard and Teather 1997; Rhodes and Frye 2006). Posttraining administration of estrogen is useful to distinguish between its actions on non-mnemonic factors like anxiety, motor activity, attention, etc., that take place during training and memory consolidation after training. We found significant positive effects of both estradiol and especially the highest genistein dose (40 mg/kg) on spatial memory, as revealed by a successful probe test and short path lengths to find the escape platform in the water maze. Acute effects of estrogens or phytoestrogens on spatial memory consolidation could be linked to their non-genomic actions on estrogen receptors. Genistein has been shown to affect protein tyrosine kinases in hippocampus in vitro modulating long-term potentiation, a synaptic plasticity phenomenon associated with memory (O'Dell et al. 1991). Therefore, although we cannot discard that “genomic” or long-term mechanisms could be also involved in the effects of genistein or estradiol on spatial memory, the actual evidence based on studies using a similar experimental approach (Fernandez et al. 2008; Luine et al. 2003) suggests that rapid effects of estrogens acting on membrane-bound estrogen receptors would be critical for enhancement of hippocampal memory consolidation.
However, additional mechanisms of action of estrogens and phytoestrogens related with memory consolidation remain to be elucidated. For example, exogenous estrogen administration activates several signaling cascades in hippocampal neurons like ERK/MAPK or PI3K/Akt leading to phosphorylated CREB, a factor necessary for the translation of proteins related to memory consolidation (Blum et al. 1999). Interestingly, a recent hypothesis (Frick et al. 2010) suggests a link between rapid and classic morphological alterations in the hippocampus (thought to be caused by genomic actions on nuclear estrogen receptors) induced by memory consolidation. According to these researchers, acute treatment with estradiol induces increases in hippocampal ERK activation in minutes (possibly mediated by membrane-bound estrogen receptors), which, in turn, induces an increase in hippocampal synaptic protein levels and dendritic spine density in hours.
Therefore, phytoestrogens like genistein could be useful to attenuate memory deficits caused by loss of estrogens in adult but not aged females. Other authors have reported similar results of memory improvement after estradiol treatment only in ovariectomized adult rats but not aged females (Savonenko and Markowska 2003; Talboom et al. 2008). Accordingly, it has been reported that treatment with genistein for 1 month improved spatial memory in young ovariectomized rats and prevented partially hippocampal degeneration (Xu et al. 2007). Recent evidence also indicates that chronic treatment with high doses of isoflavones (genistein and daidzein, in particular) enhances spatial memory in adult and aged men and women (Gleason et al. 2009; Thorp et al. 2009). On the contrary, other studies in postmenopausal women under estrogen therapy showed that adding a soy diet (containing high levels of genistein) for 1 year actually counteracted the benefits of estrogens on cognitive function (Kreijkamp-Kaspers et al. 2004). Accordingly, we found that only acute treatment with high doses of genistein was significantly better than estradiol to improve spatial memory in young females. Our results suggest that mainly acute treatment with selective estrogen modulators like genistein could be beneficial for memory in adults. In addition, our results would support the “critical period hypothesis” stating that hormone replacement therapy would be only effective to improve memory only during early menopause occurring at middle-age but not later in life. However, given the side effects of high genistein doses related with insulin clearance in young animals, genistein should be cautiously considered as an alternative option for estrogen replacement therapy in adults.
Acknowledgements
This study was supported by grants from the University of Oviedo (UNOV-06-MB-516 and UNOV-07-MB06-516). Pablo Garrido has a predoctoral fellowship from the Plan de Ciencia Tecnología e Innovación del Principado de Asturias, Spain (PCTI; BP09011).
References
- Adlercreutz H. Epidemiology of phytoestrogens. Baillieres Clin Endocrinol Metab. 1998;12:605–623. doi: 10.1016/S0950-351X(98)80007-4. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Alonso A, Fernandez R, Moreno M, Ordonez P, Gonzalez-Pardo H, Conejo NM, Diaz F, Gonzalez C. Positive effects of 17beta-estradiol on insulin sensitivity in aged ovariectomized female rats. J Gerontol A Biol Sci Med Sci. 2006;61:419–426. doi: 10.1093/gerona/61.5.419. [DOI] [PubMed] [Google Scholar]
- Alonso A, Fernandez R, Ordonez P, Moreno M, Patterson AM, Gonzalez C. Regulation of estrogen receptor alpha by estradiol in pregnant and estradiol treated rats. Steroids. 2006;71:1052–1061. doi: 10.1016/j.steroids.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Alonso A, Moreno M, Ordonez P, Fernandez R, Perez C, Diaz F, Navarro A, Tolivia J, Gonzalez C. Chronic estradiol treatment improves brain homeostasis during aging in female rats. Endocrinology. 2008;149:57–72. doi: 10.1210/en.2007-0627. [DOI] [PubMed] [Google Scholar]
- Altavilla D, Crisafulli A, Marini H, Esposito M, D'Anna R, Corrado F, Bitto A, Squadrito F. Cardiovascular effects of the phytoestrogen genistein. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:179–186. doi: 10.2174/1568016043477297. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Moreno A, Carrero P, Palacios S, Garcia-Segura LM. Neuroprotective effects of soy phytoestrogens in the rat brain. Gynecol Endocrinol. 2006;22:63–69. doi: 10.1080/09513590500519161. [DOI] [PubMed] [Google Scholar]
- Bang OY, Hong HS, Kim DH, Kim H, Boo JH, Huh K, Mook-Jung I. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol Dis. 2004;16:21–28. doi: 10.1016/j.nbd.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am J Physiol. 1995;269:E591–E597. doi: 10.1152/ajpendo.1995.269.3.E591. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Rossetti L. Age-related changes in body composition are associated with hepatic insulin resistance in conscious rats. Am J Physiol. 1996;270:E930–E936. doi: 10.1152/ajpendo.1996.270.6.E930. [DOI] [PubMed] [Google Scholar]
- Basu R, Breda E, Oberg AL, Powell CC, Dalla MC, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, Bosch MA, Rønnekleiv OK, Dorsa DM. 17-Beta estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience. 2005;133:343–352. doi: 10.1016/j.neuroscience.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Chiappetta O, Gliozzi M, Siviglia E, Amantea D, Morrone LA, Berliocchi L, Bagetta G, Corasaniti MT. Evidence to implicated early modulation of interleukin-1beta expression in the afford neuroprotection by 17beta-estradiol in male rats undergone transient middle cerebral artery occlusion. Int Rev Neurobiol. 2007;82:357–372. doi: 10.1016/S0074-7742(07)82019-8. [DOI] [PubMed] [Google Scholar]
- Choi JS, Song J. Effect of genistein on insulin resistance, renal lipid metabolism, and antioxidative activities in ovariectomized rats. Nutrition. 2009;25:676–685. doi: 10.1016/j.nut.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Colacurci N, Zarcone R, Mollo A, Russo G, Passaro M, Seta L, Franciscis P. Effects of hormone replacement therapy on glucose metabolism. Panminerva Med. 1998;40:18–21. [PubMed] [Google Scholar]
- Corasaniti MT, Amantea D, Russo R, Piccirilli S, Leta A, Corazzari M, Nappi G, Bagetta G. 17beta-estradiol reduces neuronal apoptosis induced by HIV-1 gp120 in the neocortex of rat. Neurotoxicology. 2005;26:893–903. doi: 10.1016/j.neuro.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Cotter A, Cashman KD. Genistein appears to prevent early postmenopausal bone loss as effectively as hormone replacement therapy. Nutr Rev. 2003;61:346–351. doi: 10.1301/nr.2003.oct.346-351. [DOI] [PubMed] [Google Scholar]
- Cruz MN, Luksha L, Logman H, Poston L, Agewall S, Kublickiene K. Acute responses to phytoestrogens in small arteries from men with coronary heart disease. Am J Physiol Heart Circ Physiol. 2006;290:H1969–H1975. doi: 10.1152/ajpheart.01065.2005. [DOI] [PubMed] [Google Scholar]
- Diel P, Geis RB, Caldarelli A, Schmidt S, Leschowsky UL, Voss A, Vollmer G. The differential ability of the phytoestrogen genistein and of estradiol to induce uterine weight and proliferation in the rat is associated with a substance specific modulation of uterine gene expression. Mol Cell Endocrinol. 2004;221:21–32. doi: 10.1016/j.mce.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faughnan MS, Hawdon A, Ah-Singh E, Brown J, Millward DJ, Cassidy A. Urinary isoflavone kinetics: the effect of age, gender, food matrix and chemical composition. Br J Nutr. 2004;91:567–574. doi: 10.1079/BJN20041087. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti P, Erba D, Riso P, Spadafranca A, Criscuoli F, Testolin G. Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Arch Biochem Biophys. 2005;433:421–427. doi: 10.1016/j.abb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Harburger LL (2010) A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta. In press (doi:10.1016/j.bbagen.2009.11.004) [DOI] [PMC free article] [PubMed]
- Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause. 2004;11:290–298. doi: 10.1097/01.GME.0000097845.95550.71. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, Doncarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/S0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Carlsson CM, Barnet JH, Meade SA, Setchell KD, Atwood CS, Johnson SC, Ries ML, Asthana S. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing. 2009;38:86–93. doi: 10.1093/ageing/afn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Alonso A, Alvarez N, Diaz F, Martinez M, Fernandez S, Patterson AM. Role of 17beta-estradiol and/or progesterone on insulin sensitivity in the rat: implications during pregnancy. J Endocrinol. 2000;166:283–291. doi: 10.1677/joe.0.1660283. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Alonso A, Grueso NA, Esteban MM, Fernandez S, Patterson AM. Effect of treatment with different doses of 17-beta-estradiol on the insulin receptor. Life Sci. 2002;70:1621–1630. doi: 10.1016/S0024-3205(02)01489-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Alonso A, Diaz F, Patterson AM. Dose- and time-dependent effects of 17beta-oestradiol on insulin sensitivity in insulin-dependent tissues of rat: implications of IRS-1. J Endocrinol. 2003;176:367–379. doi: 10.1677/joe.0.1760367. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gupta G, Cases JA, She L, Ma XH, Yang XM, Hu M, Wu J, Rossetti L, Barzilai N. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am J Physiol Endocrinol Metab. 2000;278:E985–E991. doi: 10.1152/ajpendo.2000.278.6.E985. [DOI] [PubMed] [Google Scholar]
- Ho KP, Li L, Zhao L, Qian ZM. Genistein protects primary cortical neurons from iron-induced lipid peroxidation. Mol Cell Biochem. 2003;247:219–222. doi: 10.1023/A:1024142004575. [DOI] [PubMed] [Google Scholar]
- Huntley AL, Ernst E. Soy for the treatment of perimenopausal symptoms–a systematic review. Maturitas. 2004;47:1–9. doi: 10.1016/S0378-5122(03)00221-4. [DOI] [PubMed] [Google Scholar]
- Iossa S, Lionetti L, Mollica MP, Barletta A, Liverini G. Energy intake and utilization vary during development in rats. J Nutr. 1999;129:1593–1596. doi: 10.1093/jn/129.8.1593. [DOI] [PubMed] [Google Scholar]
- Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- Jurdak N, Lichtenstein AH, Kanarek RB. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci. 2008;11:48–56. doi: 10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- Kaaja RJ. Metabolic syndrome and the menopause. Menopause. 2008;14:21–25. doi: 10.1258/mi.2007.007032. [DOI] [PubMed] [Google Scholar]
- Karjalainen A, Paassilta M, Heikkinen J, Backstrom AC, Savolainen M, Kesaniemi YA. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrinol (Oxf) 2001;54:165–173. doi: 10.1046/j.1365-2265.2001.01208.x. [DOI] [PubMed] [Google Scholar]
- Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003;51:7632–7635. doi: 10.1021/jf034427b. [DOI] [PubMed] [Google Scholar]
- Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824–836. doi: 10.1097/01.AOG.0000140688.71638.d3. [DOI] [PubMed] [Google Scholar]
- Kreijkamp-Kaspers S, Kok L, Grobbee DE, Haan EH, Aleman A, Lampe JW, Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/en.139.10.4252. [DOI] [PubMed] [Google Scholar]
- Kumagai S, Holmang A, Bjorntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149:91–97. doi: 10.1111/j.1748-1716.1993.tb09596.x. [DOI] [PubMed] [Google Scholar]
- Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- Larkin LM, Reynolds TH, Supiano MA, Kahn BB, Halter JB. Effect of aging and obesity on insulin responsiveness and glut-4 glucose transporter content in skeletal muscle of Fischer 344 x Brown Norway rats. J Gerontol A Biol Sci Med Sci. 2001;56:B486–B492. doi: 10.1093/gerona/56.11.b486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Dorsa DM. 17beta-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67:1029–1040. doi: 10.1016/S0039-128X(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, Adlercreutz H, Lephart ED. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001;2:20. doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nogowski L, Nowak KW, Kaczmarek P, Mackowiak P. The influence of coumestrol, zearalenone, and genistein administration on insulin receptors and insulin secretion in ovariectomized rats. J Recept Signal Transduct Res. 2002;22:449–457. doi: 10.1081/RRS-120014613. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Kandel ER, Grant SG. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Oge A, Sezer ED, Ozgonul M, Bayraktar F, Sozmen EY. The effects of estrogen and raloxifene treatment on the antioxidant enzymes and nitrite-nitrate levels in brain cortex of ovariectomized rats. Neurosci Lett. 2003;338:217–220. doi: 10.1016/S0304-3940(02)01416-7. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Clarkson TB. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proc Soc Exp Biol Med. 1999;221:118–125. doi: 10.1046/j.1525-1373.1999.d01-64.x. [DOI] [PubMed] [Google Scholar]
- Park D, Huang T, Frishman WH. Phytoestrogens as cardioprotective agents. Cardiol Rev. 2005;13:13–17. doi: 10.1097/01.crd.0000126084.68791.32. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Melby M, Whitten PL, Young LJ. Genistein affects ER beta- but not ER alpha-dependent gene expression in the hypothalamus. Endocrinology. 2002;143:2189–2197. doi: 10.1210/en.143.6.2189. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Santizo R, Baughman VL, Wang Q. Cerebral vasodilating capacity during forebrain ischemia: effects of chronic estrogen depletion and repletion and the role of neuronal nitric oxide synthase. Neuroreport. 1998;9:3285–3291. doi: 10.1097/00001756-199810050-00026. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79:265–271. doi: 10.1210/jc.79.1.265. [DOI] [PubMed] [Google Scholar]
- Rasgon N, Jarvik L. Insulin resistance, affective disorders, and Alzheimer's disease: review and hypothesis. J Gerontol A Biol Sci Med Sci. 2004;59:178–183. doi: 10.1093/gerona/59.2.m178. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Insulin signaling effects on memory and mood. Curr Opin Pharmacol. 2007;7:633–637. doi: 10.1016/j.coph.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM, Reaven EP. Age, glucose intolerance, and non-insulin-dependent diabetes mellitus. J Am Geriatr Soc. 1985;33:286–290. doi: 10.1111/j.1532-5415.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Reinli K, Block G. Phytoestrogen content of foods–a compendium of literature values. Nutr Cancer. 1996;26:123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Roder ME, Schwartz RS, Prigeon RL, Kahn SE. Reduced pancreatic B cell compensation to the insulin resistance of aging: impact on proinsulin and insulin levels. J Clin Endocrinol Metab. 2000;85:2275–2280. doi: 10.1210/jc.85.6.2275. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/S0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA. Transcriptional regulation by phytoestrogens in neuronal cell lines. Mol Cell Endocrinol. 2005;231:13–22. doi: 10.1016/j.mce.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Singh M, Bishop J. The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer's disease. Neurobiol Aging. 1994;15(Suppl 2):S195–S197. doi: 10.1016/0197-4580(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Liu R, Perez E, Cai ZY, Covey DF, Green PS. Estrogen-like compounds for ischemic neuroprotection. Stroke. 2004;35:2648–2651. doi: 10.1161/01.STR.0000143734.59507.88. [DOI] [PubMed] [Google Scholar]
- Sonee M, Sum T, Wang C, Mukherjee SK. The soy isoflavone, genistein, protects human cortical neuronal cells from oxidative stress. Neurotoxicology. 2004;25:885–891. doi: 10.1016/j.neuro.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Stoney RM, O'Dea K, Herbert KE, Dragicevic G, Giles GG, Cumpston GN, Best JD. Insulin resistance as a major determinant of increased coronary heart disease risk in postmenopausal women with Type 2 diabetes mellitus. Diabet Med. 2001;18:476–482. doi: 10.1046/j.1464-5491.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–88. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp AA, Sinn N, Buckley JD, Coates AM, Howe PR (2009) Soya isoflavone supplementation enhances spatial working memory in men. Br J Nutr, 1-7. [DOI] [PubMed]
- Weber M, Mapstone M. Memory complaints and memory performance in the menopausal transition. Menopause. 2009;16:694–700. doi: 10.1097/gme.0b013e318196a0c9. [DOI] [PubMed] [Google Scholar]
- Williams B, Granholm AC, Sambamurti K. Age-dependent loss of NGF signaling in the rat basal forebrain is due to disrupted MAPK activation. Neurosci Lett. 2007;413:110–114. doi: 10.1016/j.neulet.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LE., Jr Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci. 2004;81:69–77. doi: 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu J, Shi C, Guo K, Yew DT. Effects of genistein on hippocampal neurodegeneration of ovariectomized rats. J Mol Neurosci. 2007;31:101–112. doi: 10.1007/s12031-007-0010-y. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chen Q, Zhao B. Genistein ameliorates beta-amyloid peptide (25-35)-induced hippocampal neuronal apoptosis. Free Radic Biol Med. 2004;36:180–188. doi: 10.1016/j.freeradbiomed.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Zhao WK, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–34. doi: 10.1016/S0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen Q, Diaz BR. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp Biol Med (Maywood ) 2002;227:509–519. doi: 10.1177/153537020222700716. [DOI] [PubMed] [Google Scholar]