Abstract
Serotonin receptor 1A and 2A positive cells in postmortem brainstems were demonstrated via immunohistochemistry in eight control age-matched elderly individuals and eight Alzheimer patients. The 5-HT1A positive cells were found in substantia nigra, pontile nucleus, and vagal as well as dorsal raphe nucleus, while 5-HT2A receptor positive cells were found in motor, sensory and spinal trigeminal nuclei, pontile nucleus, substantia nigra, and nucleus solitarius. A comparison in density of positive cells per unit area was made between control age-matched and Alzheimer individuals. Statistically significant differences (p ≤ 0.01) in density were observed in 5-HT1A cells in pontile, dorsal raphe, and vagal nuclei between control age-matched and Alzheimer, and in 5-HT2A positive cells in the sensory trigeminal nucleus, between control and Alzheimer. This de novo study indicated the presence of 5-HT1A and 5-HT2A receptor positive cells in the above nuclei of human brainstem and revealed differences in density between control age-matched and Alzheimer, indicating possible functional derangements in Alzheimer patients in these areas. In addition, colocalization studies indicated that 5-HT1A receptors were in cholinergic cells and gamma-aminobutyric acid positive fibers were linked to 5-HT2A receptor positive cells. It is hoped that understanding these two important 5-HT receptors and their localization might lead to advances in future therapeutic development.
Keywords: Human, Brainstem, 5-HT1A, 5-HT2A, Control, Age-matched, Alzheimer
Introduction
Serotonin (5-hydroxytryptamine 5-HT) has a wide range of neurological functions (Banerjee et al. 2007; Kroeze et al. 2002) and is mediated by seven receptors (Saxena 1995) termed 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7. 5-HT1 class is subdivided into 1A, 1B, 1C, and 1D (Hoyer et al. 1994; Kroeze et al. 2002). The serotonin receptors or 5-HT receptors consist of a group of G-protein-coupled receptors and ligand gate ion channels, which exist in both the CNS and PNS. Except for 5-HT3, which is a ligand-gated ion channel, all others are G-protein-coupled receptors that need a second messenger to work (Hoyer et al. 1994; Frazer and Hensler 1999). Among these proteins, 5-HT1A and 5-HT2A appear to have a great effect on the major functions of the central nervous system (Lorke et al. 2006; Müller et al. 2007; Wesołowska 2002).
5-HT1A and 5-HT2A receptors are of interest in the pathology of several psychiatric disorders, such as anxiety and depression (Kroeze et al. 2002; Raote et al. 2007; Saxena 1995). 5-HT2A receptors are present in many regions of the human cortex (Lorke et al. 2006; Wai et al. 2008). Both 5-HT1A and 5-HT2A receptors have been associated with symptoms and cognitive disturbances in a wide range of psychoses, including schizophrenia (Sumiyoshi et al. 2008). Functionally, it is possible these receptors might act through other neurotransmitters, e.g., dopamine, acetylcholine, glutamine, and gamma-aminobutyric acid (GABA) (Sumiyoshi et al. 2008). Pathologically, 5-HT2A receptor upregulation has recently been documented in postmortem brain tissue of depressed patients in comparison with control and has correlated inversely with protein kinase A activities of the specimens (Shelton et al. 2009). In the brainstem, Hall et al. (1997) used labeling to report limited binding of 5-HT1A and 5-HT2A receptors. However, using a range of techniques, other studies have demonstrated 5-HT1A and 5-HT2A receptor binding sites in brainstems in general (Burnet et al. 1995) and have shown that 5-HT1A and 5-HT2A are important for both normal function and abnormal malfunction of the brainstem (Kroeze et al. 2002; Saxena 1995). The working hypothesis is that the brainstem of the human may be modulated by serotonin receptors. To prove this hypothesis in our study, the widely studied 5-HT1A and 5-HT2A nervous system receptors were used as markers to decipher possible serotonin receptor involvement in specific nuclei of the brainstem.
Materials and methods
The brainstems of eight normal aging specimens (age 63–93, mean = 83.4) and eight age-matched Alzheimer cases (age 69–94, mean = 83.3) were used. Specimens were obtained from aged homes and hospitals of Guangzhou, China, with consent from the family, the aged homes, and/or hospitals involved. The project was approved by the ethics committee of the Chinese University of Hong Kong (CUHK). The Alzheimer patients were clinically diagnosed by DSM-IV as senile dementia of the Alzheimer type (stages IV and V of Braak’s scale), and the diagnosis was confirmed by the presence of plaques and tangles in their corresponding pathological specimens. The midbrain, pons, and medulla of each individual of the two groups were separated and fixed in 4% paraformaldehyde, 1 to 6 h after death. They were then processed in graded alcohol followed by xylene clearing and then embedded in paraffin. Sections of 6 μm were cut from the specimen. For each specimen from each individual, five sections, each of 200 μm apart, were selected. Immunohistochemistry of 5-HT1A and 5-HT2A was performed. The antibodies for 5-HT1A and 5-HT2A were purchased from Santa Cruz Biotechnology (sc1459; Santa Cruz, CA, USA) and Abcam (ab16028; Cambridge, UK), respectively. The sections were then incubated with primary antibodies against 5-HT1A (1:500) and 5-HT2A (1:500). After washing three times with phosphate buffered saline (PBS), the sections were subsequently incubated with biotinylated anti-goat IgG antibody (5-HT1A) (1:500) and anti-rabbit IgG antibody (5-HT2A) (1:500) and then reacted with ABC reagents (PK 6101, Vector Laboratories, CA, USA). The resulting sections were finally observed under a photomicroscope (Axioplan 2 Photomicroscope, Zeiss, Germany). The positive reactive cells were brown in color, and some slides were counterstained with cresyl violet for our study. For comparison of density, five random fields from each of the five sections per individual at ×200 were chosen from each individual of both groups. The following regions were used for 5-HT1A positive cells counting: substantia nigra, pontile, dorsal raphe nucleus, and vagal nucleus. The substantia nigra, sensory trigeminal nucleus, and solitary nucleus were selected for 5-HT2A positive cells counting. These regions represented the major collections of positive 5-HT1A or 5-HT2A cells and were therefore selected. Identification and selection of these regions were performed according to Brodal (1981).
Statistical analysis
Mean ± standard error was computed for 30 total fields in each region of each individual of both control and Alzheimer groups. Statistical analysis was done using SPSS 13.0 software. The data were submitted to one-way ANOVA followed by the Scheffe’s method for multiple comparisons. Mean and standard deviation were computed for each group. P ≤ 0.01 was assigned as statistically significant.
Colocalization
Five random sections (each of 6 μm) from all brainstem regions of each individual from both groups of normal and Alzheimer patients were selected for colocalization. The tissue sections were first immersed in PBS with 0.1% Trition X-100 for 10 min, rinsed with PBS, incubated with 3% hydrogen peroxide (H2O2) in absolute methanol for 30 min, and then rinsed again. Bovine serum albumin (BSA) of 0.1% was used as non-specific antigen blocking solution. After blocking with 0.1% BSA for 1 h, the sections were incubated either with diluted polyclonal antibody 5HT1A (1:200; sc1459; Santa Cruz Biotechnology Inc.) or diluted 5HT2A (1:200; ab16028; Abcam Inc.) overnight at 4°C. On the next day, the sections were rinsed with PBS and incubated in corresponding diluted biotinylated secondary antibody (1:200; Zymed Laboratories, Inc., San Francisco, CA, USA) for 2 h. The sections were then rinsed and incubated in diluted streptavidin-HRP (horseradish peroxidise) conjugated solution (1:200; Zymed Laboratories, Inc.) for 2 h, then the first color was developed with Vector VIP Substrate Kit (Vector Laboratories). Subsequently, the tissue sections were rinsed with PBS and incubated in 0.1% BSA again for 1 h. The second primary polyclonal antibody, either diluted cholinacetyltransferase (ChAT, 1:200; ab68779; Abcam Inc.), TH (tyrosine hydroxylase 1:200; sc1459; Santa Cruz Biotechnology Inc.), GABA (1:200; ab65269; Abcam Inc.), or serotonin (1:200; ab10385; Abcam Inc.) was added and incubated overnight at 4°C. After rinsing with PBS, the sections had 2-h incubation with the corresponding biotinylated secondary antibody (1:200; Zymed Laboratories, Inc.). The tissue sections were then rinsed, followed by 2-h incubation in dilute streptavidin-HRP conjugated solution (1:200; Zymed Laboratories, Inc.). The second color was developed with vector DAB Substrate Kit (Vector Laboratories), and the sections were dehydrated and mounted. Tissue sections for negative control were processed similarly but without the first and secondary primary antibodies added.
Results
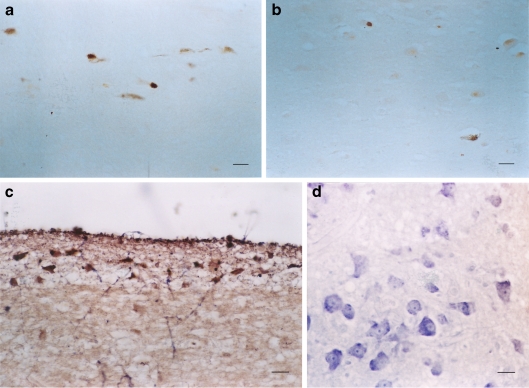
In the normal control aged, serotonin 1A receptor positive cells were observed in the periaqueductal gray of the midbrain (Fig. 1a–c). The superior colliculus displayed few 5-HT1A positive cells (Fig. 2). Significant amounts of 5-HT1A receptor positive cells were demonstrated in the substantia nigra (Fig. 3a), and while no positive cells were seen in the red nucleus, there were many positive 5-HT1A fibers (Fig. 3a, b). In the pons, pontile nuclei had many 1A receptor positive cells (Fig. 4a). High magnification revealed that the majority of the positive sites were in the soma of the cells (Fig. 4b). In the medulla, the hypoglossal nucleus displayed very few 1A receptor positive cells, while some 1A receptor positive cells were seen in the dorsal vagal nucleus (Fig. 5a). The midline regions of the brainstem, e.g., the dorsal raphe nucleus, had scattered 1A receptor positive cells (Fig. 5b, c), while the inferior olive had almost no 1A receptor positive cells (Fig. 5d).
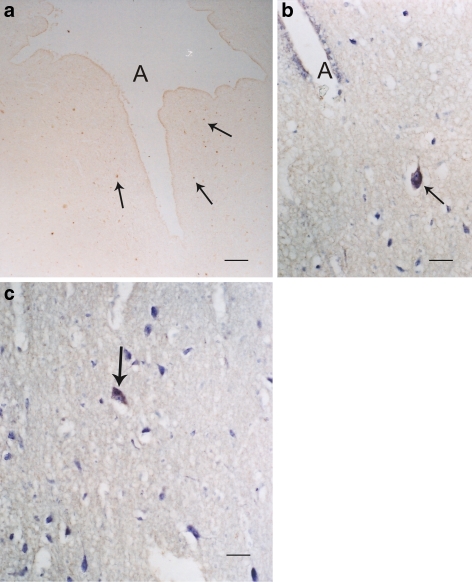
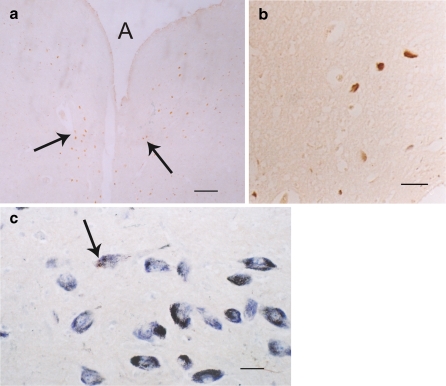
Fig. 1.
Most immunostained sections were not counterstained, unless otherwise stated (e.g. cresyl violet). a–c Periaqueductal gray of the human midbrain showing groups of 5-HT1A receptor positive cells (arrows). A denotes aqueduct. aBar = 40 μm; bbar = 5 μm; cbar = 5 μm. b and c Neurons show blue (cresyl violet stain) and brown (5-HT1A immunoreaction) (DAB) sites, while a is not counterstained and only shows brown reaction sites
Fig. 2.
The superior colliculus shows very few 5-HT1A positive cells (brown) with long branches in the deep layer (arrow). Bar = 5 μm
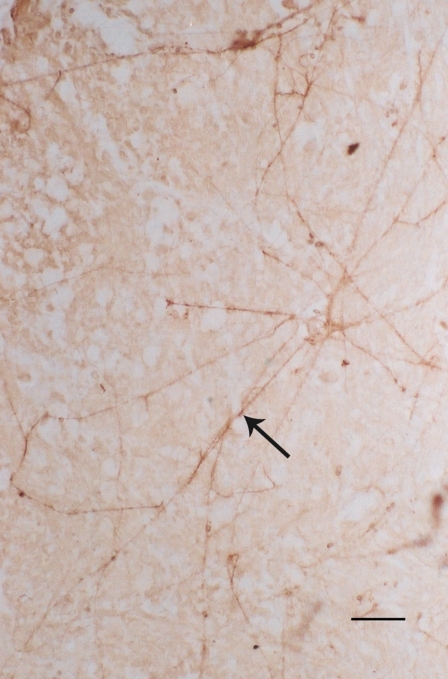
Fig. 3.
a A lot of 5-HT1A positive cells (arrows) in the substantia nigra of human midbrain. These cells had brown reaction products. Light blue was cresyl violet stain. Bar = 5 μm. b Positive brown fibers passing through red nucleus (N). These fibers are 5-HT1A positive fibers. Bar = 5 μm
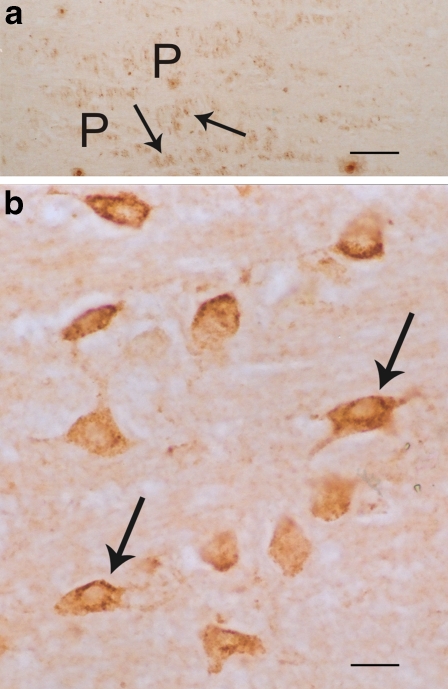
Fig. 4.
a and b The human pontile nuclei (P) also exhibit large numbers of positive 5-HT1A (brown) cells (arrows). aBar = 40 μm. b Higher magnification of these brown positive 5-HT1A soma of cells (arrows); bar = 5 μm
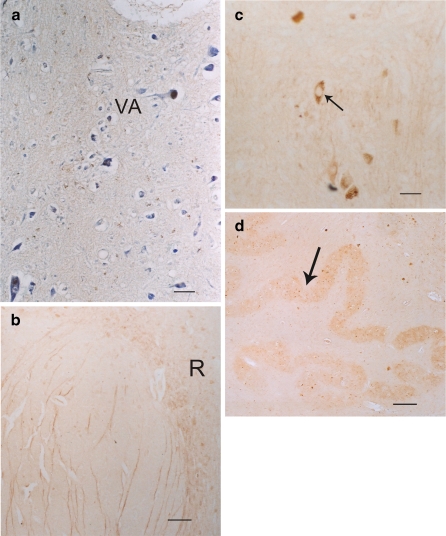
Fig. 5.
a The human medulla illustrating some positive 5-HT1A cells (brown stain) in the floor of the IV ventricles, e.g., dorsal vagal nucleus (VA). The blue counterstain was creyl violet. Bar = 5 μm. b and c The dorsal raphe nucleus (R) of the human in the midline of the brainstem also displays many scattered positive 5-HT1A cells (brown cells). bBar = 40 μm. c Higher magnification shows positive 5-HT1A dorsal raphe cells (arrow); bar = 5 μm. d The 5-HT1A positive cells (arrow) in the inferior olivary nucleus (dentate shaped) of the human medulla shows no obvious brown reaction. Bar = 40 μm
In the normal aged, the 2A receptor positive cells again appeared in the periaqueductal region of the midbrain (Fig. 6a, b). There were no 5-HT2A positive cells in the red nucleus, but there were a few positive cells in the substantia nigra (Fig. 6c). In the pons, some 2A receptors positive cells were seen in the pontile nuclei (Fig. 6d). There were also 2A receptor positive cells in the motor, sensory, and spinal trigeminal nuclei (Fig. 6e, f), while no positive 5-HT2A cells were seen in the floor of the IV ventricle (the hypoglossal and vagal nuclei) (Fig. 6g). The solitary nucleus in the medulla exhibited some 2A receptor positive cells (Fig. 7a, b). No 2A receptor positive cell was observed in the inferior olive (Fig. 7a), but positive 5-HT2A cells were demonstrated in the dorsal raphe and the other parts of the reticular formation (Fig. 7a).
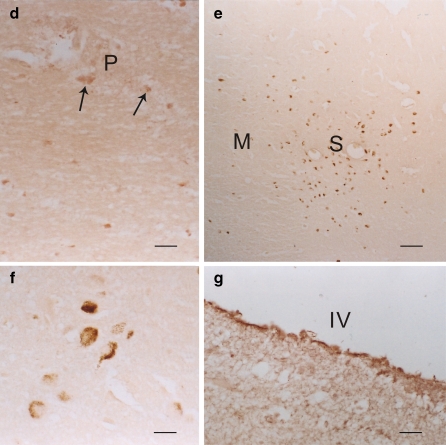
Fig. 6.
a and b Some 5-HT2A positive (brown) cells were found periaqueductally (arrows) in the midbrain. A denotes aqueduct. a Bar = 40 μm. b A higher power of the positive (brown) cells of 5-HT2A; bar = 5 μm. c A few of the 5-HT2A positive (brown) cells (arrow) was observed in the substantia nigra (counterstained with cresyl violet). Bar = 5 μm. d The pontile nuclei (P) contained some positive 5-HT2A (brown) cells of different sizes (arrows). Bar = 5 μm. e–g Some positive 5-HT2A (brown) cells were spotted in the motor V nucleus (M) and the sensory V nucleus (S) in the pons. eBar = 40 μm. f The positive 5-HT2A (brown) cells in the spinal trigeminal nucleus; bar = 5 μm. g No 5-HT2A positive cells in floor of the fourth ventricle (IV the fourth ventricle); bar = 5 μm
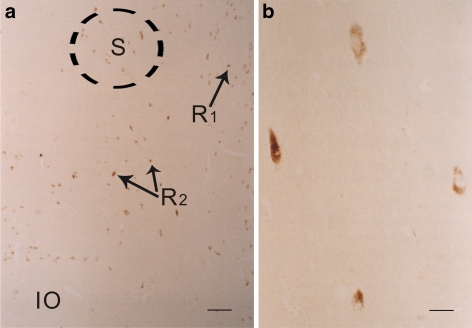
Fig. 7.
a Some positive 5-HT2A (brown) cells were seen in the solitary nucleus (S), in the dorsal raphe (R1), and the rest of the reticular formation (R2), while the inferior olivary nucleus (IO) had no reaction. Bar = 40 μm. b High power of the positive 5-HT2A (brown) cells in solitary nucleus. Bar = 5 μm
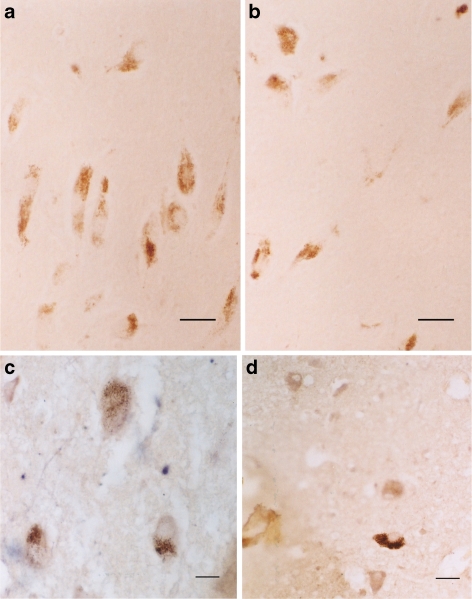
Colocalization was performed between 5-HT1A and 5-HT2A with cholinergic neurons (using ChAT as a marker), dopaminergic neurons (using tyrosine hydroxylase as a marker), GABA neurons (using GABA as a marker), and serotonin neurons (using serotonin as a marker). To date, we have observed only colocalization of 5-HT1A receptors on cholinergic neurons (Fig. 8a, b) and GABA positive fibers ending on 5-HT2A neurons (Fig. 8c). 5-HT1A and 5-HT2A receptor positive cells were compared in the normal and Alzheimer brainstems as follows: Fig. 9a, b showed comparisons of 5-HT2A receptor positive cells in the sensory trigeminal nucleus of normal aged versus Alzheimer patients; Fig. 9c, d showed the comparison of 5-HT1A receptor positive cells in the pontile nuclei of normal aged versus Alzheimer patients; Fig. 10a, b showed 5-TH1A receptor positive cells in raphe nucleus of normal aged versus Alzheimer patients; Fig. 10c, d demonstrated the comparison of 5-HT1A receptor positive cells in the vagal nucleus of normal aged versus Alzheimer patients. The trend was a decrease of receptor positive cells in Alzheimer patients.
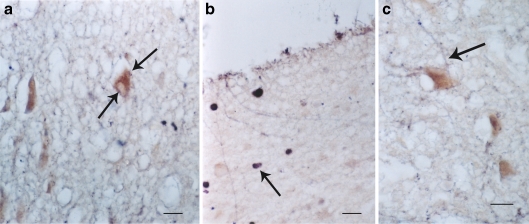
Fig. 8.
a and b A ChAT marker positive (brown) neuron had also blue dots (VIP reaction) of 5-HT1A (arrows) indicating a colocalization of 5-HT1A receptors in cholinergic (ChAT marker) neurons. aBar = 5 μm. b Another cell (arrow) colocalized with 5-HT1A (blue VIP reaction) and cholinergic enzyme (ChAT) (brown) neuron; bar = 5 μm. c GABA positive fibers (blue, VIP reaction) (arrow) ends on a 5-HT2A positive neurons. Bar = 5 μm
Fig. 9.
a and b The positive 5-HT2A (brown) cells in the sensory trigeminal nucleus. Normal aged (a) versus Alzheimer patients (b). Note more positive cells in normal aged (a) than in Alzheimer patients (b). a and bBar = 5 μm. c and d The positive 5-HT1A (brown) cells in the pontile nuclei of normal aged (c) versus Alzheimer patients (d). Note more positive cells in normal aged (c) than in Alzheimer patients (d). c and dBar = 5 μm
Fig. 10.
a and b The positive 5-HT1A (brown) cells in the raphe nucleus of normal aged (a) versus Alzheimer patients (b). Note more positive cells in normal aged (a) than in Alzheimer patients (b). a and bBar = 5 μm. c and d The positive 5-HT1A (brown) cells (arrow) of the vagal nucleus (floor of IV ventricle) of normal aged (a) versus Alzheimer patients (b). Note more positive cells in normal aged (a) than in Alzheimer patients (b). c and dBar = 5 μm
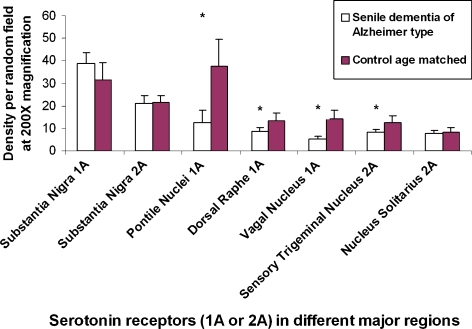
Comparison of the densities of 1A and 2A receptor positive cells in the different brainstem regions between normal age-matched and Alzheimer patients were computed (Fig. 11). The statistical difference (p < 0.01) was particularly significant in 1A receptors of the pontile nuclei, dorsal raphe nuclei, and vagal nuclei. For 2A receptor positive cells, the difference (p < 0.01) was significant only for the sensory trigeminal nuclei. For the substantia nigra and the nucleus solitarius, differences in the density of 2A receptor positive cells were not significant between control age-matched and Alzheimer brainstems.
Fig. 11.
Comparison of density per random field under ×200 of either positive 1A or positive 2A receptor cells in different major regions of distribution in the brainstem between normal aged matched individuals and those of Alzheimer patients. *p < 0.01 and therefore statistically significant between groups
Discussion
Our results revealed the extensive localization of the serotonergic 1A and 2A receptor positive cells inside the human brainstem and therefore inferred the involvement of these serotonin receptors in the functioning of many nuclei in the brainstem. There was also a general decrease of these positive cells in all the nuclei selected for study in Alzheimer patients, with the exception of the substantia nigra. These findings in the human are de novo information and must be kept in mind in future therapeutic studies with serotonin-related psychiatric drugs that will also influence the brainstem. Further, serotonin 1A receptor positive cells were located only in substantia nigra, pontile, dorsal raphe, and vagal nuclei. It is unknown at this time whether this phenomenon was related to a general loss of neurons in Alzheimer disease or to specific loss of particular groups of neurons, such as those of the cortex (Li et al. 1997; Lorke et al. 2006; Yew et al. 1999a, b). 5-HT1A and 5-HT2A positive cells have been documented to exist in the dorsal raphe nucleus in animals via pharmacological studies (Curran and Leiter 2007; Freitas et al. 2008) and in humans with PET scans (Lundberg et al. 2007). Using immunohistochemistry, our study verified the results of Lundberg et al. (2007) showing 5-HT1A receptor positive cells in the dorsal raphe of the human. We, however, could not see any significant amount of 5-HT2A positive cells in the same human nucleus as reported in animals (Freitas et al. 2008). The function of both 5-HT receptor positive cells in this study may be involved with antinociception (Freitas et al. 2008); their roles in behavior anxiety and depression have been illustrated by others (Delgado et al. 2005; Lira et al. 2003). 5-HT1A and 2A positive cells in the dorsal raphe might also control sleep and thus have an important relationship in depression (Ulak et al. 2010).
Serotonin immunoreactive neurons have been reported in the trigeminal nucleus of mammals (Lazarov 2002). This study has pinpointed the localization of serotonin 2A receptor cells in the same nucleus, suggesting specific serotonergic modulation or function in the trigeminal complex. In addition, presence of 5-HT1A receptor positive cells in the vagal nucleus and 5-HT2A receptor positive cells in the solitary nucleus indicates the role of these receptor positive cells in internal regulation of the viscera, as in cardiac control (Jordan 2005), baroreceptor reflex (Raul 2003), and respiration (Ozawa and Okado 2002). Serotonin 1A and 2A receptor participation in important visceral functions such as cardiovascular respiratory regulation has also been documented by other workers (Kennett et al. 1987; Meyer et al. 2006; Nalivaiko et al. 2005; Ogren et al. 2008; Popa et al. 2005; Schreiber et al. 1998; Ulak et al. 2010). The present study showed that the positive 5-HT1A cells were in the dorsal vagal nucleus, while 5-HT2A cells were in the solitary nucleus. Both are known as important areas for visceral function (Yew et al. 1990). Whether these 1A and 2A receptors are also involved in sleep apnea is still controversial (Sawaguchi et al. 2003). On the other hand, the participation of serotonin-related cells in depression and suicide has been frequently suggested (see review Arango et al. 2002). Past experiments have mostly been concerned with the cortex, e.g., in the prefrontal cortex (Lorke et al. 2006), while the involvement of these diseases of the brainstem remains elusive. The presence of 5-HT1A receptor positive cells in the pontile nucleus in this study is interesting because of the cerebellar connections (Brodal 1981). It is highly likely that at least 5-HT1A plays a role in the cortical-cerebellar-vestibular functions. Apoptosis in cells with different transmitters in Alzheimer brainstem versus those of age-matched controls has been documented in the cerebral cortex in other studies (Li et al. 1997; Yew et al. 1999a, b). In this study, decrease of serotonin receptor 1A and 2A receptor positive cells in the brainstem of these patients may also mean either cell death of specific types of neurons or downregulation of receptor production. Thus, the present study further strengthens the need to review the brainstem as vigorously as the cortex.
While 5-HT1A and 5-HT2A were observed in common regions like the periaqueductal gray, in the substantia nigra, the pontile nucleus, and the dorsal raphe, they might exist individually and not together, e.g., 5-HT1A in the dorsal vagal nucleus and 5-HT2A in the trigeminal nucleus and the solitary nucleus. There were suggestion where 1A and 2A were always present together in the cortex (Yuen et al. 2008); this, however, might not be the case in our study, as either 5-HT1A or 5-HT2A receptors might be present solely in some nuclei. The decrease of serotonin 5-HT1A receptors in nuclei like the pontile nuclei, the dorsal vagal nucleus, and the dorsal raphe nucleus has pointed to cerebellar (ponto cerebellar link) and autonomic (vagal) disturbances and also disturbances leading to insomnia and depression (dorsal raphe). The presence of the serotonin receptors in the trigeminal nucleus is interesting, perhaps equivalent to the pain pathway as documented in the substantia gelatinosa of the spinal cord (Abe et al. 2009). The decrease of 5-HT2A receptors in the sensory trigeminal nucleus might point to derangement of facial sensation. These downregulations elicited in the brainstem might combine with those of the cortex to form a group of complex syndromes that could become apparent in the Alzheimer patients. This is something we should note with care. Related studies on receptors 5-HT1A and 1B on the brainstem nuclei have been documented in the postnatal rat: The positive sites were mainly in the raphe nucleus, nucleus ambiguus, hypoglossal nucleus, and the reticular, and solitary nucleus (Liu and Wong-Riley 2010). In this adult human study, we have additional positive locations, e.g., pontile nucleus, vagal nucleus, and trigeminal nucleus. This may be due to the differences between adult versus postnatal or simply the differences in species. Finally, although many transmitters have been documented in the human brainstem (Li et al. 2007; Lorke et al. 2003; Shen et al. 1989; Tiu et al. 1993; Wai et al. 2004; Yew et al. 1990, 1991, 1992, 1996, 1999c; Yew and Chan 1999), results have been scattered along the cortex and different nuclei, which has made correlation difficult. For example, it had been documented that many sites in the medulla contained catecholaminergic neurons (Lorke et al. 2003) and NPY positive cells (Wai et al. 2004), while the midbrain raphe nucleus and reticular formation contained serotonin cells (Shen et al. 1989). However, attempting to colocalize specific types of neurons in this study with different transmitters resulted in finding that only the cholinergic neurons have serotonin 5-HT1A receptors, while on the other hand, GABAergic fibers innervated 5-HT2A positive neurons. This latter interaction of GABA and 5-HT2A receptors has been suggested in the cortex (Yan 2002). Since this is the first study in this area, the interaction of 5-HT receptors with different neurons certainly needs to be pursued in greater detail in future research.
There had been very few studies on 5-HT1A and 5-HT2A receptors in Alzheimer’s disease. Salmon (2007) reported that in these patients, there was a loss of 5-HT2A receptors in the association cortex and 5-HT1A receptors in the hippocampus. He suggested that these might be related to hyperactivity, aggression, and memory lost (Salmon 2007). Our study here pointed out that there were 5-HT1A and 5-HT2A changes in the brainstem as well. Costall et al. (1990) reported that 5-TH1A receptors were related to cholinergic projections. In this study, we found that cholinergic neurons themselves had serotonin receptors (see above). Rocchi et al. (2003) reported that 5-HT2A polymorphosis were related to psychosis. Ramanathan and Glatt (2009) suggested that at least 5-HT2A receptors were involved in psychosis and delusion. Similar changes in isolated mice showed reduction of 5-HT receptors in prefrontal cortex, hippocampus, hypothalamus, and midbrain and those associated with hyperactive and aggressive behavior (Bibancos et al. 2007). It is hoped that in the future there are more studies in these directions.
References
- Abe K, Kato G, Katafuchi T, Tamae A, Furue H, Yoshimura M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience. 2009;159:316–324. doi: 10.1016/j.neuroscience.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/S0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Mehta M, Kanjilal B. The 5-HT receptor: a signaling 1A hub linked to emotional balance. In: Chattopadhyay A, editor. Serotonin receptors in neurobiology. Boca Raton: CRC; 2007. pp. 133–156. [PubMed] [Google Scholar]
- Bibancos T, Jardim DL, Aneas I, Chiavegatto S. Social isolation and expression of serotonergic neurotransmission-related genes in several brain areas of male mice. Genes Brain Behav. 2007;6:529–539. doi: 10.1111/j.1601-183X.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- Brodal A (1981) Neurological anatomy, 3rd edn. Oxford University Press, Oxford, 1053pp
- Burnet PW, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res. 1995;676:157–168. doi: 10.1016/0006-8993(95)00104-X. [DOI] [PubMed] [Google Scholar]
- Costall B, Barnes JM, Hamon M, Müller WE, Briley M. Biochemical models for cognition enhancers. Pharmacopsychiatry. 1990;23(Suppl 2):85–88. doi: 10.1055/s-2007-1014540. [DOI] [PubMed] [Google Scholar]
- Curran AK, Leiter JC. Baroreceptor-mediated inhibition of respiration after peripheral and central administration of a 5-HT1A receptor agonist in neonatal piglets. Exp Physiol. 2007;92:757–767. doi: 10.1113/expphysiol.2007.037481. [DOI] [PubMed] [Google Scholar]
- Delgado M, Caicoya AG, Greciano V, Benhamú B, López-Rodríguez ML, Fernández-Alfonso MS, Pozo MA, Manzanares J, Fuentes JA. Anxiolytic-like effect of a serotonergic ligand with high affinity for 5-HT1A, 5-HT2A and 5-HT3 receptors. Eur J Pharmacol. 2005;511:9–19. doi: 10.1016/j.ejphar.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Frazer A, Hensler JG. Serotonin receptors. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic neurochemistry: molecular, cellular, and medical aspects. Philadelphia: Lippincott-Raven; 1999. pp. 263–292. [Google Scholar]
- Freitas RL, Bassi GS, Oliveira AM, Coimbra NC. Serotonergic neurotransmission in the dorsal raphe nucleus recruits in situ 5-HT(2A/2C) receptors to modulate the post-ictal antinociception. Exp Neurol. 2008;213:410–418. doi: 10.1016/j.expneurol.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikström H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res. 1997;745:96–108. doi: 10.1016/S0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol. 2005;90:175–181. doi: 10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dourish CT, Curzon G. Antidepressant-like action of 5-HT1A agonists and conventional antidepressants in an animal model of depression. Eur J Pharmacol. 1987;134:265–274. doi: 10.1016/0014-2999(87)90357-8. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. doi: 10.1016/S0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- Li WP, Chan WY, Lai HW, Yew DT. Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J Mol Neurosci. 1997;8:75–82. doi: 10.1007/BF02736774. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu G, Antonio GE, Mak YT, Rudd JA, Fan M, Yew DT. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int. 2007;50:848–857. doi: 10.1016/j.neuint.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/S0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience. 2010;165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorke DE, Kwong WH, Chan WY, Yew DT. Development of catecholaminergic neurons in the human medulla oblongata. Life Sci. 2003;73(10):1315–1331. doi: 10.1016/S0024-3205(03)00430-2. [DOI] [PubMed] [Google Scholar]
- Lorke DE, Lu G, Cho E, Yew DT. Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 2006;7:36. doi: 10.1186/1471-2202-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J, Borg J, Halldin C, Farde L. A PET study on regional coexpression of 5-HT1A receptors and 5-HTT in the human brain. Psychopharmacology (Berl) 2007;195:425–433. doi: 10.1007/s00213-007-0928-3. [DOI] [PubMed] [Google Scholar]
- Meyer LC, Fuller A, Mitchell D. Zacopride and 8-OH-DPAT reverse opioid-induced respiratory depression and hypoxia but not catatonic immobilization in goats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R405–R413. doi: 10.1152/ajpregu.00440.2005. [DOI] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, Huston JP, Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in the medullary raphe reduces cardiovascular changes elicited by acute psychological and inflammatory stresses in rabbits. Am J Physiol Regul Integr Comp Physiol. 2005;289:R596–R604. doi: 10.1152/ajpregu.00845.2004. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekström JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Popa D, Léna C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M, Adrien J. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25:11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Glatt SJ. Serotonergic system genes in psychosis of Alzheimer dementia: meta-analysis. Am J Geriatr Psychiatry. 2009;17:839–846. doi: 10.1097/JGP.0b013e3181ab8c3f. [DOI] [PubMed] [Google Scholar]
- Raote I, Bhattacharya A, Panicker MM. Serotonin 2A (5-HT2A) receptor function: ligand-dependent mechanisms and pathways. In: Chattopadhyay A, editor. Serotonin receptors in neurobiology. Boca Raton: CRC; 2007. pp. 105–132. [PubMed] [Google Scholar]
- Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol. 2003;23:709–726. doi: 10.1023/A:1025096718559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi A, Micheli D, Ceravolo R, Manca ML, Tognoni G, Siciliano G, Murri L. Serotoninergic polymorphisms (5-HTTLPR and 5-HT2A): association studies with psychosis in Alzheimer disease. Genet Test. 2003;7:309–314. doi: 10.1089/109065703322783662. [DOI] [PubMed] [Google Scholar]
- Salmon E. A review of the literature on neuroimaging of serotoninergic function in Alzheimer's disease and related disorders. J Neural Transm. 2007;114:1179–1185. doi: 10.1007/s00702-007-0636-5. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Ozawa Y, Patricia F, Kadhim H, Groswasser J, Sottiaux M, Takashima S, Nishida H, Kahn A. Serotonergic receptors in the midbrain correlated with physiological data on sleep apnea in SIDS victims. Early Hum Dev. 2003;75(Suppl):S65–S74. doi: 10.1016/j.earlhumdev.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Saxena PR. Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol Ther. 1995;66:339–368. doi: 10.1016/0163-7258(94)00005-N. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Melon C, Vry J. The role of 5-HT receptor subtypes in the anxiolytic effects of selective serotonin reuptake inhibitors in the rat ultrasonic vocalization test. Psychopharmacology (Berl) 1998;135:383–391. doi: 10.1007/s002130050526. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WZ, Luo ZB, Zheng DR, Yew DT. Immunohistochemical studies on the development of 5-HT (serotonin) neurons in the nuclei of the reticular formations of human fetuses. Pediatr Neurosci. 1989;15:291–295. doi: 10.1159/000120485. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Bubenikova-Valesova V, Horacek J, Bert B. Serotonin1A receptors in the pathophysiology of schizophrenia: development of novel cognition-enhancing therapeutics. Adv Ther. 2008;25:1037–1056. doi: 10.1007/s12325-008-0102-2. [DOI] [PubMed] [Google Scholar]
- Tiu SC, Li WY, Luo CB, Yew DT. Habenulo-interpeduncular descending pathways and their relationship to enkephalin- and somatostatin-immunoreactive neurons in the interpeduncular nucleus of human fetuses. Neuroscience. 1993;53:489–493. doi: 10.1016/0306-4522(93)90213-Y. [DOI] [PubMed] [Google Scholar]
- Ulak G, Mutlu O, Tanyeri P, Komsuoglu FI, Akar FY, Erden BF. Involvement of serotonin receptor subtypes in the antidepressant-like effect of trim in the rat forced swimming test. Pharmacol Biochem Behav. 2010;95:308–314. doi: 10.1016/j.pbb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Wai SM, Kindler PM, Lam ET, Zhang A, Yew DT. Distribution of neuropeptide Y-immunoreactive neurons in the human brainstem, cerebellum, and cortex during development. Cell Mol Neurobiol. 2004;24:667–684. doi: 10.1023/B:CEMN.0000036404.39432.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai MS, Shi C, Kwong WH, Zhang L, Lam WP, Yew DT. Development of the human insular cortex: differentiation, proliferation, cell death, and appearance of 5HT-2A receptors. Histochem Cell Biol. 2008;130:1199–1204. doi: 10.1007/s00418-008-0497-5. [DOI] [PubMed] [Google Scholar]
- Wesołowska A. In the search for selective ligands of 5-HT5, 5-HT6 and 5-HT7 serotonin receptors. Pol J Pharmacol. 2002;54:327–341. [PubMed] [Google Scholar]
- Yan Z. Regulation of GABAergic inhibition by serotonin signaling in prefrontal cortex: molecular mechanisms and functional implications. Mol Neurobiol. 2002;26:203–216. doi: 10.1385/MN:26:2-3:203. [DOI] [PubMed] [Google Scholar]
- Yew DT, Chan WY. Early appearance of acetylcholinergic, serotoninergic, and peptidergic neurons and fibers in the developing human central nervous system. Microsc Res Tech. 1999;45:389–400. doi: 10.1002/(SICI)1097-0029(19990615)45:6<389::AID-JEMT6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Yew DT, Luo CB, Zheng DR, Guan YL, Lin YQ, Chen WZ. Development and localization of enkephalin and substance P in the nucleus of tractus solitarius in the medulla oblongata of human fetuses. Neuroscience. 1990;34:491–498. doi: 10.1016/0306-4522(90)90157-Y. [DOI] [PubMed] [Google Scholar]
- Yew DT, Pang KM, Mok YC. Immunohistochemical and ultrastructural studies of the various nuclei of the trigeminal complex in the human newborn. Neuroscience. 1991;45:23–35. doi: 10.1016/0306-4522(91)90100-3. [DOI] [PubMed] [Google Scholar]
- Yew DT, Luo CB, Shen WZ. Immunohistochemical localization of enkephalin and substance P in the nucleus caudalis of the spinal trigeminal V in the medulla oblongata of the human fetus. Neuroscience. 1992;51:185–190. doi: 10.1016/0306-4522(92)90483-I. [DOI] [PubMed] [Google Scholar]
- Yew DT, Webb SE, Lam ET. Neurotransmitters and peptides in the developing human facial nucleus. Neurosci Lett. 1996;206:65–68. doi: 10.1016/0304-3940(96)12435-6. [DOI] [PubMed] [Google Scholar]
- Yew DT, Li WP, Webb SE, Lai HW, Zhang L. Neurotransmitters, peptides, and neural cell adhesion molecules in the cortices of normal elderly humans and Alzheimer patients: a comparison. Exp Gerontol. 1999;34:117–133. doi: 10.1016/S0531-5565(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Yew DT, Wong HW, Li WP, Lai HW, Yu WH. Nitric oxide synthase neurons in different areas of normal aged and Alzheimer's brains. Neuroscience. 1999;89:675–686. doi: 10.1016/S0306-4522(98)00383-2. [DOI] [PubMed] [Google Scholar]
- Yew DT, Chan WY, Luo CB, Zheng DR, Yu MC. Neurotransmitters and neuropeptides in the developing human central nervous system. A review. Biol Signals Recept. 1999;8:149–159. doi: 10.1159/000014586. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008;283:17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]