Abstract
Hydrogen sulfide gas (H2S) is a putative signaling molecule that causes diverse effects in mammalian tissues including relaxation of blood vessels and regulation of perfusion in the liver, but the effects of aging on H2S signaling are unknown. Aging has negative impacts on the cardiovascular system. However, the liver is more resilient with age. Caloric restriction (CR) attenuates affects of age in many tissues. We hypothesized that the H2S signaling system is negatively affected by age in the vasculature but not in the liver, which is typically more resilient to age, and that a CR diet minimizes the age affect in the vasculature. To investigate this, we determined protein and mRNA expression of the H2S-producing enzymes cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS), H2S production rates in the aorta and liver, and the contractile response of aortic rings to exogenous H2S. Tissue was collected from Fisher 344 × Brown Norway rats from 8–38 months of age, which had been maintained on an ad libitum (AL) or CR diet. The results demonstrate that age and diet have differential effects on the H2S signaling system in aorta and liver. The aorta showed a sizeable effect of both age and diet, whereas the liver only showed a sizeable effect of diet. Aortic rings showed increased contractile sensitivity to H2S and increased protein expression of CSE and CBS with age, consistent with a decrease in H2S concentration with age. CR appears to benefit CSE and CBS protein in both aorta and liver, potentially by reducing oxidative stress and ameliorating the negative effect of age on H2S concentration. Therefore, CR may help maintain the H2S signaling system during aging.
Keywords: H2S, Gasotransmitter, Aging, Caloric restriction, Aorta, Liver
Introduction
Hydrogen sulfide (H2S) is proposed to function as a gasotransmitter (Wang 2002) in the nervous, cardiovascular, and gastrointestinal systems. The two other recognized gasotransmitters are nitric oxide (NO) and carbon monoxide (CO). All three gasotransmitters share several similarities: they are endogenously produced, small gas molecules that are capable of physiological action; they can easily diffuse across cell membranes to exert their function; and they do not require a mechanism of degradation or reuptake because they are all very reactive (Wang 2002). In terms of the dose response, H2S is the least potent of the known gasotransmitters, having physiological effects at micromole per liter concentrations, whereas the physiological effects of CO and NO occur at nanomole per liter concentrations (Pryor et al. 2006).
Abe and Kimura (Abe and Kimura 1996) first demonstrated that H2S is produced in the brain and that it increases N-methyl-d-aspartic acid receptor-mediated responses and facilitates hippocampal long-term potentiation. Since then a variety of physiological roles have been proposed for H2S (Lowicka and Beltowski 2007; Szabo 2007), and at least some capacity for H2S production has been demonstrated in several vertebrate tissues (Dombkowski et al. 2006; Olson 2005; Olson et al. 2006; Zhao et al. 2003), including human mammary artery (Webb et al. 2008). When exogenously applied to isolated blood vessels of vertebrates, H2S elicits responses that range from a monophasic relaxation or contraction, to multiphasic contraction-relaxation-contraction responses, depending on the phylogenetic class and type of vessel examined (Dombkowski et al. 2005). Vascular response to H2S also depends on other experimental factors, such as the presence of an intact endothelium, the method of pre-constriction used, and the oxygen tension of the tissue baths (Koenitzer et al. 2007; Lim et al. 2008; Olson et al. 2008). In the liver, H2S has been proposed to regulate perfusion and maintain portal venous pressure, with deficiencies in H2S production pathways resulting in increases in intra-hepatic resistance (Fiorucci et al. 2005b). H2S may also have a role in regulating biliary bicarbonate secretion (Fujii et al. 2005).
H2S is endogenously produced from l-cysteine predominantly by the enzymes cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS) in a variety of animals, from invertebrates to mammals (Fiorucci et al. 2005b; Gainey and Greenberg 2005; Geng et al. 2004b; Julian et al. 2005; Julian et al. 2002; Kimura 2000; Kimura et al. 2005; Wang 2002; Webb et al. 2008). Recently, 3-mercaptopyruvate sulfurtransferase (3-MST), in conjunction with cysteine aminotransferase, has also been found to produce H2S from cysteine (Shibuya et al. 2009; Shibuya et al. 2008). In mammals, CSE and CBS are differentially expressed (Zhao et al. 2003; Zhao et al. 2001): of the two enzymes, CSE expression is higher in the cardiovascular system, whereas CBS expression is higher in the nervous system (Ebrahimkhani et al. 2005; Geng et al. 2004b; Hosoki et al. 1997; Kimura et al. 2005). In liver and kidney, CSE and CBS are both expressed in high amounts compared with other tissues (Fujii et al. 2005; Lowicka and Beltowski 2007). 3-MST is expressed in vascular endothelial cells and is highly expressed in the brain (Shibuya et al. 2009; Shibuya et al. 2008).
Aging influences the action and/or efficacy of many signaling molecules, including norepinephrine and the more well-studied gasotransmitter NO (Lakatta 1993; Tanaka et al. 2006; van der Loo et al. 2000). A recent study of plasma H2S concentration in humans over 50–80 years of age reported that H2S levels may decline with age (Chen et al. 2005), but whether aging affects the H2S signaling system is unknown. Furthermore, any affect of age on the H2S signaling system may differ among tissues. For example, substantial physiological changes occur with age in the cardiovascular system, leading to endothelial dysfunction (Castello et al. 2005) and dysregulation of blood vessel tone (Anantharaju et al. 2002; Kitani 1991; Kitani 1994). In comparison, the liver is less susceptible to functional changes with age. While liver size and the number of proliferating hepatocytes decline with age, the liver enzymatic activity does not appear to change substantially until very old age (Anantharaju et al. 2002; Kitani 1991; Kitani 1994; Schmucker 1998; Zeeh and Platt 2002).
Caloric restriction (CR) is an intervention that attenuates many effects of aging (Anderson and Weindruch 2010; Labinskyy et al. 2006; Leeuwenburgh and Prolla 2006), including cardiovascular morbidity (van der Loo et al. 2000), cross-linking of cardiac and skeletal muscle proteins (Leeuwenburgh et al. 1997), mitochondrial dysfunction (Aspnes et al. 1997; Payne et al. 2003), loss of skeletal muscle mass (Payne et al. 2003; van der Loo et al. 2000), and endothelial dysfunction (Barja 2002; Castello et al. 2005; Gredilla et al. 2004; Gredilla et al. 2001; Taddei et al. 2006). Furthermore, CR attenuates the age-related impairment of the NO signaling system (Minamiyama et al. 2007; Sharifi et al. 2008; Yang et al. 2004). However, it is unknown whether CR affects the H2S signaling system and whether CR specifically attenuates any affects of age on the H2S signaling system.
In this study, we investigated the effects of aging and CR on the H2S signaling system in liver and aorta in the Fisher 344 × Brown Norway hybrid rat. We hypothesized that the H2S signaling system is negatively affected by age in the vasculature but not in the liver, which is typically more resilient to age, and that a CR diet minimizes the age affect in the vasculature. Liver and aorta tissue obtained from rats of various ages were assayed for CSE and CBS protein and mRNA expression and H2S production rates. Contraction of aorta smooth muscle was used as a functional indicator to investigate the effects of age and CR on functional responses to H2S.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), except where noted otherwise.
Animals
Two groups of male Fisher 344 × Brown Norway hybrid rats of 8, 18, 29, 38 months of age and 14, 27, 34 months of age were obtained from the National Institute on Aging (NIA) rodent colony. These animals were part of larger studies on the biological markers of skeletal muscle fitness and physical function and the relationship between cognition and brain fitness. Additional data resulting from these animals has also been published (Carter et al. 2009; Hofer et al. 2009; Marzetti et al. 2008). At NIA, all rats were fed the NIH31 diet ad libitum (AL) until 14 weeks of age. For CR animals, the calorie restricted regimen was incrementally established by 10% CR per week for 4 weeks, reaching 40% CR by 17 weeks. The NIH31 diet provided 60% of the calories and 100% of the vitamins compared with the diet consumed by AL animals. After arriving at our facilities (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care), animals were housed under standard conditions (12 h light, 12 h dark) and provided with food and water using either the NIH31 diet or the NIH31-NIA fortified diet at 40% CR, for AL and CR animals, respectively. Myography studies were performed on tissues from animals of 14, 27, and 34 months of age. All other studies were performed on tissues from animals of 8, 18, 29, and 38 months of age. The method of anesthesia affects the H2S response in vascular tissues from a variety of vertebrates (Dombkowski et al. 2005). To reduce this potential artifact, euthanasia was by guillotine under IACUC-approved methods for all animals used in this study.
Western blotting
Western blotting was used to evaluate CSE and CBS protein expression in liver and aorta samples. Liver and aorta were removed within 5 min of euthanasia, frozen in liquid nitrogen and stored at -80°C. Samples were suspended in a lysis buffer consisting of 50 mmol/L Tris base, 2% (w/v) sodium dodecyl sulfate, 10 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L dithiothreitol, 0.5 mmol/L sodium tetraborate, and 1% (v/v) Halt protease inhibitor cocktail (Thermo Fisher Scientific Rockford, IL USA). Western blotting was performed using commercially available monoclonal antibodies for CSE and CBS (Abnova, Neihu District, Taipei City, Taiwan), a monoclonal antibody for β-actin (Cell Signaling Technology, Danvers, MA, USA), and a secondary antibody conjugated to alkaline phosphatase (Sigma-Aldrich St. Louis, MO, USA). Protein was separated using SDS-PAGE and transferred onto PVDF membrane (Immobilon P, Millipore, Billerica, MA USA) using a semidry blotter (BioRad, Hercules, CA USA). Using the Snap-ID system (Millipore, Billerica, MA USA) at room temperature, membranes were blocked in 0.05% non-fat milk in phosphate-buffered saline with 0.05% Tween-20 (PBST), washed in PBST and incubated 10 min with appropriate primary antibodies (diluted 1:333 in PBST). Membranes were then washed in PBST four times and subsequently incubated 10 min with secondary antibody diluted 1:3,333 in blocking buffer. Membranes were washed two times in PBST, once in phosphate-buffered saline, and once in 100 mmol/L Tris-HCl, pH 9.5. Chemiluminescent substrate (DuoLux, Vector Laboratories, Burlingame, CA USA) was used to generate a signal that was captured with a digital imager (GeneSnap, Syngene, Frederick, MD USA). Images were analyzed using commercial software (Quantity One, BioRad, Hercules, CA USA), with which band intensity was determined using local background subtraction.
RNA extraction
RNA was extracted from liver and aorta samples to evaluate mRNA expression for CSE and CBS using real-time polymerase chain reaction (PCR), for comparison to the protein expression data. Liver and aorta tissues were disrupted into powder under liquid nitrogen. All samples for each tissue were processed on the same day. For liver, total RNA was extracted using an Arum total RNA mini kit (BioRad, Hercules, CA USA). For aorta, total RNA was extracted using Tri reagent and a glass homogenizer (Kontes Glass, Vineland, NJ USA).
Real-time PCR
Relative quantitative real time PCR was run in 96-well PCR plates in an Applied Biosystems 7300 Real-time PCR system (Life Technologies, Carlsbad, CA USA). Primers were designed using commercial software (Premier Biosoft International, Palo Alto, CA USA) and synthesized by Integrated DNA Technologies (Coralville, IA USA). The primers used were as follows: CSE sense 5′-TGGGCTTAGTGTCTGTTAATTCC-3′, CSE antisense 5′-GGCAGCAGAGGTAACAATCG-3′, CBS sense 5′-TGCCTGAGAAGATGAGTATG-3′, CBS antisense 5′-GGTCCAGAATGTGAGAATTG-3′, Actin sense 5′-CTCCCAGCACACTTAACTTAG C-3′, Actin antisense 5′-AAAGCCACAAGAAACACTCAGG-3′, 18S rRNA sense 5′-CGAGGAATTCCCAGTAAGTGC-3′, 18S rRNA antisense 5′-CCATCCAATCGCTAGTAGCG-3′. For both liver and aorta plates, a standard curve for the housekeeping genes β-actin (run with CSE samples) or 18S rRNA (run with CBS samples) was run with a standard curve for the gene of interest (GOI), with all samples in triplicate. The remainder of the plate was filled with eight random samples plus one calibrator sample for plate-to-plate corrections, loaded in triplicate for both the housekeeping gene and the GOI. Additional plates were run containing the calibrator sample on each plate until all samples had been processed. All plates for a given tissue-GOI combination were made and run on the same day, using the same real-time PCR machine.
Hydrogen sulfide production
H2S production was measured essentially as previously described (Dombkowski et al. 2006; Julian et al. 2002; Stipanuk and Beck 1982). Frozen tissues were ground into a fine powder under liquid nitrogen with mortar and pestle. For each sample, two small scoops (approximately 50 mg wet weight) were added to 1.5 mL 100 mmol/L potassium phosphate buffer (pH 7.4) and homogenized with a Polytron homogenizer (Kinematica, Bohemia, NY USA). A 1 mL aliquot of this homogenate solution was placed in the outer well of a 25 mL glass flask containing an additional plastic center well (Kontes Glass, Vineland, NJ USA), which contained 0.5 mL 1% (w/v) zinc acetate and a piece of chromatography paper (Whatman grade 3MM Chr, Maldstone England) cut to 2.5 by 4.5 cm and folded into a fan shape. The CSE and CBS substrate l-cysteine (10 mmol/L) and cofactor pyridoxal-5′-phosphate (PLP; 2 mmol/L) were added to the outer well, after which the flask was sealed with a septum stopper and flushed with N2 gas for 30 s. Each flask was incubated on a shaker at 37°C for 1.5 h for liver and 8 h for aorta. After incubation, 1 mL 50% (w/v) trichloroacetic acid was added to the outer well to stop enzyme activity and to convert all S2− and HS− to H2S. The zinc acetate solution on the chromatography paper reacts with volatilized H2S to form zinc sulfide, which is relatively stable. The flasks were then incubated for an additional hour to allow remaining H2S to volatilize and form zinc sulfide. The filter paper was then removed from the center well and placed in a test tube containing 3.5 mL of de-ionized water, 0.4 mL 20 mmol/L N,N-dimethyl-p-phenylenediamine oxalate in 7.2 mol/L HCl and 0.4 mL of 30 mmol/L FeCl3 in 1.2 mol/L HCl. The test tubes were gently vortexed and incubated for 20 min at room temperature. Absorbance of the solution in the test tube was read at 670 nm with a plate reader (BioTek Instruments, Inc., Winooski, VT USA). Absorbance measurements were calibrated against a standard curve generated from NaHS in deoxygenated, de-ionized water that was added to the outside wells, incubated at 37°C for 1.5 h, and assayed as above. To ensure that all measured H2S production is by tissue enzymes, several controls were run in addition to sample homogenates: a negative control of homogenate but no cofactors or substrate; and a blank of all cofactors and substrate in phosphate buffer, but without homogenate. Inhibitors of CSE (20 mmol/L propargylglycine, Pgly) and CBS (1 mmol/L aminooxyacetic acid, AOAA) were added to confirm the H2S production was through these, and not alternate pathways (Julian et al. 2002). Protein concentration was quantified with a NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA USA), to calculate nanomole H2S production per hour per milligram (nmol H2S/h/mg) protein.
Myography
The effect of H2S on vascular tone was measured in 5 mm width aorta rings from 41 animals, with five to eight rings tested for each age × diet treatment combination. Immediately after euthanasia, the aorta was removed, cleaned of fat and connective tissue, and sectioned into rings. Rings were attached with stainless steel wire to force transducers and mounted in a 37°C tissue bath system (Radnoti Glass Technology, Monrovia, Ca USA) containing Krebs bicarbonate buffer. Rings were allowed to equilibrate after mounting for a minimum of 1 h, and a baseline tension of 1.5 g was maintained throughout each experiment. At the start of an experiment, each ring was maximally contracted with two sequential additions of 80 mmol/L KCl, with a wash step in between each addition. Acetylcholine (1 µmol/L) was used to check for a functional endothelium. If the aortic rings did not constrict to KCl or relax to acetylcholine, they were assumed to be damaged and were discarded. After washing with Krebs buffer and returning to baseline tension, the rings were incubated with 10 µmol/L propranolol (to block β-adrenergic receptor relaxation and maximize α-adrenergic receptor contraction), and precontracted with 1 µmol/L norepinephrine (NE). After the NE precontraction stabilized, rings were exposed to 100 µmol/L H2S (diluted from a 100 mmol/L NaHS stock solution). After 30–45 min, the tissue bath was drained and the rings were washed twice with Krebs buffer and allowed to return to baseline tension. This sequence of propranolol, NE and H2S addition was repeated for 300, 600, and 900 µmol/L H2S. Data for H2S-induced contractions for each aortic ring were standardized to the weight of the ring.
Actual total H2S concentrations, as measured by a methylene blue assay (Gilboa-Garber 1971), were approximately 75% of the predicted value (presumably due mostly to oxidation). In physiological saline at pH 7.4, the dissociation of H2S results in approximately 1/3 of total H2S as H2S and 2/3 as HS− (Beauchamp et al. 1984), and it is unknown whether the physiological effects of hydrogen sulfide are mediated only through H2S gas, or if the HS- anion is involved as well (Olson and Donald 2009).
Statistics
All statistical analyses were performed using JMP statistical software (JMP 7.0, SAS Institute, Cary, NC, USA), with alpha ≤0.05 considered significant. Two-way analyses of variance (ANOVAs) were performed with a Tukey' post-hoc test, when possible. However, the functional response data were not always normally distributed, and therefore these data violated the assumptions of the ANOVA. In those cases, significant effects of age and diet were tested using the Kruskal–Wallis nonparametric ANOVA, and post-hoc testing between these groups was done with a Wilcoxon's non-parametric t test. If the two-way ANOVA showed no significant effects or interactions, data were pooled and run using a one-way ANOVA (pooled by age) or t test (pooled by diet). For real-time PCR, statistical analyses were performed on the average cycle threshold (Ct) of each sample, since the Cts are normally distributed (Wood et al. 2005).
Results
CSE and CBS protein expression
As expected, the relative expression of CSE and CBS protein differed between aorta and liver. In aorta, CSE protein expression (relative to β-actin) was 1.43 fold higher than CBS protein expression on average, whereas in liver, CBS protein expression (relative to β-actin) was 1.18 fold higher than CSE protein expression, on average.
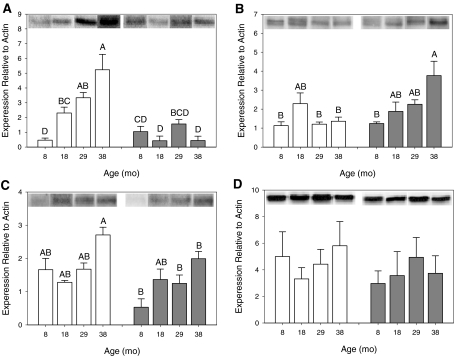
CSE In both aorta and liver, CSE protein expression was significantly affected by age, diet, and an interaction between age and diet (Fig. 1a, b). In aorta, CSE protein expression increased with age in AL animals, but it was unchanged with age in CR animals (Fig. 1a, bars; 2-way ANOVA with Tukey's post-hoc, F = 13.9, p < 0.0001). Specifically, at ages 18, 29, and 38 months, CSE protein expression was significantly lower in CR animals than in AL animals, whereas CSE expression at 8 months was not affected by diet (Fig. 1a, asterisks). In liver, CSE protein expression was unchanged with age in AL animals but increased with age in CR animals (Fig. 1b, bars; 2-way ANOVA with Tukey's post-hoc, F = 4.90, p = 0.0041). At 38 months, AL CSE protein expression was significantly lower than CR (Fig. 1b, asterisks).
Fig. 1.
Effect of age and diet on CSE and CBS protein expression. CSE expression is shown in aorta (a, n = 3–5 per group) and liver (b, n = 3 per group). CBS expression is shown in aorta (c, n = 3–4 per group) and liver (d, n = 3 per group). The y-axis shows CSE (a and b) or CBS (c and d) protein expression relative to β-actin. The x-axis shows the age in months (mo). AL data are in white columns and CR data are in grey columns. Representative blots are aligned along the top, above each column. Data are represented as mean ± SE and were analyzed by 2-way ANOVA with Tukey's post-hoc tests. Means that do not share a common letter are significantly different
CBS In aorta but not liver, CBS expression was significantly affected by age, diet, and an interaction between age and diet (Fig. 1c, d). In aorta, CBS protein expression at 38 months was significantly higher in AL animals than in CR animals (Fig. 1c, asterisks; 2-way ANOVA with Tukey post-hoc, F = 4.34, p = 0.0056). In liver, there was no significant effect of age, diet, or an interaction between age and diet on CBS expression (Fig. 1d, 2-way ANOVA, F = 1.44, p = 0.257).
CSE and CBS mRNA expression
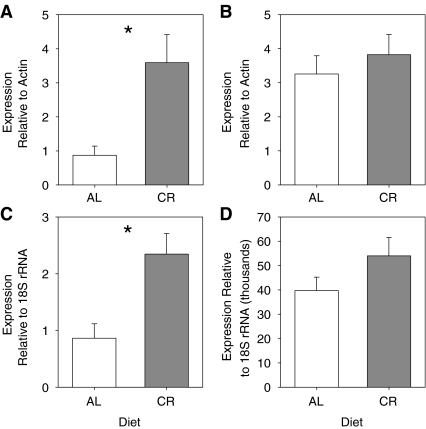
There were no interactions between age and diet on mRNA expression of CSE or CBS in aorta or liver using a two-way ANOVA (Fig. 2). Therefore, data were pooled by age and pooled by diet and tested with one-way ANOVA and t tests, respectively. CSE and CBS mRNA expression was affected by diet, but not age. In aorta, the CR diet significantly increased expression of both CSE mRNA (Fig. 2a, one-tailed t test, p = 0.0013) and CBS mRNA (Fig. 2c, one-tailed t test, p = 0.0258) compared with the AL diet. In liver, diet did not affect the expression of CSE mRNA (Fig. 2b, pooled t test, p = 0.142) or CBS mRNA (Fig. 2d, pooled t test, p = 0.261).
Fig. 2.
Effect of diet on CSE and CBS mRNA expression. CSE expression is shown in aorta (a, n = 13 AL, n = 12 CR) and liver (b, n = 12 per group). CBS expression is shown in aorta (c, n = 12 AL, n = 11 CR) and liver (d, n = 12 per group). The y-axis shows CSE (a and b) protein expression relative to β-actin and CBS (c and d) protein expression relative to 18S rRNA. The x-axis shows the diet. AL data are in white columns and CR data are in grey columns. Data are represented as mean ± SE and were analyzed by one-tailed or pooled t tests. Means that are significantly different are indicated by asterisks
Hydrogen sulfide production
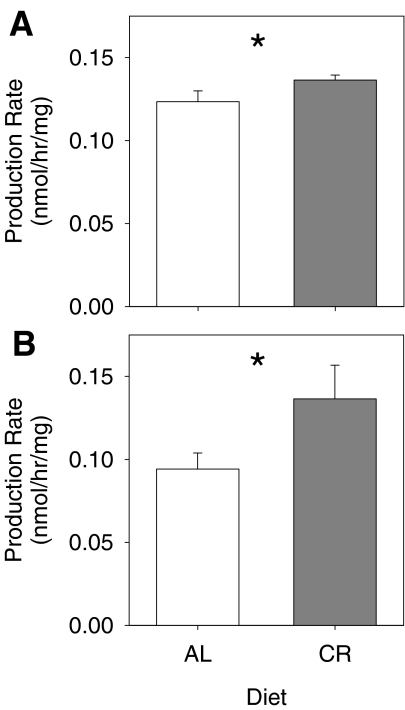
There was no effect of age, diet, or an interaction between age and diet on the capacity for H2S production in either aorta or liver using a two-way ANOVA (Fig. 3). Therefore, data were pooled by age and pooled by diet and tested with one-way ANOVA and t tests, respectively. The capacity for H2S production was affected by diet, but not age. In both aorta and liver, H2S production was higher in tissues of CR animals than AL animals (Fig. 3a, one-tailed t test, p = 0.0407 and Fig. 3b, one-tailed t test, p = 0.0411, respectively). Inhibitors of the pyridoxal-5'-phosphate (PLP)-dependent enzymes CSE and CBS were added to confirm that H2S production was occurring via the actions of these enzymes (N = 4). Addition of the CSE inhibitor Pgly (20 mmol/L) decreased H2S production by 57% in aorta (Ctrl = 0.143 ± 0.006 nmol H2S/hr/mg protein vs. Pgly = 0.061 ± 0.002 nmol H2S/h/mg protein) and by 94% in liver (Ctrl = 0.439 ± 0.026 nmol H2S/h/mg protein vs. Pgly = 0.024 ± 0.005 nmol H2S/h/mg protein). Addition of the CBS inhibitor AOAA (1 mmol/L) decreased H2S production by 50% in aorta (Ctrl = 0.143 ± 0.006 nmol H2S/h/mg protein vs. AOAA = 0.071 ± 0.008 nmol H2S/h/mg protein) and by 81% in liver (Ctrl = 0.331 ± 0.041 nmol H2S/h/mg protein vs. AOAA = 0.064 ± 0.011 nmol H2S/h/mg protein).
Fig. 3.
Effect of diet on H2S production. H2S production rates are shown in aorta (a, n = 17 AL, n = 21 CR) and liver (b, n = 12 per group). The y-axis shows production as nmol H2S/h/mg protein. The x-axis shows the diet. AL data are in white columns and CR data are in grey columns. Data are represented as mean ± SE and were analyzed by one-tailed t tests. Means that are significantly different are indicated by asterisks
Contractile response to hydrogen sulfide in aorta
Application of KCl and NE initiated contractions that were used to ascertain ring viability and responsiveness. Age did not significantly affect the contractions in response to KCl or norepinephrine (data not shown, one-way ANOVA, p > 0.05 for both). However, the contractions in response to KCl and NE were significantly stronger in rings from CR animals than from AL animals (Fig. 4a, b, one-tailed t test, p = 0.0282 and p < 0.0001, respectively).
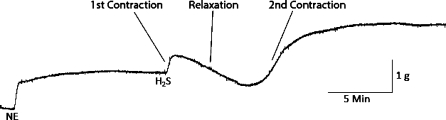
Fig. 4.
Representative tension tracing of a rat aorta ring pre-contracted with norepinephrine (NE) and exposed to H2S (H2S, 300 µmol/L). Scale bar shows time in minutes and tension in grams
Application of H2S to isolated aorta rings typically elicited a triphasic, contraction-relaxation-contraction response (Fig. 5). The contraction in the third phase was larger in magnitude and longer lasting than the contraction in the first phase, although at the lowest H2S concentration used (100 µmol/L) the third phase contraction was typically very weak or absent. Contractile response data that were not normally distributed were analyzed using non-parametric tests (see “Materials and methods”).
Fig. 5.
Effect of age the first-phase contraction and second-phase relaxation to 100 µM H2S. The first-phase contraction (a, n = 12, 15, and 14, from 14–34 month, respectively) and second phase relaxation (b, n = 12, 15, and 14, from 14–34 month, respectively) to H2S significantly increase in magnitude with age at 100 µmol/L H2S addition. The y-axis represents the H2S-induced tension of aortic rings (grams per milligram) pooled from both diets. The x-axis shows the age (month). Data are presented as means ± SE (a) or as box plots showing the median as a horizontal line, the box covering the 25th and 50th percentiles, and error bars defining the 10th and 90th percentiles (b). Data were analyzed by one-way ANOVA with Tukey's post-hoc test (a) or a Kruskal–Wallis ANOVA with a Wilcoxon's t test for post-hoc comparison (b). Means that do not share a common letter are significantly different
Age significantly affected the first phase of the triphasic contract-relax-contract response to H2S. After application of 100 µmol/L H2S, the first-phase contraction was stronger in rings from 34 month rats than from 14 month rats (Fig. 6a, one-way ANOVA with Tukey's post-hoc, p = 0.0062), even though the first-phase contraction to 300–900 µmol/L H2S did not significantly increase with age (data not shown; one-way ANOVA, p ≥ 0.182 for 300–600 µmol/L; Kruskal–Wallis ANOVA, p = 0.216 for 900 µmol/L). As with the first-phase contraction, age significantly affected the second phase relaxation after application of 100 µmol/L H2S (Fig. 6b, Kruskal–Wallis ANOVA, p = 0.038) and post-hoc testing with a Wilcoxon's non-parametric t test showed significant differences between 14 and 34 months (p = 0.0109), but there was no affect of age at 300–900 µmol/L H2S (data not shown; one-way ANOVA, p ≥ 0.255 for 300–600 µmol/L; Kruskal–Wallis ANOVA, p = 0.0792 for 900 µmol/L). The third-phase contraction was not significantly affected by age at any H2S concentration tested (data not shown; Kruskal–Wallis ANOVA, p = 0.147 for 100 µmol/L; one-way ANOVA, p ≥ 0.517 for 300–900 µmol/L).
Fig. 6.
Effect of diet on KCl-and norepinephrine (NE)-induced contractions. CR causes an increase in contraction magnitude in response to KCl (a, n = 41) and NE (b, n = 41). The y-axis represents the KCl- or NE-induced tension of aortic rings (grams per milligram) pooled from all ages. The x-axis represents age diet: ad libitum (AL) or caloric restricted (CR). AL data are in white columns and CR data are in grey columns. Data are represented as mean ± SE and were analyzed by one-tailed t tests. Means that are significantly different are indicated by asterisks
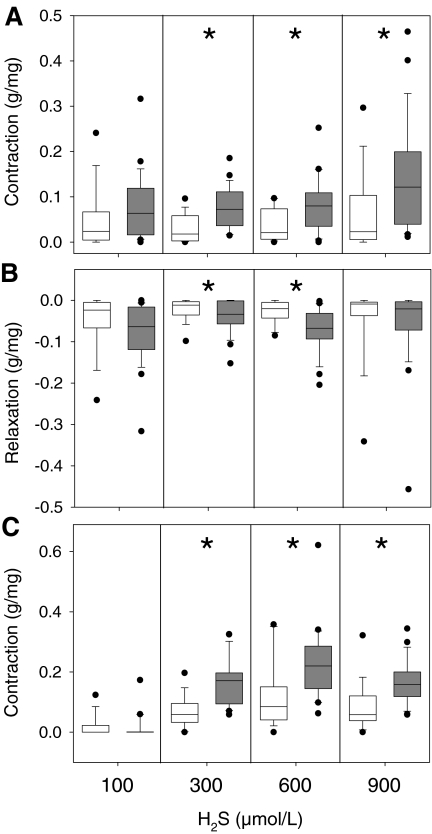
Diet significantly affected all phases of the triphasic response to H2S. Compared with rings from AL animals, CR increased the magnitude of the first-phase contraction after application of 300–900 µmol/L H2S (Fig. 7a, pooled t test, p = 0.0006, p = 0.0079, and 0.0192, respectively), the magnitude of the second-phase relaxation after application of 300 and 600 µmol/L H2S (Fig. 7b, one-tailed t test, p = 0.0492 and Wilcoxon's test, p = 0.0027, respectively), and the magnitude of the third-phase contraction after application of 300–900 µmol/L H2S (Fig. 7c, pooled t test, p < 0.0001, p = 0.0025, and p = 0.0008, respectively).
Fig. 7.
Effect of diet on H2S-induced contractions. CR causes an increase in contraction magnitude in response to H2S in the first-phase contraction (a, n = 18 AL, n = 23 CR), second-phase relaxation (b, n = 18 AL, n = 23 CR) and third-phase contraction (c, n = 18 AL, n = 23 CR). The y-axis represents the H2S-induced tension of aortic rings (grams per milligram) pooled from all ages. The x-axis represents H2S concentration. Columns show diet for ad libitum (AL, white) or caloric restricted (CR, grey) animals at each concentration tested. Data are presented as box plots showing the median as a horizontal line, the box covering the 25th and 50th percentiles, and error bars defining the 10th and 90th percentiles. Data were analyzed by one-tailed t test, pooled t test, or Wilcoxon's test. Medians that are significantly different are indicated by asterisks
Discussion
These data support our hypothesis that the H2S signaling system is negatively affected by age in the vasculature but not in the liver, which is typically more resilient to age, and that a CR diet minimizes the age affect in the vasculature. We also observed a difference in the effect of age between CSE and CBS protein, and finally, we observed a positive effect of the CR diet that led to increased H2S production in aorta and liver homogenates and increased vascular reactivity in aorta rings. Increased age was associated with increased expression of CSE protein in both aorta and liver, whereas CBS protein remained unchanged. Increased age was not associated with changes in mRNA expression of CSE and CBS or changes in H2S production in aorta and liver. Compared with an AL diet, the CR diet resulted in decreased expression of CSE and CBS protein in aorta, whereas liver from CR rats showed increased expression of CSE protein at 38 months of age. CR also substantially increased mRNA expression of CSE and CBS in aorta but not liver and increased H2S production in both aorta and liver. Age and diet both affected the triphasic response of aorta to H2S: the first-phase contraction and second-phase relaxation each increased in magnitude with age, and all three phases of the aortic response increased in magnitude with CR.
CSE and CBS protein expression
In aorta, CSE protein expression increased with age in rats maintained on an AL diet, but remained constant in rats maintained on a CR diet. CBS protein expression in aorta showed a similar trend, but with lower expression levels. It is perhaps not surprising that changes were more evident for CSE than for CBS, since CSE is expressed at higher levels in the endothelium and smooth muscle cells of aorta and is believed to make the largest functional contribution to regulation of vascular tone (Hosoki et al. 1997; Wang et al. 2008; Zhao et al. 2003; Zhao et al. 2001).
The increased CSE protein expression with age in aorta is similar to changes in eNOS protein expression with age in peripheral arteries, although the increase in eNOS expression is typically larger (Cernadas et al. 1998; Goettsch et al. 2001; van der Loo et al. 2005; van der Loo et al. 2000). It has been proposed that the change in eNOS is a homeostatic correction for decreased NO bioavailability or NO concentration, and that this is caused by increased superoxide reacting with NO to form a peroxynitrite (van der Loo et al. 2005; van der Loo et al. 2000). Interestingly, NO and H2S can interact, and therefore changes in one system could impact the other (Hosoki et al. 1997; Wang et al. 2008). Additionally, H2S may also react with, and be quenched by, increased superoxide (Geng et al. 2004a) as well as increased peroxynitrite produced from the reaction of NO with superoxide (Whiteman et al. 2004). CR has been proposed to reduce age-related endothelial dysfunction by reducing the increase in superoxide production associated with aging, thereby maintaining a more youthful state (Barja 2002; Castello et al. 2005; Gredilla et al. 2004; Gredilla et al. 2001; Taddei et al. 2006). Consistent with the beneficial effects of CR, the expression of CSE and to a lesser extent CBS did not significantly increase with age in CR animals.
In liver, CSE protein expression increased with age in CR animals whereas CBS protein expression did not change. In addition to producing H2S, CSE, and CBS are involved in cysteine metabolism (Zhao et al. 2001) and are present in relative abundance in the liver compared with other tissues. However, since CBS is the primary enzyme responsible for H2S production in the liver (Zhao et al. 2003), any changes in CSE expression may have only a small effect on H2S signaling. These data are also not surprising given that liver enzymatic activity generally does not change substantially until very old age (Anantharaju et al. 2002; Kitani 1991; Kitani 1994).
CSE and CBS mRNA expression
While there were complex changes with age and diet in CSE protein expression in aorta and liver, there were no significant changes in mRNA message for CSE or CBS with age, but CR did increase CSE and CBS mRNA levels in aorta. This seems to contradict the protein expression data, in which CR reduced the expression of those proteins. Conversely, liver CSE and CBS mRNA message did not significantly change with age or diet, which was more consistent with the protein data. We cannot account for the discrepancy between mRNA and protein data, except to note that mRNA message does not always translate directly into protein (Gygi et al. 1999). We conclude that while CR causes increases in mRNA for CSE and CBS in the vasculature, this does not translate into the synthesis of more new protein.
Hydrogen sulfide production
Extracellular concentrations of total H2S (representing the sum of H2S, HS−, and S2−) have been reported in the range of 50–160 µmol/L in the brain (Abe and Kimura 1996; Goodwin et al. 1989) and ∼50 µmol/L in the plasma (Zhao et al. 2001). However, more recent measurements in plasma indicate that true H2S concentrations are much lower (<100 nmol/L) (Whitfield et al. 2008). This discrepancy is likely due to methodological differences in how free H2S is differentiated from bound or “acid-labile” H2S (Olson 2009).
While age did not affect H2S production in homogenates of aorta or liver, diet did, with CR increasing H2S production in both aorta and liver. For aorta, this is despite CR causing decreased CSE and CBS protein in aorta compared with AL. The fact that CR animals with low expression of CSE and CBS can produce as much or more H2S than AL animals with higher expression of CSE and CBS implies that CR maintains CSE and CBS function with age. The mechanism for this is unknown, but the reduction of oxidative stress may reduce the amount of oxidative damage to these enzymes that would otherwise occur with an AL diet, allowing maintenance of enzyme function.
Addition of the CBS inhibitor AOAA and the CSE inhibitor Pgly reduced but did not completely block H2S production in both aorta and liver. Therefore, CSE and CBS both contribute to H2S production in these tissues, but a small amount of the measured H2S production may have come from PLP-independent sources, such as “acid labile” H2S. The recovery rate of H2S using the assay is probably 33–59%, depending on the H2S production rate of the tissue (Julian et al. 2002). Therefore, these rates should only be used for comparisons between the treatment groups and not as an absolute rate of H2S production. Furthermore, note that the H2S production rates were calculated as nmol H2S/h/mg protein, and that when H2S production is instead calculated as nanomole H2S production per hour per gram tissue or nanomole H2S production per hour per milliliter of homogenate, liver has a higher production rate than aorta.
Contractile response to hydrogen sulfide in aorta
While aorta was used in this study and is commonly used to examine the vascular actions of hydrogen sulfide (Dombkowski et al. 2005; Hosoki et al. 1997; Koenitzer et al. 2007; Olson et al. 2006; Yang et al. 2008; Zhao et al. 2001), it should be noted that this is a conduit vessel, not a resistance vessel. Changing the tone of the aorta will effectively alter blood pressure and flow to the entire vascular system, not specific vascular beds, and therefore would not be as effective in regulating blood flow as it would in a resistance vessel. However, the triphasic response we observed in aorta has been observed before in pulmonary arteries (Dombkowski et al. 2005). Therefore, we propose that it is reasonable to use the aorta as an experimental proxy for the responses to H2S in smaller resistance vessels.
Although triphasic responses to H2S application have been reported in rat pulmonary artery (Olson et al. 2006), previous studies of rat aorta have shown only a monophasic relaxation or contraction with H2S (Dombkowski et al. 2005; Kubo et al. 2007; Zhao and Wang 2002). We found that the development of all three phases typically required 15–30 min to develop after a bolus of H2S. Consequently, the triphasic response may not have been observed in previous studies of aorta because in those studies vessel tone was measured soon after H2S addition. Additional methodological variations, such as tissue preparation (ring vs. strip), method of euthanasia, and lag time between tissue dissection and H2S application, may have also contributed to differences in aorta response.
High O2 concentrations in the tissue bath may lead to formation of vasoactive H2S oxidation products, leading to contraction of the vessel instead of relaxation (Koenitzer et al. 2007). Since our experiments were run at ambient O2 concentrations, oxidation products may have contributed to the triphasic response. However, H2S-induced contractions in the dorsal aorta of cyclostomes are enhanced at low O2 concentrations, suggesting that H2S oxidation products are not the only cause of H2S-induced contractions (Olson et al. 2008). Additionally, in rat aorta rings we observed the same triphasic response during hypoxia (Predmore et al. unpublished data), which is presumably mediated in part by reduced oxidation of H2S under the extremely low oxygen conditions (Olson et al. 2006).
We found that the response of rat aorta rings to H2S increases in magnitude with age, particularly in the first-phase contraction but also in the second phase relaxation. The mechanism of the first-phase contraction is unknown, but a likely candidate is the reaction of H2S with NO to form a nitrosothiol (Whiteman et al. 2006), which would occur rapidly, reducing free NO and thereby lead to contraction (Ali et al. 2006). At higher H2S concentrations, sufficient H2S may remain to activate KATP channels, causing the second phase relaxation (Zhao et al. 2001). The concentration of NO is reduced with age (Cernadas et al. 1998; Goettsch et al. 2001; van der Loo et al. 2005; van der Loo et al. 2000), and therefore it follows that less H2S would be required to significantly reduce free NO in tissue of older animals. This would have the effect of increasing the apparent sensitivity of the first-phase contraction to H2S, consistent with our observations.
CR dramatically increased the response to H2S in all three phases, as well as the response to KCl. Therefore, rather than representing a specific action on H2S-signaling, the effect of CR may represent an overall enhancement of the smooth muscle function, as has been reported in skeletal muscle (Payne et al. 2003). CR is proposed to prevent endothelial dysfunction, by reducing oxidative stress (Raitakari et al. 2004; Sasaki et al. 2002) at least in part by the nuclear transcription factor nuclear factor-E2-related factor 2 (Nrf-2), and by maintaining the tonic release of NO in blood vessels (Ungvari et al. 2008). This would protect the vasculature by inhibiting platelet aggregation and inflammatory cell adhesion to endothelial cells, as well as by inhibiting pro-inflammatory cytokine-induced signaling pathways (Ungvari et al. 2008). While we are not implying that CR works via H2S, it would not be surprising if reduced oxidative stress, enhanced endothelial function and NO release were to result in enhanced function of the H2S-signaling system, given the synergy between NO and H2S (Hosoki et al. 1997; Zhao et al. 2001). In fact, H2S has anti-oxidant and anti-inflammatory affects (Fiorucci et al. 2005a; Wallace 2007; Zanardo et al. 2006). For example, by acting through Nrf-2 and other cytoprotective signaling pathways, H2S can upregulate phase II detoxifying genes and a variety of antioxidant enzymes (Calvert et al. 2009). Therefore, the possibility exists that H2S may also play a role in the enhanced function observed with CR.
Limitations
While this study is the first to investigate the effects of both aging and caloric restriction on the hydrogen sulfide-signaling system, it is not without its limitations. Hydrogen sulfide is produced in the human cardiovascular system, but the evidence is largely from animal models that do not undergo the same cardiovascular pathology as humans; i.e., rats do not develop atherosclerotic plaques with progression of cardiovascular disease or age. This necessarily limits inferences to a human model. Additionally, since conduit vessels were used rather than resistance vessels, these data may not represent the changes in reactivity that are occurring in the vascular beds responsible for fine blood pressure regulation. Finally, the CR regimen imposed on the experimental animals was quite strict (40%) and is difficult to attain in humans as a therapeutic intervention. Therefore investigation at a lower level of calorie restriction (5–10%) may be warranted.
Conclusions
These results demonstrate that age and diet have differential effects on the H2S signaling system, with age and CR causing greater changes in aorta than liver. Aging leads to increased free radical production, and oxidatively damaged CSE and CBS protein may lead to decreased H2S production and concentration, potentially reducing the chemical interactions between H2S and other compounds, such as NO. CR seems to benefit CSE and CBS protein in both aorta and liver, potentially ameliorating the negative effect of age on H2S concentration. Therefore, CR may help maintain the H2S signaling system during aging. The sensitization of the aorta's triphasic contractile response to H2S with CR may result from indirect effects on the NO signaling system. Therefore, elucidating the interactions between the known gasotransmitters (NO, CO, and H2S) will be essential to understating the ramifications of aging and diet on gas signaling.
Acknowledgments
We would like to thank Dr. Christy Carter for donation of rat aorta tissue used in the myography experiments. This work was supported by National Institute of Health T32 HL083810 to BP, National Institute of Health AG21042 to CL and National Science Foundation IBN-0422139 to DJ.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol. 2006;146:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaju A, Feller A, Chedid A. Aging liver. A review. Gerontology. 2002;48(6):343–353. doi: 10.1159/000065506. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21(3):134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11(7):573–581. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev. 2002;1(3):397–411. doi: 10.1016/S1568-1637(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Beauchamp RO, Bus JS, Popp JS, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105(4):365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64(8):850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J. 2005;19(13):1863–1865. doi: 10.1096/fj.04-2864fje. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83(3):279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, Tang CS. Endogenous hydrogen sulfide in patients with COPD. Chest. 2005;128(5):3205–3211. doi: 10.1378/chest.128.5.3205. [DOI] [PubMed] [Google Scholar]
- Dombkowski RA, Russell MJ, Schulman AA, Doellman MM, Olson KR. Vertebrate phylogeny of hydrogen sulfide vasoactivity. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R243–R252. doi: 10.1152/ajpregu.00324.2004. [DOI] [PubMed] [Google Scholar]
- Dombkowski RA, Doellman MM, Head SK, Olson KR. Hydrogen sulfide mediates hypoxia-induced relaxation of trout urinary bladder smooth muscle. J Exp Biol. 2006;209(Pt 16):3234–3240. doi: 10.1242/jeb.02376. [DOI] [PubMed] [Google Scholar]
- Ebrahimkhani MR, Mani AR, Moore K. Hydrogen sulphide and the hyperdynamic circulation in cirrhosis: a hypothesis. Gut. 2005;54(12):1668–1671. doi: 10.1136/gut.2004.056556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005a;129(4):1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005b;42(3):539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- Fujii K, Sakuragawa T, Kashiba M, Sugiura Y, Kondo M, Maruyama K, Goda N, Nimura Y, Suematsu M. Hydrogen sulfide as an endogenous modulator of biliary bicarbonate excretion in the rat liver. Antioxid Redox Signal. 2005;7(5–6):788–794. doi: 10.1089/ars.2005.7.788. [DOI] [PubMed] [Google Scholar]
- Gainey LF, Jr, Greenberg MJ. Hydrogen sulfide is synthesized in the gills of the clam Mercenaria mercenaria and acts seasonally to modulate branchial muscle contraction. Biol Bull. 2005;209(1):11–20. doi: 10.2307/3593138. [DOI] [PubMed] [Google Scholar]
- Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004a;318(3):756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004b;313(2):362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N. Direct spectrophotometric determination of inorganic sulfide in biological materials and in other complex mixtures. Anal Biochem. 1971;43:129–133. doi: 10.1016/0003-2697(71)90116-3. [DOI] [PubMed] [Google Scholar]
- Goettsch W, Lattmann T, Amann K, Szibor M, Morawietz H, Munter K, Muller SP, Shaw S, Barton M. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun. 2001;280(3):908–913. doi: 10.1006/bbrc.2000.4180. [DOI] [PubMed] [Google Scholar]
- Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, Reiffenstein RJ, Dowling G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol. 1989;13(2):105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15(9):1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Phaneuf S, Selman C, Kendaiah S, Leeuwenburgh C, Barja G. Short-term caloric restriction and sites of oxygen radical generation in kidney and skeletal muscle mitochondria. Ann N Y Acad Sci. 2004;1019:333–342. doi: 10.1196/annals.1297.057. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19(3):1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, Wohlgemuth SE, Leeuwenburgh C. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130(5):297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237(3):527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Julian D, Statile JL, Wohlgemuth SE, Arp AJ. Enzymatic hydrogen sulfide production in marine invertebrate tissues. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(1):105–115. doi: 10.1016/S1095-6433(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Julian D, Statile J, Roepke TA, Arp AJ. Sodium nitroprusside potentiates hydrogen-sulfide-induced contractions in body wall muscle from a marine worm. Biol Bull. 2005;209(1):6–10. doi: 10.2307/3593137. [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun. 2000;267(1):129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- Kimura H, Nagai Y, Umemura K, Kimura Y. Physiological roles of hydrogen sulfide: synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid Redox Signal. 2005;7(5–6):795–803. doi: 10.1089/ars.2005.7.795. [DOI] [PubMed] [Google Scholar]
- Kitani K. Aging of the liver: facts and theories. Arch Gerontol Geriatr. 1991;12(2–3):133–154. doi: 10.1016/0167-4943(91)90024-K. [DOI] [PubMed] [Google Scholar]
- Kitani K. Aging and the liver: functional aspects. Arch Gerontol Geriatr. 1994;19(2):145–158. doi: 10.1016/0167-4943(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR, Jr, Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol. 2007;292(4):H1953–H1960. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007;232(1–2):138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Labinskyy N, Csiszar A, Veress G, Stef G, Pacher P, Oroszi G, Wu J, Ungvari Z. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem. 2006;13(9):989–996. doi: 10.2174/092986706776360987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73(2):413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Prolla TA. Genetics, redox signaling, oxidative stress, and apoptosis in Mammalian aging. Antioxid Redox Signal. 2006;8(3–4):503–505. doi: 10.1089/ars.2006.8.503. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346(1):74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- Lim JJ, Liu Y, Khin Sandar Win E, Bian JS. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2008;295(5):C1261–C1270. doi: 10.1152/ajpcell.00195.2008. [DOI] [PubMed] [Google Scholar]
- Lowicka E, Beltowski J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59(1):4–24. [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129(9):542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamiyama Y, Bito Y, Takemura S, Takahashi Y, Kodai S, Mizuguchi S, Nishikawa Y, Suehiro S, Okada S. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J Pharmacol Exp Ther. 2007;320(2):535–543. doi: 10.1124/jpet.106.110460. [DOI] [PubMed] [Google Scholar]
- Olson KR. Vascular actions of hydrogen sulfide in nonmammalian vertebrates. Antioxid Redox Signal. 2005;7(5–6):804–812. doi: 10.1089/ars.2005.7.804. [DOI] [PubMed] [Google Scholar]
- Olson KR. Is hydrogen sulfide a circulating “Gasotransmitter” in vertebrate blood? Biochim Biophys Acta. 2009;1787(7):856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Olson KR, Donald JA. Nervous control of circulation—the role of gasotransmitters, NO, CO, and H(2)S. Acta Histochem. 2009;111(3):244–256. doi: 10.1016/j.acthis.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. 2006;209(Pt 20):4011–4023. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- Olson KR, Forgan LG, Dombkowski RA, Forster ME. Oxygen dependency of hydrogen sulfide-mediated vasoconstriction in cyclostome aortas. J Exp Biol. 2008;211(Pt 14):2205–2213. doi: 10.1242/jeb.016766. [DOI] [PubMed] [Google Scholar]
- Payne AM, Dodd SL, Leeuwenburgh C. Life-long calorie restriction in Fischer 344 rats attenuates age-related loss in skeletal muscle-specific force and reduces extracellular space. J Appl Physiol. 2003;95(6):2554–2562. doi: 10.1152/japplphysiol.00758.2003. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24(1):124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Sasaki S, Hara K, Matsuura H, Goto C, Oshima T, Chayama K. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15(4 Pt 1):302–309. doi: 10.1016/S0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- Schmucker DL. Aging and the liver: an update. J Gerontol A Biol Sci Med Sci. 1998;53(5):B315–B320. doi: 10.1093/gerona/53a.5.b315. [DOI] [PubMed] [Google Scholar]
- Sharifi AM, Mohseni S, Nekoparvar S, Larijani B, Fakhrzadeh H, Oryan S. Effect of caloric restriction on nitric oxide production, ACE activity, and blood pressure regulation in rats. Acta Physiol Hung. 2008;95(1):55–63. doi: 10.1556/APhysiol.95.2008.1.3. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2008;11(4):703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146(5):623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206(2):267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Versari D, Salvetti A. Endothelium, aging, and hypertension. Curr Hypertens Rep. 2006;8(1):84–89. doi: 10.1007/s11906-006-0045-4. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Funabiki M, Michikawa H, Koike K. Effects of aging on alpha1-adrenoceptor mechanisms in the isolated mouse aortic preparation. J Smooth Muscle Res. 2006;42(4):131–138. doi: 10.1540/jsmr.42.131. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102(5):519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo B, Bachschmid M, Labugger R, Schildknecht S, Kilo J, Hahn R, Palacios-Callender M, Luscher TF. Expression and activity patterns of nitric oxide synthases and antioxidant enzymes reveal a substantial heterogeneity between cardiac and vascular aging in the rat. Biogerontology. 2005;6(5):325–334. doi: 10.1007/s10522-005-4807-1. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007;28(10):501–505. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Wang YF, Mainali P, Tang CS, Shi L, Zhang CY, Yan H, Liu XQ, Du JB. Effects of nitric oxide and hydrogen sulfide on the relaxation of pulmonary arteries in rats. Chin Med J (Engl) 2008;121(5):420–423. [PubMed] [Google Scholar]
- Webb GD, Lim LH, Oh VM, Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, Wong PS, Caleb MG, Salto-Tellez M, Bhatia M, Chan ES, Taylor EA, Moore PK. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Ther. 2008;324(2):876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90(3):765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun. 2006;343(1):303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. A Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:1930–1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- Wood CE, Chen GF, Keller-Wood M. Expression of nitric oxide synthase isoforms is reduced in late-gestation ovine fetal brainstem. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R613–R619. doi: 10.1152/ajpregu.00722.2004. [DOI] [PubMed] [Google Scholar]
- Yang H, Shi M, Story J, Richardson A, Guo Z. Food restriction attenuates age-related increase in the sensitivity of endothelial cells to oxidized lipids. J Gerontol A Biol Sci Med Sci. 2004;59(4):316–323. doi: 10.1093/gerona/59.4.b316. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20(12):2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zeeh J, Platt D. The aging liver: structural and functional changes and their consequences for drug treatment in old age. Gerontology. 2002;48(3):121–127. doi: 10.1159/000052829. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283(2):H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81(9):848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]