Abstract
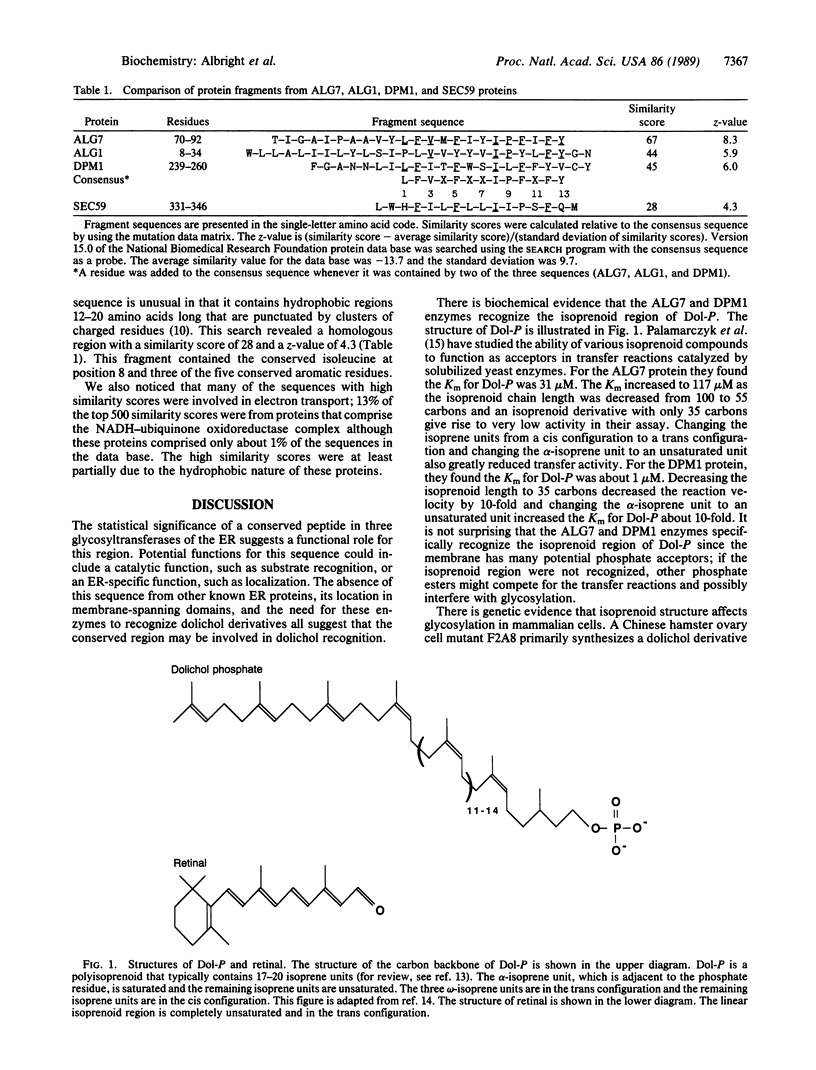
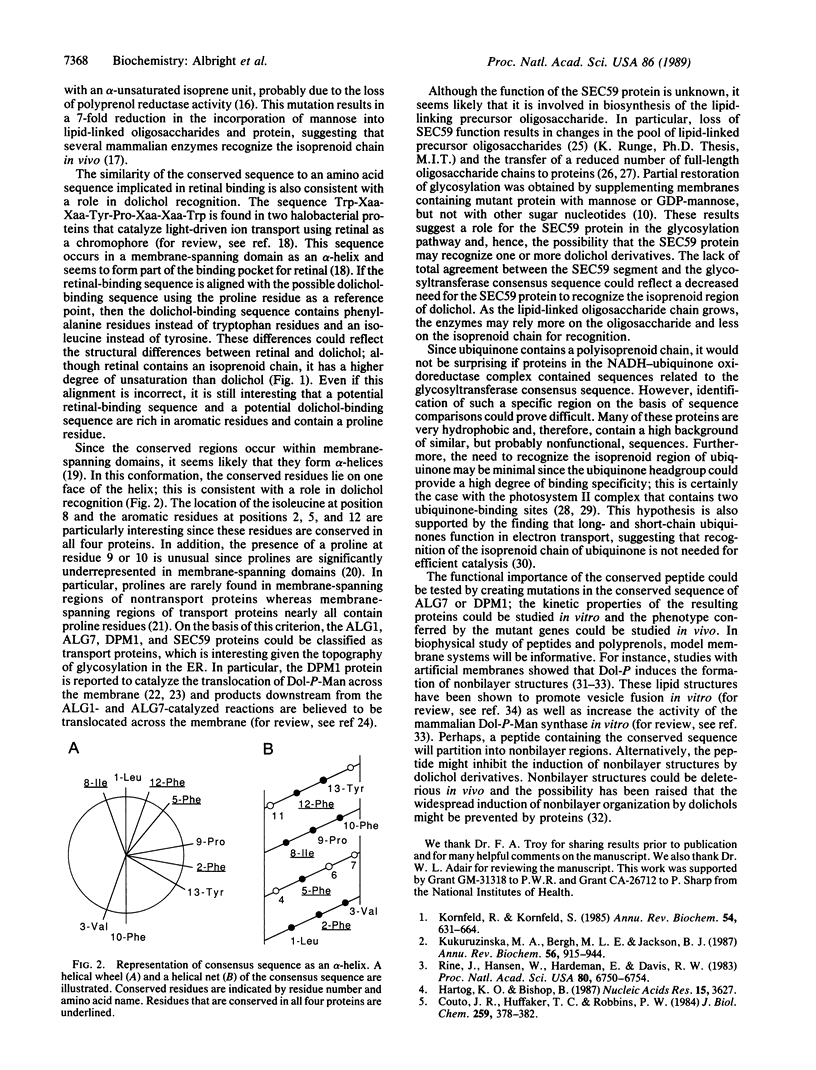
A 13-amino acid peptide was identified in three glycosyltransferases of the yeast endoplasmic reticulum. These enzymes, the products of the ALG1, ALG7, and DPM1 genes, catalyze the transfer of sugars from nucleotide sugars to dolichol phosphate derivatives. The consensus sequence for the conserved peptide was Leu-Phe-Val-Xaa-Phe-Xaa-Xaa-Ile-Pro-Phe-Xaa-Phe-Tyr. A sequence resembling the conserved peptide was also found in the predicted SEC59 protein, which is suspected to participate in assembly of the lipid-linked precursor oligosaccharide, although its specific function is unknown. All of the identified sequences contain an isoleucine at position 8 and phenylalanine or tyrosine at positions 2, 5, and 12. We believe this peptide may be involved in dolichol recognition for the following reasons. (i) The conserved sequence occurs in potential membrane-spanning regions. (ii) The ALG7 and DPM1 proteins are known to recognize the isoprenoid region of dolichol phosphate specifically; this recognition presumably occurs in the membrane since dolichol is very hydrophobic. (iii) The consensus sequence is similar to a region of two halobacterial proteins implicated in binding of the isoprenoid region of retinal. (iv) If the consensus sequence is represented as an alpha-helix, the conserved residues lie on one face of the helix. An alpha-helical structure is likely since the conserved regions are in potential membrane-spanning domains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein M., Kepes F., Schekman R. Sec59 encodes a membrane protein required for core glycosylation in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Mar;9(3):1191–1199. doi: 10.1128/mcb.9.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl C. J., Deber C. M. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):917–921. doi: 10.1073/pnas.83.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki T., Dallner G. The biological role of dolichol. Biochem J. 1988 Apr 1;251(1):1–9. doi: 10.1042/bj2510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Hyman R., Bron C. Anchoring of membrane proteins via phosphatidylinositol is deficient in two classes of Thy-1 negative mutant lymphoma cells. EMBO J. 1986 Dec 1;5(12):3291–3296. doi: 10.1002/j.1460-2075.1986.tb04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell B. A., Keniry M. A., Post A., Robertson R. N., Weir L. E., Westerman P. W. Location and activity of ubiquinone 10 and ubiquinone analogues in model and biological membranes. Biochemistry. 1987 Dec 1;26(24):7702–7707. doi: 10.1021/bi00398a025. [DOI] [PubMed] [Google Scholar]
- Couto J. R., Huffaker T. C., Robbins P. W. Cloning and expression in Escherichia coli of a yeast mannosyltransferase from the asparagine-linked glycosylation pathway. J Biol Chem. 1984 Jan 10;259(1):378–382. [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. Hydrophilic anchor-deficient Thy-1 is secreted by a class E mutant T lymphoma. Cell. 1986 Aug 29;46(5):653–657. doi: 10.1016/0092-8674(86)90340-5. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S., Novick P., Field C., Schekman R. Yeast secretory mutants that block the formation of active cell surface enzymes. J Cell Biol. 1984 Jan;98(1):35–43. doi: 10.1083/jcb.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog K. O., Bishop B. Genomic sequence coding for tunicamycin resistance in yeast. Nucleic Acids Res. 1987 Apr 24;15(8):3627–3627. doi: 10.1093/nar/15.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselbeck A., Tanner W. Dolichyl phosphate-mediated mannosyl transfer through liposomal membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1520–1524. doi: 10.1073/pnas.79.5.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Dunger I., Oesterhelt D., Lottspeich F. The 'light' and 'medium' subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the genes, nucleotide and amino acid sequence. EMBO J. 1986 Jun;5(6):1149–1158. doi: 10.1002/j.1460-2075.1986.tb04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 1989 Feb;14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Orlean P., Albright C., Robbins P. W. Cloning and sequencing of the yeast gene for dolichol phosphate mannose synthase, an essential protein. J Biol Chem. 1988 Nov 25;263(33):17499–17507. [PubMed] [Google Scholar]
- Palamarczyk G., Lehle L., Mankowski T., Chojnacki T., Tanner W. Specificity of solubilized yeast glycosyl transferases for polyprenyl derivatives. Eur J Biochem. 1980 Apr;105(3):517–523. doi: 10.1111/j.1432-1033.1980.tb04527.x. [DOI] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll J., Krag S. S. A mutant of Chinese hamster ovary cells with a reduction in levels of dolichyl phosphate available for glycosylation. J Biol Chem. 1988 Aug 5;263(22):10766–10773. [PubMed] [Google Scholar]
- Stoll J., Rosenwald A. G., Krag S. S. A Chinese hamster ovary cell mutant F2A8 utilizes polyprenol rather than dolichol for its lipid-dependent asparagine-linked glycosylation reactions. J Biol Chem. 1988 Aug 5;263(22):10774–10782. [PubMed] [Google Scholar]
- Tanner W., Lehle L. Protein glycosylation in yeast. Biochim Biophys Acta. 1987 Apr 27;906(1):81–99. doi: 10.1016/0304-4157(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Valtersson C., van Duÿn G., Verkleij A. J., Chojnacki T., de Kruijff B., Dallner G. The influence of dolichol, dolichol esters, and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J Biol Chem. 1985 Mar 10;260(5):2742–2751. [PubMed] [Google Scholar]



