Abstract
Purpose
Aim was to evaluate the accuracy of computed tomography colonography (CTC) for detection of colorectal neoplasia in a Fecal Occult Blood Test (FOBT) positive screening population.
Methods
In three different institutions, consecutive FOBT positives underwent CTC after laxative free iodine tagging bowel preparation followed by colonoscopy with segmental unblinding. Each CTC was read by two experienced observers. For CTC and for colonoscopy the per-polyp sensitivity and per-patient sensitivity and specificity were calculated for detection of carcinomas, advanced adenomas, and adenomas.
Results
In total 22 of 302 included FOBT positive participants had a carcinoma (7%) and 137 had an adenoma or carcinoma ≥10 mm (45%). CTC sensitivity for carcinoma was 95% with one rectal carcinoma as false negative finding. CTC sensitivity for advanced adenomas was 92% (95% CI: 88–96) vs. 96% (95% CI: 93–99) for colonoscopy (P = 0.26). For adenomas and carcinomas ≥10 mm the CTC per-polyp sensitivity was 93% (95% CI: 89–97) vs. 97% (95% CI: 94–99) for colonoscopy (P = 0.17). The per-patient sensitivity for the detection of adenomas and carcinomas ≥10 mm was 95% (95% CI: 91–99) for CTC vs. 99% (95% CI: 98–100) for colonoscopy (P = 0.07), while the per-patient specificity was 90% (95% CI: 86–95) and 96% (95% CI: 94–99), respectively (P < 0.001).
Conclusion
CTC with limited bowel preparation performed in an FOBT positive screening population has high diagnostic accuracy for the detection of adenomas and carcinomas and a sensitivity similar to that of colonoscopy for relevant lesions.
Keywords: CT colonography, Colorectal neoplasia, Advanced adenoma, Colorectal cancer, FOBT
Colorectal cancer screening is aimed mainly at detecting advanced adenomas and early stage colorectal cancer [1]. Although a large part of adenomas will not develop into a carcinoma, a specific subgroup of advanced adenomas is believed to have an increased risk of developing into carcinoma [2–4]. Such advanced adenomas are now understood to include adenomas of 10 mm or larger and adenomas with advanced histological features: villous histology or high-grade dysplasia [5]. The removal of these adenomas leads to a reduction in the expected incidence of CRC [6]. Screening for colorectal neoplasia and subsequent removal of adenomas identified through screening can therefore reduce cancer mortality.
In screening for colorectal cancer, the Fecal Occult Blood Test (FOBT) is the only screening test with a documented reduction in CRC-related mortality [7, 8]. It is a cheap test, well accepted and has a high negative predictive value [9]. Unfortunately, the FOBT has a limited positive predictive value. Large randomized trials have shown that the prevalence of carcinomas in an FOBT positive screening population is between 5.6% and 17.7%, while the prevalence of adenomas ranges between 15% and 55% [8, 10–12]. These numbers indicate that a large number of positive FOBTs are false positives: these screening participants have a positive FOBT test but no colorectal neoplasia.
In principle, CT colonography can image both adenomas and carcinomas. In an FOBT positive screening population, CT colonography might therefore be able to detect all relevant lesions that should be removed or that need follow-up. As a consequence, CT colonography might be used as a triage instrument in FOBT positives, but in our previous study this did not seem efficient [13]. Another possibility is to use CT colonography only in patients that are unfit for or are unwilling to undergo colonoscopy. All screening participants with a positive FOBT without relevant lesions at CT colonography could then be safely withheld colonoscopy.
Recent studies have reported a high sensitivity for the detection of colorectal neoplasia on CT colonography in average risk screening participants [14–16]. One recent study investigated the accuracy of CT colonography in FOBT positives (no screening) and found a sensitivity of 87% for advanced neoplasia [17]. Another study found lesions 6 mm and larger in 40% of patients at CT colonography in an FOBT screening population [18]. Colonoscopy was not performed in all patients, so no accuracy could be calculated. Up to our knowledge no previous study has assessed the accuracy of CT colonography for detection of advanced neoplasia in population-based FOBT screening.
The main aim of the present study was to assess the sensitivity and specificity of CT colonography with a limited bowel preparation for detecting colorectal neoplasia in an FOBT positive screening population.
Methods
Study population
Data were collected in an invitational FOBT pilot screening trial in the Netherlands. A cohort of approximately 30,000 individuals between 50 and 75 years was randomly allocated to receive either an immunochemical test with a 50 ng/mL cut-off (I-FOBT, OC-sensor, Eiken Chemical Co, Tokyo, Japan) or a non-rehydrated guaiac test (G-FOBT; Haemoccult II, Beckman Coulter, Fullerton, CA) [12, 19]. Details about the invitation, participant recruitment, and evaluation of eligibility have been described in detail elsewhere [13].
All FOBT positives invited to undergo colonoscopy at the gastroenterology department were asked to undergo additionally CT colonography before colonoscopy. Excluded were participants with a terminal illness, those who had had a colonoscopy or an FOBT in the previous 2 years, inflammatory bowel disease or an examination with radiation exposure in the last 12 months, participants with hyperthyroidism, with an iodine contrast allergy or a pregnancy, as well those persons unable to give informed consent. Institutional review board approval was obtained before study initiation. Eligible participants were informed about the study purpose and asked for written consent.
CT colonography
Bowel preparation
The first 153 participants received a 2-day preparation with ingestion of 7 times 50 mL high-osmolar ionic monomer meglumine-ioxithalamate during meals (Telebrix Gastro 300 mg I/mL; Guerbet, Cedex, France) and a low-fiber diet. The other 149 participants received a 1-day preparation with 4 times 50 mL of meglumine-ioxithalamate and a low-fiber diet. No laxatives were used and patients were encouraged to drink additional glasses of water. The reason to reduce the amount of ingested contrast agent during this study was that new studies on CT colonography bowel preparation showed that image quality and polyp detection remained sufficient with only 1 day of bowel preparation [20–22]. After evaluation we found that both preparations had a high acceptance and good image quality [23].
CT colonography examination
Scans were made on two 64-slice CT scanners in supine and prone position. The scan protocol for the first scanner (Brilliance, Philips Medical Systems, Best, the Netherlands) was: 120 kV, pitch 1.2, rotation time 0.4 s and a 40 reference mAs with dose-modulation. For the other scanner (SOMATOM Sensation, Siemens Medical Solutions, Erlangen, Germany) this was: 120 kV, 1.4, 0.5 s, and 32 reference mAs with dose-modulation. Before starting the insufflation, a smooth muscle-relaxant (20 mg of butylscopalamine bromide (Buscopan; Boehringer-Ingelheim, Ingelheim, Germany)) was injected intravenously. When contraindicated 1 mg of glucagon hydrochloride was injected intravenously (Glucagen; Novo-Nordisk, Bagsvaerd, Denmark). In participants with contraindications for both smooth muscle-relaxants, no bowel relaxant was administrated. CO2 gas was insufflated into the colon using a flexible balloon-tipped rectal catheter (20 French Gauge) using an automated insufflator (ProtoCO2l, Bracco Diagnostics, Princeton, NY). The total amount of CO2 insufflated and the in-room time were noted per participant.
CT colonography analysis
Evaluations were performed on a workstation with specialized software (View Forum, Philips Medical Systems, Best, the Netherlands; Aquarius Workstation, TeraRecon, STAD, USA). A primary 2D axial evaluation (window setting 1500, −250 HU) was done with 3D problem solving. Additionally a quick uni-directional 3D fly through was performed after the primary 2D evaluation. Electronic cleansing software was not available. Lesions were identified at each CT colonography by two out of seven experienced observers (radiologists and research fellows) who had evaluated at least 100 CT colonographies with colonoscopic verification (range 100–700 CT colonographies). All observers had passed a CT colonography exam with 25 cases by scoring above a predefined sensitivity threshold of 90% for lesions ≥10 mm. The observers reported observing time per viewing method (2D or 3D). The maximal diameter of each lesion was measured by using electronic calipers applied to a multiplanar reformatted (MPR) setting. The location, morphology, size, and probability were noted for each lesion. According to the Paris criteria, flat polyps were defined as lesions that protrude less than 2.5 mm from the mucosa [24]. Both observers scored distension (four-point scale: 1 poorly distended to 4 good distension) and quality of bowel preparation (five-point scale: 1 very inhomogeneous tagging to 5 excellent preparation). When one of these was judged insufficient (score 1 or 2) by both readers in both scans, the participant was excluded for analysis.
Colonoscopy
Colonoscopy was planned within 3 weeks after CT colonography. Four liters of polyethylene glycol electrolyte solution (KleanPrep; Helsinn Birex Pharmaceuticals, Dublin, Ireland) or 4 L of macrogol solution (Colofort; Laboratoires Macors, Auxerre, France) and a clear liquid diet the preceding evening were used for colonoscopy. The examination was performed by experienced gastroenterologists, gastroenterology fellows and colonoscopy nurses with supervision, using a standard colonoscope (Olympus Medical Systems Europe, Hamburg Germany). With the segmental unblinding technique, lesions 6 mm or larger found at CT colonography were revealed to the colonoscopist by a research fellow [ML] after completing the examination of one segment. Colonoscopies were videotaped starting from the cecum. The colonoscopist estimated lesion size by an opened biopsy forceps or by a linear measure probe (Olympus Medical Systems Europe, Hamburg Germany). Histology of lesions was classified as normal, inflammatory, hyperplastic, adenoma (serrated, tubular, tubolovillous, or villous), or carcinoma [25]. Advanced neoplasias were defined as adenomas 10 mm or larger in diameter, with a villous architecture or high-grade dysplasia on histology, or an invasive carcinoma [5].
Polyp matching
Matching of all lesions and tumors of 6 mm and larger found on CT colonography was done by a research fellow [ML] by reviewing the colonoscopy video’s and reports. Colonoscopy with segmental unblinding served as an enhanced reference standard. A lesion on CT colonography was classified as a true positive when colonography and colonoscopy lesion size were within 50% margin, lesions were in the same or adjacent segment and the morphology at CT colonography resembled morphology of the lesion seen on the videotaped colonoscopy. Lipoma and normal mucosa were considered false positives for CT colonography when scored as a lesion by the CT colonography observer. False negative lesions at CT colonography could be due to a perceptive error or a technical error. Perceptive errors were visible on retrospect at CT colonography, whereas technique related errors were not.
Statistical analysis
The results of the two observers were combined in a double reading procedure. If at least one observer had detected a lesion this was considered a positive finding. We also calculated the mean result for both observers.
The CT colonography per-polyp sensitivity was calculated by using all true positive lesions at CT colonography and the false negative lesions found at colonoscopy. This was done separately for adenomatous polyps and for carcinomas ≥10 mm, for adenomas and carcinomas between 6 and 9 mm, as well as for all lesions in these size categories, also including hyperplastic and inflammatory polyps. In addition, we evaluated the per-polyp sensitivity for advanced neoplasia and per lesion morphology, classified as flat, sessile, or pedunculated. Differences were tested with the Chi-square test statistic. The per-polyp sensitivity of colonoscopy was calculated by using the true positive and false negative lesions, found after unblinding of the CT colonography results. Comparisons between CT colonography and colonoscopy sensitivity and influence of additional 3D reading were evaluated using the McNemar test statistic.
The per-patient sensitivity was defined as the number of participants with at least one true positive adenoma or a carcinoma ≥10 and ≥6 mm at CT colonography relative to all participants with an adenoma or carcinoma in that size category identified after colonoscopy with segmental unblinding. The per-patient specificity was defined as the number of participants negative on CT colonography relative to all participants with a negative colonoscopy result. A participant with both one true positive and one false negative finding at CT colonography was defined as a true positive. All non-adenomatous lesions were counted as false positives, also for the calculation of the colonoscopy specificity.
We estimated interobserver agreement by calculating kappa statistics with corresponding 95% confidence intervals for the two readers and by calculating the total number of concordant cases. The two reviewers were considered to agree if they both recorded at least one true lesion in the same participant or if both recorded no findings. The kappa values were interpreted as follows: <0.20 poor agreement; 0.21–0.40 fair; 0.41–0.60 moderate; 0.61–0.80 good; 0.81–1.00 excellent. All calculations were performed using a statistical software package (SPSS for Windows version 15.0.1, Chicago, IL).
Results
A total of 314 FOBT positive participants underwent CT colonography before colonoscopy between June 2006 and May 2008. Twelve participants had an incomplete CT colonography or colonoscopy. In two colonoscopies the cecum was not reached because of extreme pain and a colonic stricture. In four CT colonographies the bowel preparation was insufficient and in six the distension was insufficient. Finally 302 participants could be included in the analysis (54 guaiac FOBT, 248 immunochemical FOBT). Totally 187 males and 115 females were included (see article [13], for additional demographic characteristics and a flow-diagram of the study).
Colonoscopy
Colonoscopy was successful in 312 participants. One participant (0.3%) had a bleeding after polypectomy for which hospitalization was required. With colonoscopy 22 carcinomas were detected in 22 participants (7%), two carcinomas were smaller than 10 mm; 184 adenomas and carcinomas 10 mm or larger were detected in 137 participants (45%). Another 138 adenomas and carcinomas between 6 and 9 mm were detected in 60 participants (20%). In total 180 advanced adenomas were found, of which 164 were 10 mm or larger. Furthermore, three lipomas and one hamartoma were found. Table 1 summarizes the distribution of lesions according to size and morphology. Lesions with no histology available were not considered as an adenoma or carcinoma. Six adenomas ≥10 mm were found with colonoscopy only after unblinding of the CT colonography results. The latter also resulted in 8 lesions between 6 and 9 mm that were additionally found, of which 4 were adenomas. In Table 2 the per-polyp sensitivity for colonoscopy is presented.
Table 1.
Distribution of adenomatous or non-adenomatous lesions per size category and per type of morphology
| <6 mm | 6–9 mm | ≥10 mm | |
|---|---|---|---|
| Pedunculated | |||
| Adenoma or carcinoma | 22 | 40 | 109 |
| All lesion typesa | 24 | 45 | 118 |
| Sessile | |||
| Adenoma or carcinoma | 228 | 86 | 63 |
| All lesion typesa | 418 | 126 | 76 |
| Flat | |||
| Adenoma or carcinoma | 23 | 12 | 12 |
| All lesion typesa | 46 | 19 | 14 |
| Total | |||
| All polyps and carcinomas | 488 | 190 | 208 |
a All lesion types: adenomas, carcinomas, hyperplastic, hamartomous or infectious lesions. These also include polyps that were lost after polypectomy and polyps with unclear histology
Table 2.
Per-polyp sensitivity for CT colonography compared to colonoscopy
| Sensitivity | CT colonography | Colonoscopy | P values |
|---|---|---|---|
| Advanced adenomas | 92% (88–96) | 96% (93–99) | P = 0.26 |
| 165/178 | 173/180 | ||
| Adenomas and carcinomas 6–9 mm | 78% (71–85) | 97% (94–100) | P < 0.001 |
| 108/138 | 134/138 | ||
| Adenomas and carcinomas ≥10 mm | 93% (89–97) | 97% (94–99) | P = 0.17 |
| 171/184 | 178/184 | ||
| All lesionsa 6–9 mm | 75% (69–81) | 96% (93–99) | P < 0.001 |
| 142/190 | 190/198 | ||
| All lesionsa ≥10 mm | 92% (88–96) | 97% (95–99) | P = 0.035 |
| 191/208 | 208/214 |
a All lesion types: adenoma, carcinoma, hyperplasia, hamartomous or infectious. Also includes not-removed polyps and polyps with unclear histology. Between brackets is the 95% confidence interval
CT colonography
The mean in-room time for CT colonography was 21′58′′ (SD 7′20′′) per participant. In total, 260 participants (86%) received an intravenous injection of Buscopan while 29 (9.7%) received Glucagen. The average amount of CO2 insufflated was 4.1L (SD 2.1L). No complications were associated with the CT colonography examination. The mean reading time for the 2D read was 10′43′′ (SD 5′21′′) and the additional 3D accounted for 4′16′′ (SD 1′30′′).
Per-polyp analysis
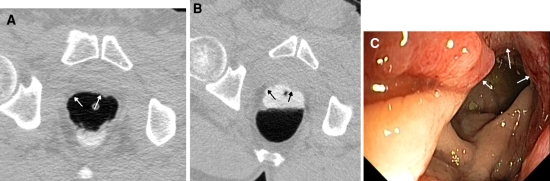
Of the 22 detected cancers at colonoscopy, 21 were also found at CT colonography with double reading, resulting in a sensitivity of 97% (95% CI: 87–100). The one missed colorectal cancer was a flat rectal carcinoma of 20 mm, hardly visible in retrospect (see Fig. 1). CT colonography detected 171 of 184 adenomas and carcinomas ≥10 mm (sensitivity 93%; 95% CI: 89–97). This was not significantly different from the colonoscopy sensitivity (P = 0.17). See Table 2 for results on per-polyp sensitivity of CT colonography compared to colonoscopy. For lesions ≥10 mm CT colonography sensitivity was 92% (95% CI: 88–96), which was lower than the colonoscopy sensitivity (P = 0.035).
Fig. 1.
False negative rectal carcinoma images of the supine (A) and prone scans (B) of a patient with a rectal carcinoma. This lesion was not detected at CT colonography by both observers and retrospectively hardly visible. Arrows indicate the tumor seen at CT colonography and colonoscopy (C).
In this study, 165 of the 180 advanced adenomas were detected at CT colonography, resulting in an estimated sensitivity of 92% (95% CI: 88–96The colonoscopy sensitivity for advanced adenomas was 96% (95% CI: 93–99) (P = 0.26). In Table 3 the sensitivity of CT colonography per morphology and size category is given separately. CT colonography sensitivity in detecting flat lesions was lower than that of pedunculated lesions in both size categories (P < 0.001). The sensitivity of sessile lesions 6–9 mm was lower than that for pedunculated lesions (P < 0.01). Figures 2 and 3 show examples of a pedunculated and flat lesion found at CT colonography and colonoscopy.
Table 3.
Per-polyp sensitivity of CT colonography per size category and per lesion type for adenomatous polyps and for all histology types (double read)
| 6–9 mm | ≥10 mm | |
|---|---|---|
| Pedunculated | ||
| Adenomas and carcinomas | 95% (88–100) | 95% (91–99) |
| 38/40 | 104/109 | |
| All lesionsa | 96% (90–100) | 95% (91–99) |
| 43/45 | 112/118 | |
| Sessile | ||
| Adenomas and carcinomas | 76% (67–85)* | 95% (90–100) |
| 65/86 | 60/63 | |
| All lesionsa | 71% (64–79)* | 93% (88–99)* |
| 90/126 | 71/76 | |
| Flat | ||
| Adenomas and carcinomas | 42% (14–70)* | 58% (30–86)* |
| 5/12 | 7/12 | |
| All lesionsa | 47% (25–70)* | 57% (31–83)* |
| 9/19 | 8/14 | |
a All lesion types: adenoma, carcinoma, hyperplasia, hamartomous or infectious. Also includes not-removed polyps and polyps with unclear histology. Between brackets is the 95% confidence interval
* Significantly different sensitivity compared to the pedunculated lesions P < 0.05
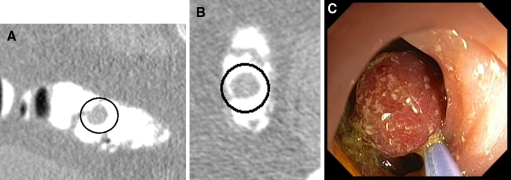
Fig. 2.
Pedunculated tubulovillous adenoma 15 mm, submerged in tagged feces: A is an MPR view of the supine position, B the axial supine view, C the lesion is removed at colonoscopy.
Fig. 3.
Flat serrated adenoma 12 mm. This flat adenoma was detected by both CT colonography observers: A is the 2D axial image of the prone scan and B is the 3D view. Arrows indicate the flat lesion, C is the lesion seen at colonoscopy.
In Table 4, the false negatives are categorized in false negatives due to perspective error and false negatives due to technical error. The largest percentage of technical false negatives consisted of flat lesions (5 out of 14 flat lesions; 36% for ≥10 mm and 8 out of 19; 42% for 6–9 mm).
Table 4.
Number of false negatives per size category and per lesion type
| 6–9 mm | ≥10 mm | |
|---|---|---|
| Perceptive false negative | ||
| Pedunculated | 0 (0%) | 2 (1.7%) |
| Sessile | 7 (5.6%) | 1 (1.3%) |
| Flat | 2 (10.5%) | 2 (14.3%) |
| Technical false negative | ||
| Pedunculated | 2 (4.4%) | 3 (2.6%) |
| Sessile | 28 (22.4%) | 2 (2.7%) |
| Flat | 8 (42.1%) | 5 (35.7%) |
Percentages are the number of false negatives related to the total number of lesions of this type
One lesion ≥10 mm and 15 lesions of 6–9 mm were seen at secondary 3D reading only. Of these lesions, four had a flat histology, eleven were sessile, and one pedunculated. The additional 3D read detected lesions increased the sensitivity from 67% (95% CI: 60–74) to 75% (95% CI: 69–81) for lesions between 6 and 9 mm (P < 0.000), without a similar increase for lesions ≥10 mm: 91% (95% CI: 88–95) vs. 92% (95% CI: 88–96) (P = 1).
Per-patient sensitivity and specificity
In Table 5 the CT colonography and colonoscopy sensitivity and CT colonography specificity per patient for adenomas and carcinomas of both size categories are given. The per-patient sensitivity of CT colonography was 95% (95% CI: 91–99) for adenomas and carcinomas ≥10 mm and 93% (95% CI: 89–96) for adenomas ≥6 mm. The specificity for the identification of participants without any adenoma or carcinoma of ≥10 mm was 90% (95% CI: 86–95), for ≥6 mm this was 70% (95% CI: 62–79). Comparing CT colonography and colonoscopy no significant difference was found in the sensitivity for adenomas and carcinomas ≥10 mm.
Table 5.
Per-patient sensitivity and specificity for adenoma detection
| Adenomas and carcinomas ≥6 mm | Adenomas and carcinomas ≥10 mm | |
|---|---|---|
| Sensitivity (per patient) | ||
| CTC double read | 93% (89–96)* | 95% (91–99) |
| CTC mean 2 readers | 89% (85–94)* | 92% (88–97)* |
| Colonoscopy | 98% (96–100) | 99% (98–100) |
| Specificity | ||
| CTC double read | 70% (62–79)* | 90% (86–95)* |
| CTC mean 2 readers | 77% (69–85)* | 93% (90–97)* |
| Colonoscopy | 93% (89–98) | 96% (94–99) |
Lesions that were not adenomatous or carcinoma were counted as false positive. Between brackets is the 95% confidence interval
* Indicates significant difference compared to colonoscopy
CTC, CT colonography
Interobserver agreement
For per-patient analysis for lesions ≥10 mm, a kappa value of 0.90 (95% CI, 0.85–0.94) was calculated for the interobserver agreement. For lesions ≥6 mm this was 0.82 (95% CI, 0.76–88).
Discussion
In the Joint Guideline for screening on colorectal cancer from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology a number of alternative colorectal screening tests are recommended, including the FOBT [9]. The FOBT is a cheap and simple screening test for colorectal cancer with a high negative predictive value, but it has the disadvantage that it generates a large number of false positives, resulting in lower positive predictive value which will result in a higher number of unnecessary invasive colonoscopic examinations. Ours is the first study to investigate the diagnostic accuracy of CT colonography for detection of colorectal neoplasia in an FOBT positive screening population. In the present study there was a high prevalence of colorectal neoplasia in the FOBT positive participants: almost two-thirds of all participants had one or more adenomas or a carcinoma. The vast majority of these lesions were detected by the CT colonography readers resulting in a high sensitivity for detection of adenomas and carcinomas.
In a recent study of Regge et al. [17], the per-patient sensitivity for detection of advanced neoplasia of 6 mm and larger in FOBT positive individuals was 87%, which is comparable to the sensitivity we found in our study. Also the specificity was similar; in both studies this was 77% (mean percentage of all readers). The FOBT positives in the previous study were no screening participants, but increased risk patients that could already have had symptoms. This is different than in our study that included FOBT positives from a population screening program. Furthermore, when compared to earlier studies that evaluated CT colonography for adenoma detection in screening participants, we found a similar sensitivity and specificity [14, 16, 26]. In these studies, sensitivities for detection of adenomas and carcinomas ≥10 mm were 90% or more. Different than in our study, these studies contained average risk screening participants with a low lesion prevalence and all patients received an extensive cathartic bowel preparation. When using a limited bowel preparation, we found a sensitivity of 93% for detection of colorectal neoplasia ≥10 mm in FOBT positive screened participants, not significantly different than the sensitivity of colonoscopy (97%). For the detection of advanced adenomas, CT colonography had a sensitivity similar to that of colonoscopy.
The prevalence of lesions is an important issue when evaluating the use of a diagnostic test in screening participants. In the present study there was a high lesion prevalence in the FOBT positive group, almost 65% of all positives had an adenoma or carcinoma of 6 mm and larger. Therefore, the CT colonography does not seem an effective triage instrument in FOBT positives (see [13]), but because of the high sensitivity it might have a role in patients with severe comorbidities that are unfit to undergo colonoscopy or patients unwilling to undergo colonoscopy.
The per-patient specificity for the detection of large lesions was lower than that of colonoscopy, 90% vs. 96%. For the medium size category (6–9 mm) we found a sensitivity of 78% which was significantly lower than that of colonoscopy. One flat rectal carcinoma was missed at CT colonography and was even retrospectively hardly visible. It is already known from earlier studies that lesions with a flat morphology are easily missed at CT colonography [27, 28]. Furthermore, the rectum is a difficult colonic segment to examine because the distension is not always optimal, especially not in supine position, and an inflated rectal balloon can mask rectal lesions [29, 30]. One previous paper described a malignant rectal lesion missed due to the presence of the inflated balloon [31]. A digital rectal examination could be performed to reduce the number of false negative rectal lesions in this segment. Its feasibility in clinical practice is questionable because a digital rectal examination has to be performed by an experienced person. In many centers CT colonography is performed independently by radiographers.
We found in the present study that sensitivity for detection of pedunculated lesions was high at CT colonography. For lesions ≥10 mm the sensitivity in detecting flat lesions was significantly lower than the sensitivity in detecting pedunculated and sessile lesions. Most of the false negative lesions were not visible retrospectively and have to be considered as technically false negatives. In this population the number of flat lesions was low, so this did not greatly affect CT colonography polyp sensitivity.
Each CT colonography was examined by two observers and their results were combined. This double reading procedure is time consuming but it resulted in a higher per-patient sensitivity. This was also found in a previous study by Johnson et al. [32]. A disadvantage of this double reading is that the number of false positives increases as well, consequently, the per-patient specificity decreases. The mean specificity of both readers for adenomas and carcinomas ≥6 mm is 77% compared to a 70% specificity for the double read. In this high lesion prevalence FOBT positive population it is important to obtain a high sensitivity so participants are not wrongly withheld colonoscopy.
Another method to improve the sensitivity of CT colonography is the use of an additional 3D reading after the primary 2D reading. We found that for detection of lesions between 6 and 9 mm, the sensitivity increased when using 3D reading after the 2D reading. In particular additional sessile and flat lesions were found after 3D viewing. Flat lesions are easily missed and probably best detected at a 3D viewing method [28]. A previous study showed that an additional 3D read resulted in a higher sensitivity for detection of polyps [33]. The main reason for not performing a primary 3D and evaluating a primary 2D review method only in the present study was the use of a limited bowel preparation without having the availability of a cleansing algorithm.
A potential limitation of the present study is that we changed the bowel preparation after half of the participants had received a CT colonography. This was done because articles had been published during the study period indicating that a 1-day bowel preparation was sufficient for qualitative fecal tagging, simultaneously reducing patient burden [20–22]. We retrospectively compared the quality of the bowel preparation in the 2- and 1-day preparation groups and found no differences in homogeneity of stool and detection of polyps while participant acceptance increased [23]. Another potential limitation is that due to a limited bowel preparation an immediate colonoscopy after a positive CT colonography is not possible. In our opinion, however, the advantage of an improved patient acceptance is more important than this disadvantage. Furthermore, we did not use a consensus read between the two observers. The main reasons for this were that we wanted to reduce time spent on examining the CT colonographies and we aimed to obtain a high sensitivity by combining the scores of both observers.
In conclusion we found that CT colonography has a high diagnostic accuracy for detection of colorectal neoplasia in an FOBT positive screening population. Even with the use of a limited bowel preparation, the sensitivity of CT colonography for detection of large adenomas and carcinomas in our study was similar to that of colonoscopy and therefore CT colonography can be used in FOBT positives that are unfit for or unwilling to undergo colonoscopy. Double reading and additional 3D reading increased the sensitivity of CT colonography.
Acknowledgments
We thank all other persons of the departments of Radiology and Gastroenterology and Hepatology of the Academic Medical Center in Amsterdam, the University Medical Center Nijmegen and the Erasmus MC University Medical Center Rotterdam who contributed to this study. Furthermore, we would like to thank Philips Medical Systems for providing all necessary workstations. Supported by the Netherlands Organization for Health Research and Development (ZonMW: project number 62300036).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 2000;95(11):3053–3063. doi: 10.1111/j.1572-0241.2000.03434.x. [DOI] [PubMed] [Google Scholar]
- 2.Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38(2):173–176. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- 3.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36(6):2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 4.Bond JH. Clinical evidence for the adenoma-carcinoma sequence, and the management of patients with colorectal adenomas. Semin Gastrointest Dis. 2000;11(4):176–184. [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56(3):143–159. doi: 10.3322/canjclin.56.3.143. [DOI] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American college of gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 8.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E (2007) Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev Jan 24(1):CD001216 [DOI] [PMC free article] [PubMed]
- 9.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 10.Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ. 1998;317(7158):559–565. doi: 10.1136/bmj.317.7158.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK colorectal Cancer Screening Pilot Group (2004) Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ doi:10.1136/bmj.38153.491887.7C [DOI] [PMC free article] [PubMed]
- 12.Van Rossum LG, Van Rijn AF, Laheij RJ, Van Oijen MG, Fockens P, Van Krieken HH, Verbeek AL, Jansen JB, Dekker E. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Liedenbaum MH, van Rijn AF, de Vries AH, et al. Using CT colonography as a triage technique after a positive faecal occult blood test in colorectal cancer screening. Gut. 2009;58(9):1242–1249. doi: 10.1136/gut.2009.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, Gopal DV, Reichelderfer M, Hsu RH, Pfau PR. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357(14):1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349(23):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 17.Regge D, Laudi C, Galatola G, et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA. 2009;301(23):2453–2461. doi: 10.1001/jama.2009.832. [DOI] [PubMed] [Google Scholar]
- 18.Neri E, Vagli P, Turini F, et al. (2009) Diagnostic accuracy of CT colonography in patients with positive faecal occult blood test: results of the Italian project Legatumori 2003–2006. Radiol Med. doi:10.1007/s11547-009-0342-x. [DOI] [PubMed]
- 19.Hol L, Wilschut JA, van BM, van Vuuren AJ, van d V, Reijerink JC, van der Togt AC, Kuipers EJ, Habbema JD, van Leerdam ME. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer. 2009;100(7):1103–1110. doi: 10.1038/sj.bjc.6604961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callstrom MR, Johnson CD, Fletcher JG, Reed JE, Ahlquist DA, Harmsen WS, Tait K, Wilson LA, Corcoran KE. CT colonography without cathartic preparation: feasibility study. Radiology. 2001;219(3):693–698. doi: 10.1148/radiology.219.3.r01jn22693. [DOI] [PubMed] [Google Scholar]
- 21.Jensch S, de Vries AH, Pot D, Peringa J, Bipat S, Florie J, van Gelder RE, Stoker J. Image quality and patient acceptance of four regimens with different amounts of mild laxatives for CT colonography. AJR Am J Roentgenol. 2008;191(1):158–167. doi: 10.2214/AJR.07.3128. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SA, Slater A, Burling DN, Tam E, Greenhalgh R, Gartner L, Scarth J, Pearce R, Bassett P, Halligan S. CT colonography: optimisation, diagnostic performance and patient acceptability of reduced-laxative regimens using barium-based faecal tagging. Eur Radiol. 2008;18(1):32–42. doi: 10.1007/s00330-007-0631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liedenbaum MH, de Vries AH, Gouw CI, van Rijn AF, Bipat S, Dekker E, Stoker J. CT colonography with minimal bowel preparation: evaluation of tagging quality, patient acceptance and diagnostic accuracy in two iodine-based preparation schemes. Eur Radiol. 2009 doi: 10.1007/s00330-009-1570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Participants in the Paris workshop The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 25.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47(2):251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58(2):241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 27.Fidler J, Johnson C. Flat polyps of the colon: accuracy of detection by CT colonography and histologic significance. Abdom Imaging. 2009;34(2):157–171. doi: 10.1007/s00261-008-9388-4. [DOI] [PubMed] [Google Scholar]
- 28.Park SH, Ha HK, Kim AY, et al. Flat polyps of the colon: detection with 16-MDCT colonography—preliminary results. AJR Am J Roentgenol. 2006;186(6):1611–1617. doi: 10.2214/AJR.04.1889. [DOI] [PubMed] [Google Scholar]
- 29.Chen SC, Lu DS, Hecht JR, Kadell BM. CT colonography: value of scanning in both the supine and prone positions. AJR Am J Roentgenol. 1999;172(3):595–599. doi: 10.2214/ajr.172.3.10063842. [DOI] [PubMed] [Google Scholar]
- 30.Yee J, Kumar NN, Hung RK, Akerkar GA, Kumar PR, Wall SD. Comparison of supine and prone scanning separately and in combination at CT colonography. Radiology. 2003;226(3):653–661. doi: 10.1148/radiol.2263010701. [DOI] [PubMed] [Google Scholar]
- 31.Choi EK, Park SH, Kim DY, Ha HK. Malignant rectal polyp overlooked on CT colonography because of retention balloon: opposing crescent appearance as sign of compressed polyp. AJR Am J Roentgenol. 2007;189(1):W1–W3. doi: 10.2214/AJR.05.1643. [DOI] [PubMed] [Google Scholar]
- 32.Johnson CD, MacCarty RL, Welch TJ, Wilson LA, Harmsen WS, Ilstrup DM, Ahlquist DA. Comparison of the relative sensitivity of CT colonography and double-contrast barium enema for screen detection of colorectal polyps. Clin Gastroenterol Hepatol. 2004;2(4):314–321. doi: 10.1016/S1542-3565(04)00061-8. [DOI] [PubMed] [Google Scholar]
- 33.Johnson CD, Fletcher JG, MacCarty RL, Mandrekar JN, Harmsen WS, Limburg PJ, Wilson LA. Effect of slice thickness and primary 2D versus 3D virtual dissection on colorectal lesion detection at CT colonography in 452 asymptomatic adults. AJR Am J Roentgenol. 2007;189(3):672–680. doi: 10.2214/AJR.07.2354. [DOI] [PubMed] [Google Scholar]