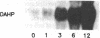Abstract
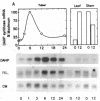
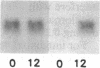
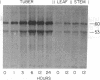
The first enzyme of the shikimate pathway, 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (EC 4.1.2.15), is induced by wounding potato or tomato tissue. The increase in enzyme activity is associated with elevated amounts of the enzyme as determined by immunoblots. The specific wound-induced protein synthesis is preceded by an increase in the mRNA encoding this enzyme. The induced mRNA of potato tuber, leaf, and stem tissue is translated into a precursor polypeptide that is recognized by antibodies raised against the mature enzyme from tuber plastids. Wounding also induces mRNA encoding phenylalanine ammonia-lyase (EC 4.3.1.5), a key enzyme of plant secondary metabolism. The time courses for the induction of the two enzymes are similar, suggesting coordinate regulation for the biosynthesis of primary and secondary aromatic compounds.
Keywords: 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudoin-Eagan L. D., Thorpe T. A. Shikimate Pathway Activity during Shoot Initiation in Tobacco Callus Cultures. Plant Physiol. 1983 Oct;73(2):228–232. doi: 10.1104/pp.73.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs M. S., Harriman R. W., Handa A. K. Changes in Gene Expression during Tomato Fruit Ripening. Plant Physiol. 1986 Jun;81(2):395–403. doi: 10.1104/pp.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen J., Varner J. E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit C. M., Meagher R. B. Expression of a gene encoding a glycine-rich protein in petunia. Mol Cell Biol. 1987 Dec;7(12):4273–4279. doi: 10.1128/mcb.7.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Kuroki G., Conn E. E. Increased Chorismate Mutase Levels as a Response to Wounding in Solanum tuberosum L. Tubers. Plant Physiol. 1988 Mar;86(3):895–898. doi: 10.1104/pp.86.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue K. F., Conn E. E. Induction of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase activity by fungal elicitor in cultures of Petroselinum crispum. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7374–7377. doi: 10.1073/pnas.86.19.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pinto J. E., Dyer W. E., Weller S. C., Herrmann K. M. Glyphosate Induces 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase in Potato (Solanum tuberosum L.) Cells Grown in Suspension Culture. Plant Physiol. 1988 Aug;87(4):891–893. doi: 10.1104/pp.87.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J. E., Suzich J. A., Herrmann K. M. 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase from Potato Tuber (Solanum tuberosum L.). Plant Physiol. 1986 Dec;82(4):1040–1044. doi: 10.1104/pp.82.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder T. B., Hedrick S. A., Bell J. N., Liang X. W., Clouse S. D., Lamb C. J. Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet. 1987 Dec;210(2):219–233. doi: 10.1007/BF00325687. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and protein metabolism in pea epicotyls : I. The aging process. Plant Physiol. 1983 Nov;73(3):809–816. doi: 10.1104/pp.73.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Suzich J. A., Ranjeva R., Hasegawa P. M., Herrmann K. M. Regulation of the shikimate pathway of carrot cells in suspension culture. Plant Physiol. 1984 Jun;75(2):369–371. doi: 10.1104/pp.75.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]