Abstract
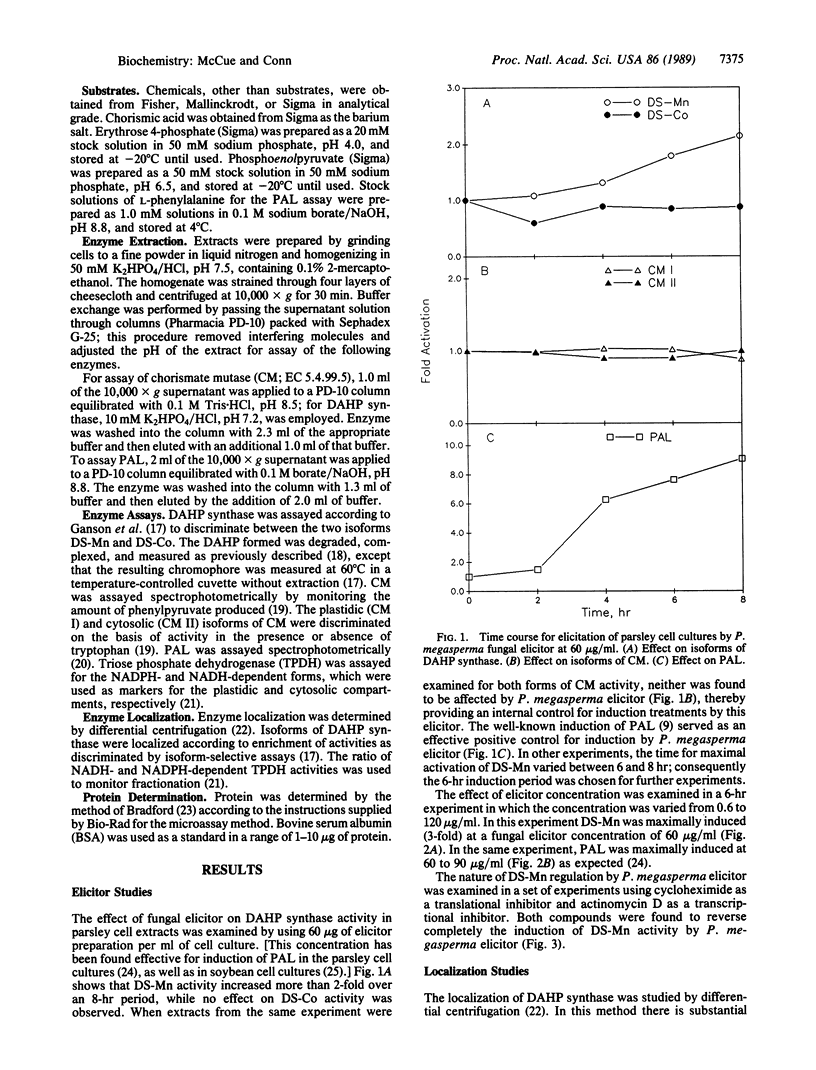
The effects of a cell wall fraction of the fungus Phytophthora megasperma on the enzymatic activities of 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) synthase (EC 4.1.2.15) in extracts of cultured parsley cells (Petroselinum crispum) were examined. The specific activity of a plastidic form of DAHP synthase, designated DS-Mn by Ganson et al. [Ganson, R. J., d'Amato, T. A. & Jensen, R. A. (1986) Plant Physiol. 82, 203-210], was increased 2- to 3-fold in extracts of treated cells, with maximum induction occurring with 60 micrograms of fungal elicitor per ml after 6-8 hr. The cytosolic form of DAHP synthase, DS-Co, was unaffected by fungal elicitor. In the same experiments, phenylalanine ammonia-lyase activity (EC 4.3.1.5) increased, while no effect on isoforms of chorismate mutase (EC 5.4.99.5) was observed. The subcellular localization of the two DAHP synthase isoforms in parsley was confirmed by differential centrifugation. Prior treatment of cultures with actinomycin D or cycloheximide prevented the increase in DS-Mn activity, indicating transcriptional regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz B., Schäfer E., Hahlbrock K. Light-induced phenylalanine ammonia-lyase in cell-suspension cultures of Petroselinum hortense. Quantitative comparison of rates of synthesis and degradation. Arch Biochem Biophys. 1978 Sep;190(1):126–135. doi: 10.1016/0003-9861(78)90259-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davis K. R., Hahlbrock K. Induction of defense responses in cultured parsley cells by plant cell wall fragments. Plant Physiol. 1987 Aug;84(4):1286–1290. doi: 10.1104/pp.84.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V., Dennis D. T. Isoenzyme of pyruvate kinase in proplastids from developing castor bean endosperm. Plant Physiol. 1978 Jun;61(6):1037–1039. doi: 10.1104/pp.61.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer W. E., Henstrand J. M., Handa A. K., Herrmann K. M. Wounding induces the first enzyme of the shikimate pathway in Solanaceae. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7370–7373. doi: 10.1073/pnas.86.19.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer W. E., Weller S. C., Bressan R. A., Herrmann K. M. Glyphosate Tolerance in Tobacco (Nicotiana tabacum L.). Plant Physiol. 1988 Nov;88(3):661–666. doi: 10.1104/pp.88.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Ayers A. R., Albersheim P. Host-Pathogen Interactions: XII. Response of Suspension-cultured Soybean Cells to the Elicitor Isolated from Phytophthora megasperma var. sojae, a Fungal Pathogen of Soybeans. Plant Physiol. 1976 May;57(5):775–779. doi: 10.1104/pp.57.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganson R. J., D'Amato T. A., Jensen R. A. The Two-Isozyme System of 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase in Nicotiana silvestris and Other Higher Plants. Plant Physiol. 1986 Sep;82(1):203–210. doi: 10.1104/pp.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K., Lamb C. J., Purwin C., Ebel J., Fautz E., Schäfer E. Rapid Response of Suspension-cultured Parsley Cells to the Elicitor from Phytophthora megasperma var. sojae: INDUCTION OF THE ENZYMES OF GENERAL PHENYLPROPANOID METABOLISM. Plant Physiol. 1981 Apr;67(4):768–773. doi: 10.1104/pp.67.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K., Ragg H. Light-induced changes of enzyme activities in parsley cell suspension cultures. Effects of inhibitors of RNA and protein synthesis. Arch Biochem Biophys. 1975 Jan;166(1):41–46. doi: 10.1016/0003-9861(75)90362-8. [DOI] [PubMed] [Google Scholar]
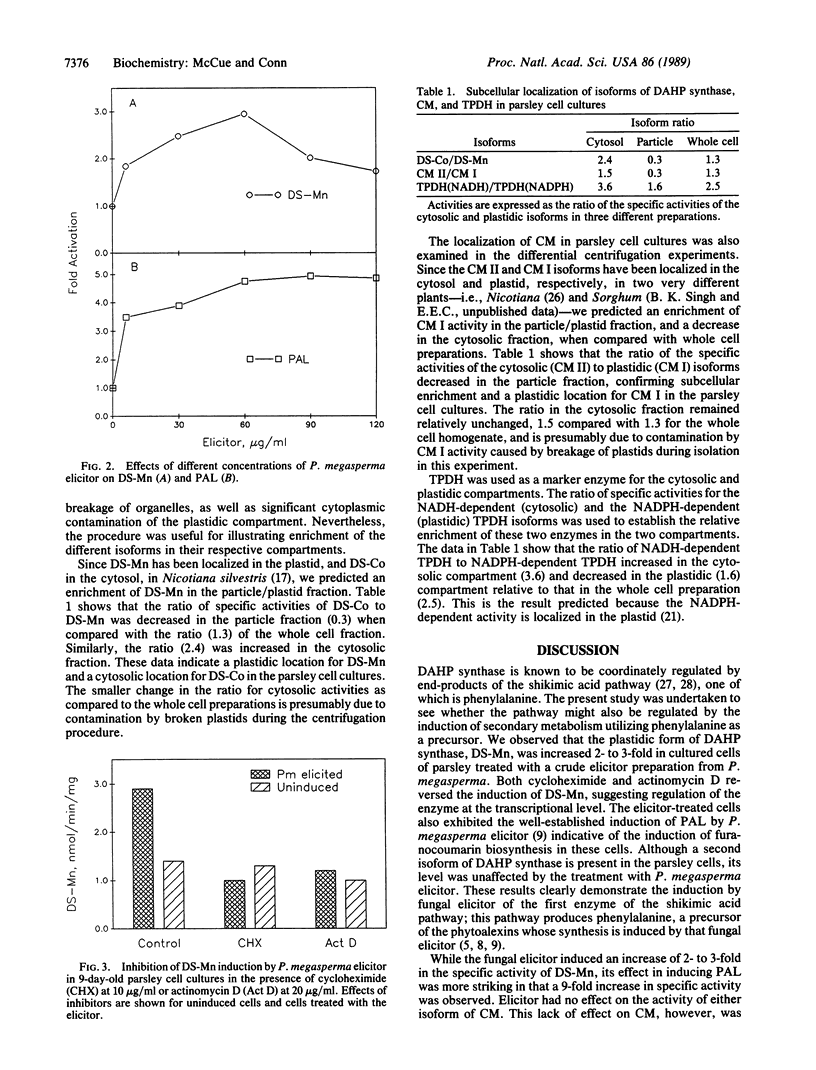
- Hahlbrock K., Schröder J. Light-induced changes of enzyme activities in parsley cell suspension cultures. Increased rate of synthesis of phenylalanine ammonia-lyase. Arch Biochem Biophys. 1975 Jan;166(1):47–53. doi: 10.1016/0003-9861(75)90363-x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Schröder J. Specific effects on enzyme activities upon ditution of petroselinum hortense cell cultures into water. Arch Biochem Biophys. 1975 Dec;171(2):500–506. doi: 10.1016/0003-9861(75)90059-4. [DOI] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J. E., Dyer W. E., Weller S. C., Herrmann K. M. Glyphosate Induces 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase in Potato (Solanum tuberosum L.) Cells Grown in Suspension Culture. Plant Physiol. 1988 Aug;87(4):891–893. doi: 10.1104/pp.87.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. L., Jensen R. A. Differentially Regulated Isozymes of 3-Deoxy-d-arabino-Heptulosonate-7-Phosphate Synthase from Seedlings of Vigna radiata [L.] Wilczek. Plant Physiol. 1985 Nov;79(3):711–718. doi: 10.1104/pp.79.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoner R., Herrmann K. M. 3-Deoxy-D-arabino-heptulosonate 7-phosphate synthase. Purification, properties, and kinetics of the tyrosine-sensitive isoenzyme from Escherichia coli. J Biol Chem. 1976 Sep 25;251(18):5440–5447. [PubMed] [Google Scholar]
- Singh B. K., Connelly J. A., Conn E. E. Chorismate mutase isoenzymes from Sorghum bicolor: purification and properties. Arch Biochem Biophys. 1985 Dec;243(2):374–384. doi: 10.1016/0003-9861(85)90514-4. [DOI] [PubMed] [Google Scholar]
- Suzich J. A., Ranjeva R., Hasegawa P. M., Herrmann K. M. Regulation of the shikimate pathway of carrot cells in suspension culture. Plant Physiol. 1984 Jun;75(2):369–371. doi: 10.1104/pp.75.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietjen K. G., Hunkler D., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur J Biochem. 1983 Mar 15;131(2):401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]
- Tietjen K. G., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 2. Effects on enzyme activities. Eur J Biochem. 1983 Mar 15;131(2):409–413. doi: 10.1111/j.1432-1033.1983.tb07278.x. [DOI] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]



