Abstract
Activation of the canonical Notch pathways has been implicated in Th cell differentiation, but the role of specific Notch ligands in Th2 mediated allergic airway responses has not been completely elucidated. In this study, we show that delta-like 4 (Dll4) was up-regulated on dendritic cells in response to cockroach allergen. Blocking Dll4 in vivo during either the primary or secondary response enhanced allergen-induced pathogenic consequences including airway hyperresponsiveness (AHR) and mucus production via increased Th2 cytokines. In vitro assays demonstrated that Dll4 regulates IL-2 in T cells from established Th2 responses as well as during primary stimulation. Interestingly, Dll4 blockade during the primary, but not the secondary response, increased IL-2 levels in lung and lymph node of allergic mice. The in vivo neutralization of Dll4 was associated with increased expansion and decreased apoptosis during the primary allergen sensitization. Moreover, Dll4-mediated Notch activation of T cells during primary stimulation in vitro increased apoptosis during the contraction/resting phase of the response, which could be rescued by exogenous IL-2. Consistent with the role for Dll4-mediated IL-2 regulation in overall T cell function, the frequency of IL-4 producing cells were also significantly altered by Dll4 both in vivo and in vitro. These data demonstrate a regulatory role of Dll4 on both initial Th2 differentiation as well as on Th2 cytokine production in established allergic responses.
Introduction
Asthma, one of the most common chronic diseases in Western society, is a pulmonary disease clinically characterized by altered lung function, peribronchial inflammation and airway hyperresponsiveness (1, 2). In most cases, asthma is believed to result from a T helper (Th)2-type reaction to an inhaled allergen from the environment (allergic asthma) (3–5). In recent decades, the prevalence of asthma and emergency room visits, especially in children has increased dramatically. Although many studies have demonstrated that Th2 responses orchestrate the pathogenesis of allergic lung disease, less is known about mechanisms that affect the development and maintenance of Th2 cells (6, 7). The initiation of pulmonary immune responses begins with recognition of inhaled antigens by antigen presenting cells (APCs) such as dendritic cells, which subsequently migrate to the draining lymph nodes (8–10). In those lymph nodes, APCs prime T cells and these activated T cells migrate into lungs where they secrete cytokines and other mediators, which direct the asthmatic response in the lung (11).
Notch is a heterodimeric cell-surface receptor that is involved in a broad range of differentiation processes, including the lineage choice between T or B lymphocytes. Mammals have four different Notch receptors (Notch1–4), which bind two conserved families of ligands, known as the delta-like ligands (consisting of DLL1, DLL3 and DLL4) and the jagged ligands (jagged1 and jagged2). In the immune system, Notch-mediated responses have been shown to regulate T cell development in the thymus (12–16). Moreover, in addition to its role in T cell lineage maturation, recent studies have also shown that Notch/Notch ligand signaling regulates mature peripheral T cells during antigen-specific response (17, 18). The requirement of Notch signaling for Th development has recently been appreciated. Amsen et al. demonstrated that MyD88-dependent Th1 stimuli increase the expression of Notch ligand delta-like 4 in dendritic cells and controls the differentiation of naïve T cells into Th1 cells, whereas MyD88-independent Th2 stimuli up-regulate the Notch ligand jagged and polarize toward Th2 cells (19). The importance of Notch signaling in T cell differentiation has been supported using inhibitors of γ-secretase (which prevent the activation of the Notch signaling pathway), to block Th1 responses through the blockade of T-bet expression (20). Furthermore, Notch intracellular domain (NICD) up-regulates IFN-γ expression (21). In contrast, Tu and colleagues showed that Notch signaling was required only for Th2 cell responses by using dominant negative mastermind-like (MAML)-CD4 transgenic mice (22). Moreover recent reports showed that Notch mediated binding of RBPJκ to GATA-3 promoter enhanced GATA-3 expression, resulting in Th2 commitment in the absence of IL-4 (23, 24). In contrast, studies have shown that delta-like ligands (Dll1 and Dll4) play a regulatory role in T cell activation by modulating Th2 cytokines (25, 26). It has also been suggested that jagged1-mediated Notch activation specifically induces Th2 responses in the lung (27). While clearly Notch ligands appear to have a significant role, the function of specific ligands and their effect of those responses during different phases of allergic disease remain unclear (28–30). Therefore, in these studies, we investigated the role of Notch ligand Dll4 in the regulation of pathogenesis of allergic pulmonary disease.
Materials and Methods
Mice
Balb/c and 4get mice were purchased from The Jackson Laboratories. All animal work was performed in accordance with the University of Michigan Committee on Use and Care of Animals policy.
Airway Response
Airway hyperreactivity was assessed as previously described using direct ventilation methodology and airway resistance measurements (31). Briefly, mice were anesthetized with sodium pentobarbital, intubated via cannulation of the trachea, and ventilated with a Harvard pump ventilator (0.3 mL tidal volume; 120 breaths/min). AHR was measured using a Buxco mouse plethysmograph and software for calculation of the measurements. After baseline measurements, mice were injected intravenously with 7.5ug of methacholine (Sigma-Aldrich, St. Louis, MO) and the peak airway resistance was recorded as a measure of airway hyperreactivity.
Cockroach Antigen (CRA) Model
CRA sensitization was performed as previously described (32, 33). Briefly, female mice, 6 to 8 weeks of age, were sensitized with a 1:1 mixture of cockroach allergen extract (CRA, Hollister-Stier, Spokane, WA) and incomplete Freund’s adjuvant (Sigma-Aldrich), both subcutaneously and intraperitoneally on day 0. This cockroach allergen is a skin test/immunotherapy grade preparation that has very little endotoxin contamination (<10 ng/ml). At day 14, mice were locally sensitized by intranasal challenges of CRA followed by intratracheal challenges on day 19.
Generation of Bone Marrow Derived Dendritic cells (BMDCs)
For generation of BMDCs, after depletion of erythrocytes with lysis buffer, BM cells were seeded in T-150 tissue culture flasks at 5×105 cells/ml in RPMI 1640–based complete media with GM-CSF 20 ng/ml (R&D Systems, Minneapolis, MN). On day 3 GM-CSF was supplemented into cultured cells again. Six days later, loosely adherent cells were collected and incubated with anti-CD11c coupled to magnetic beads for positive selection of conventional DCs from the GM-CSF cultures using a magnetic column (Miltenyi Biotec, Auburn, CA).
Anti-Dll4 antibody and in vivo administration
Rabbit anti-murine dll4 antibodies were prepared by multiple-site immunization of New Zealand White rabbits with recombinant murine dll4 (R&D Systems, Rochester, MN) and specificity verified as previously described from our laboratory (26). Briefly, polyclonal antibodies were titered by direct ELISA against dll4 coated onto 96-well plates and titered at 107 with no cross-reactivity to the other notch ligands. Antibody specificity was verified by immunofluorescent staining and flow cytometry of Notch ligand expressing OP-9 cells stably transfected with the 5 different Notch ligands, dll1, 3, 4 and jagged 1,2. Only dll4 expressing cell lines were positive for staining using our polyclonal antibody. In vivo administration used 0.5 ml of serum from pre-immunized or Dll4 immunized rabbits as previously described (26) at the time points indicated.
In vitro CD4+ T Cell Culture
CD4+ splenic T cells were purified by negative selection using CD4+ T Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s recommendations. Purified CD4+ cells were activated with plate bound anti-CD3 and anti-CD28 Abs (2 µg/ml). Plate-bound recombinant Dll4 (rDll4) was used at a final concentration of 2.5 µg/ml. Th2 conditions included IL-4 (10 ng/ml) and anti-IFN-γ (10 µg/ml). Recombinant proteins (Dll4, IL-4, and IL-2) were purchased from R&D Systems (Minneapolis, MN). Anti-IFN-γ, anti-CD3 and anti-CD28 Abs were purchased from eBiosciences (San Diego, CA).
Protein Assays
Single cell suspensions of lung draining lymph nodes were plated at a concentration of 5 × 106 cells/ml onto a 96-well plate and restimulated with cockroach antigens (3 µg/ml) for 48 hrs and supernatant were harvested for cytokine determination. Cytokines were quantified using a Bio-Plex bead-based (luminex) cytokine assay purchased from Bio-Rad Laboratories (Hercules, CA). For detection of IL-2, the ELISA (eBiosciences) was used according to manufacturer’s recommendations.
Histology and RT-PCR
Right lobes from infected mice were removed, fixed in 10% formalin, and stained with Periodic Acid Schiff (PAS) to detect mucus production. Total RNA was isolated from lower left lobes of lungs using TRIzol (Invitrogen, Carlsbad, CA). Real-time PCR was performed on cDNA using primers. Primers and probes used for the detection of mRNA in lung samples were determined using pre-developed primer/probe sets (PE Biosystems, Foster City, CA). Murine GAPDH (PE Biosystems) was used as an internal control for quantification of the total amount of cDNA used in the reaction. Results are normalized to GAPDH expression and are presented as the folds increase in mRNA expression over the naïve mice group. The Sybr primer sets for Dll1, Dll4, Jagged1, Jagged2 were purchased from Sigma-Aldrich and were described previously (19). For comparisons, dendritic cells without allergens were assigned an arbitrary value of 1.
Flow Cytometry
Lungs and lymph nodes were harvested from infected mice. They were digested with collagenase and dispersed to obtain single cell suspensions. Red blood cells were lysed and the remaining cells spun down and resuspended in PBS containing 1% BSA. Cells were Fc-blocked for 10 minutes. Cells were stained with the monoclonal antibodies: anti- Dll4 (HMD4-1) from Biolgend, anti-CD45 (30-F11), CD4 (RM4–5), CD8 (53–6.7), CD69 (H.2F3), and CD11c (N418), CD11b (M1/70), and PDCA (ebio129c) (all from eBiosciences) and analyzed by LSRII flow cytometer (Becton-Dickinson, Franklin Lakes, NJ) using Flowjo software (TreeStar, Inc., Ashland, OR). The absolute number of each cell type was determined by multiplying the percentage × total cell number isolated from each organ. For apoptosis staining of T cells, cells were collected on the indicated day and stained with Annexin V and 7-AAD (eBiosciences). CFSE staining was used by standard protocol with 9 µM incubated with 4 × 106 cells/ml.
Statistics
Statistical significance was determined by one way ANOVA with a Newman-Keuls post test. Significant differences were regarded as p < 0.05.
Results
Cockroach allergen induces Dll4 in DCs and regulates Th2 cytokine production
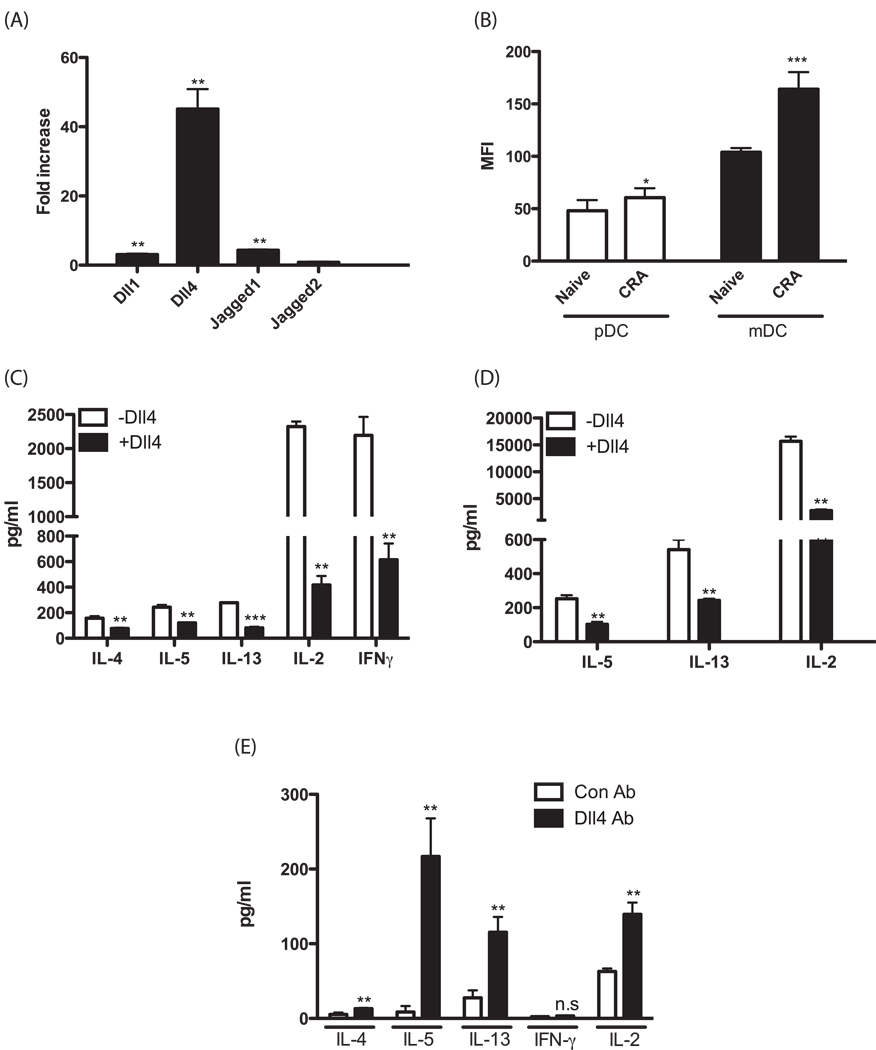
To investigate whether the expression of Notch ligands is induced in dendritic cells in response to cockroach allergen, CD11c+ bone marrow-derived DCs (BMDCs) were stimulated with cockroach allergen and the expression of Notch ligands was analyzed by real time PCR. Cockroach allergen induced the expression of several Notch ligands including Dll1, Dll4 and jagged1, but the expression of Dll4 was increased to a much higher extent compared to that of the other ligands (Fig. 1A). In addition, we have also used flow cytometric analysis to determine whether DC in the lungs of allergen challenged mice (24 hrs post-allergen challenge) express Dll4. The data in Figure 1B indicate that when we specifically examine the CD11b/CD11c+ and CD11c/PDCA+ cell populations there is a significant increase in Dll4 expression based upon mean fluorescence intensity (MFI) compared to cells from lungs of naïve mice with the most profound effect observed on the CD11b/CD11c+ subset. Recently it has been shown that Notch signaling modulates T cell cytokine production. To determine whether Dll4 alters the capacity of T cells for production of Th2 cytokines, lymph node cells were isolated from allergen challenged mice and restimulated with CD3/CD28 either in the presence or absence of Dll4 ligand coated plates. As shown in Fig. 1C, Th2 cytokines IL-4, IL-5 and IL-13 were significantly reduced in the presence of Dll4, confirming earlier reports that demonstrate regulation of Th2 cytokines by Dll4 (26). Interestingly, in the present studies IL-2 production was also decreased in the presence of Dll4 (Fig. 1C). Interestingly, when we examined IFNγ production it was also reduced in the cells from this Th2 skewed environment. We next examined the effect of Dll4 on the capacity of CD4+ T cells to produce cytokines in Th2 biased conditions using a primary skewing culture. Similar to what was observed in lymph node cells from allergic mice, Th2 cytokines and IL-2 were significantly reduced in the presence of Dll4 under Th2 skewed conditions (Fig. 1D). Thus, Dll4 appears to modulate Th2 cytokines established under allergen-induced responses as well as in primary Th2 skewing conditions.
Figure 1.
CRA induces Notch ligands in DCs and Dll4 signaling suppresses Th2 cytokines production from T cells. (A) BMDCs were cultured for 8hrs in the presence of cockroach allergen. Relative expression of Notch ligands was analyzed by real time PCR. Fold increase in Notch ligands was determined by comparison to BMDCs without allergen. (B) Flow cytometry was performed using lung digests taken from 1d after allergen challenge. The lung cell suspension was labeled with mAbs against CD11c, CD11b, PDCA, Dll4. Among CD11c+ cells, alveolar macrophages were gated out with their autofluorescent property. CD11c+CD11b+ myeloid dendritic cells(mDCs) and CD11c+PDCA+ plasmacytoid dendritic cells(pDCs) population were analyzed for mean fluorescence intensity (MFI) for Dll4 expression. (C) Lymph node cells were stimulated with CD3 and CD28 with/without Dll4 for 24hrs and cytokines levels measured. (D) CD4+ T cells isolated by MACS beads from Balb/c mice were stimulated for 3 days with Th2 biased condition either presence or absence of Dll4. (E) Mice were treated with Abs 2.5 hrs before primary cockroach allergen sensitization. At day 7 postimmunization, splenocytes were assessed for Th2 cytokines and IL-2 levels after restimulation with cockroach antigens. Cytokines were assessed by Bioplex and IL-2 ELISA kit. Each experiment was repeated 2 times and displayed similar significant results. *=p<0.05, **=p<0.01, ***=p<0.001
To address whether the changes that we observed in cells from animals fully sensitized and skewed toward a Th2 allergic response manifested itself early during the sensitization process immunized mice given anti-Dll4 antibody prior to sensitization were assessed for Th2 cytokine levels at day 7 post-immunization. To perform these studies splenocytes were isolated and rechallenged with allergen in vitro and cytokines were measured from supernatants of the in vitro cultures (Figure 1E). The data indicate that in the anti-Dll4 treated group that there was a significant increase in Th2 cytokines, including IL-4, IL-5 and IL-13, with no activation of IFNγ, which is typical with sensitization with this allergen preparation. In addition to the Th2 cytokine regulation, when IL-2 production was examined at this early time point the anti-Dll4 treated animals had a significant increase in allergen-specific IL-2 production. Thus, these data support an early alteration in the skewing of T cells in vivo and further linked a relationship with IL-2.
Anti-Dll4 Ab treatment during the primary response exacerbates allergic lung disease
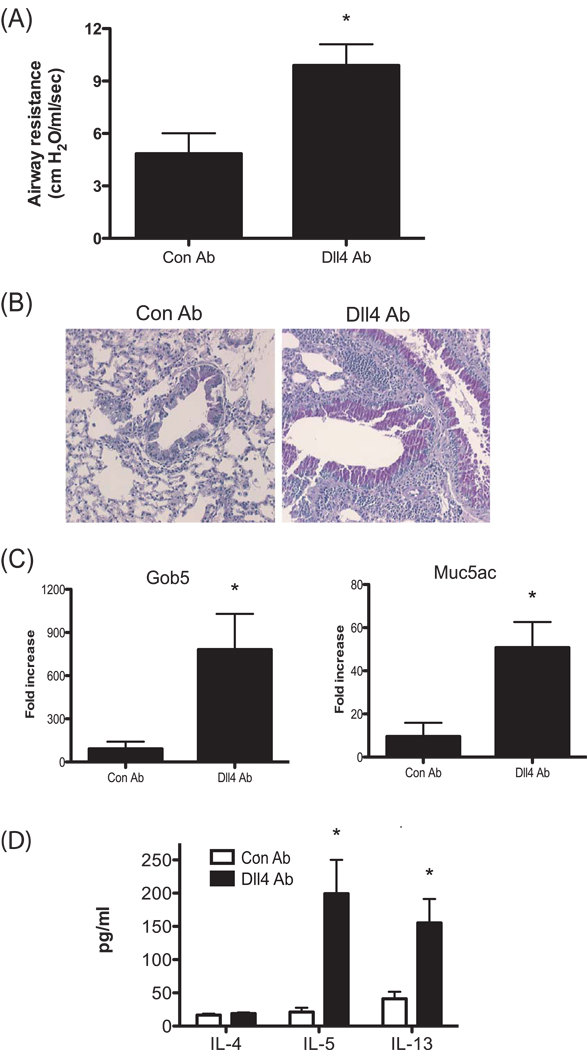
Since Dll4 suppressed Th2 cytokine production, the role of Dll4 in the development of allergic disease was investigated using an established model of cockroach allergen induced airway disease. To address whether Dll4 has a role in the development of allergic airway hyperresponsiveness during the primary response, mice were injected with anti-Dll4 Abs 2.5 hrs prior to primary cockroach allergen sensitization. Two weeks after immunization, the mice were locally sensitized with cockroach allergen through an intranasal route (IN). 5 days later, the mice were challenged with cockroach allergen through an intratracheal (IT) route and 24 hrs later, lungs and lymph nodes were harvested for histology and cytokine expression. Treatment with anti-Dll4 Abs only prior to primary sensitization resulted in a significantly enhanced airway hyperresponsivness (AHR) compared to the control Ab treated group (Fig. 2A). Moreover, histological examination of lungs showed increased mucus production in anti-Dll4 Ab treated mice compared to control Ab treated mice (Fig. 2B). Examination of the mucus-associated genes, gob5 and muc5ac, in the lung from anti-Dll4 treated mice demonstrated a substantial increase in expression of those genes compared with the control Ab-treated mice (Fig. 2C). To assess the immunologic mechanism for anti-Dll4 Ab-induced exacerbation of allergy, we examined the Th2 cytokines in draining lymph nodes. Lymph node cells were restimulated with cockroach allergen for 48 hrs and supernatant was harvested for cytokine analyses. Th2 cytokines, IL-5 and IL-13, from cockroach allergen stimulated T cells were significantly increased in anti-Dll4 Ab-treated mice compare to control Ab-treated mice (Fig. 2D). These data suggest that aggravated clinical disease in anti-Dll4 Ab treated mice is associated with enhancement of type 2 cytokines.
Figure 2.
Blockade of Dll4 signaling during primary response enhances cockroach allergen-induced airway hyperresponsiveness and mucus production in the lung. (A) Airway responses were measured in control Abs and anti-Dll4 Ab treated mice after one dose of methachline. Data are represented as mean airway resistance in H2O/ml/s± SEM. (B) Lungs were taken 1d after allergen challenge and were stained with PAS. (C) 1d after allergen challenge, lungs were isolated and assayed for Gob5 and Muc5ac expression by real-time PCR. (D) Analysis of cytokines from allergen restimulated lung draining lymph nodes assessed by Bioplex. Data represent mean ± SEM from 5 mice/group and the experiment was repeated with 5 mice/group that demonstrated a similar response. *=P<0.05
Anti-Dll4 Ab treatment during the secondary response increases severity of allergic lung disease
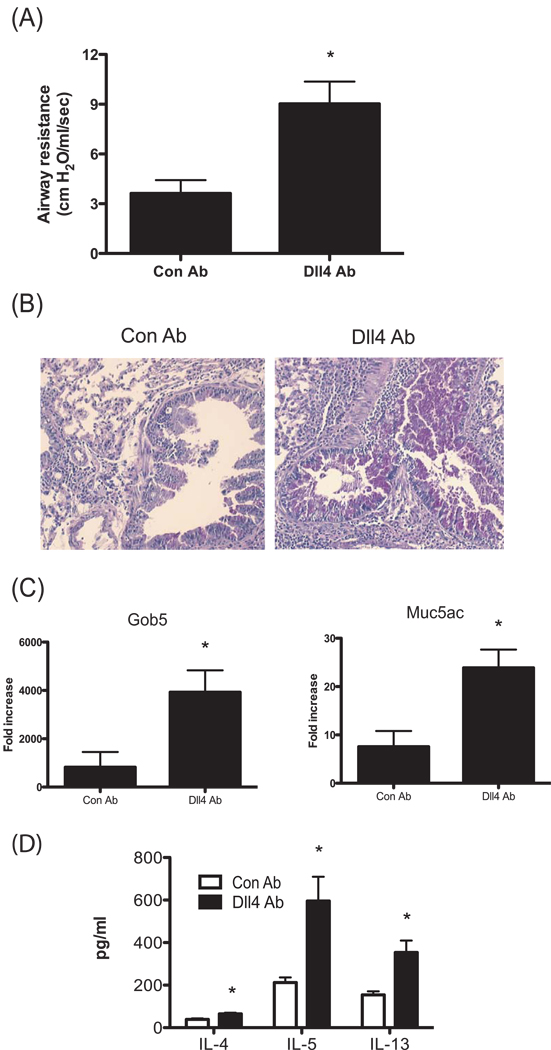
Next, to investigate whether Dll4 alters development of allergic response during the secondary response, the anti-Dll4 or control Ab was injected intraperitoneally 2.5 hrs prior to the final allergen challenge. Similar to what was observed in the mice treated with anti-Dll4 Abs only during the primary response, increased airway hyperactivity (Fig. 3A) and mucus overproduction histologically (Fig. 3B), and enhancement of muc5ac and gob5 gene expression (Fig. 3C) in the lungs from anti-Dll4 Ab mice treated were observed compared to control Ab treated mice. Allergen restimulated lymph node from anti-Dll4 Ab treated mice had increased IL-4, IL-5 and IL-13 cytokines from cockroach allergen specific T cells compared to control Ab treated mice (Fig. 3D). Overall, data thus far suggested that Dll4 modulates type 2 immunity during allergic responses, with subsequent regulation of pathophysiologic changes in the lung during the allergic reaction.
Figure 3.
Blockade of Dll4 signaling during secondary response enhances cockroach allergen-induced airway hyperresponsiveness and mucus production in the lung. (A) Airway responses were measured in control Abs and anti-Dll4 Ab-treated mice after one dose of methachline. Data are represented as mean airway resistance in H2O/ml/s± SEM. (B) Lungs were taken 1d after allergen challenge and were stained with PAS. (C) 1d after allergen challenge, lungs were isolated and assayed for Gob5 and Muc5ac expression by performing real-time PCR. (D) Analysis of cytokines from allergen restimulated lung draining lymph nodes assessed by Bioplex. Data represent mean ± SEM from 5 mice/group and the experiment was repeated with 5 mice/group that demonstrated a similar response. *=P<0.05
Dll4 signaling blockade during both primary and secondary alters the number of activated T cells in lungs and lymph nodes
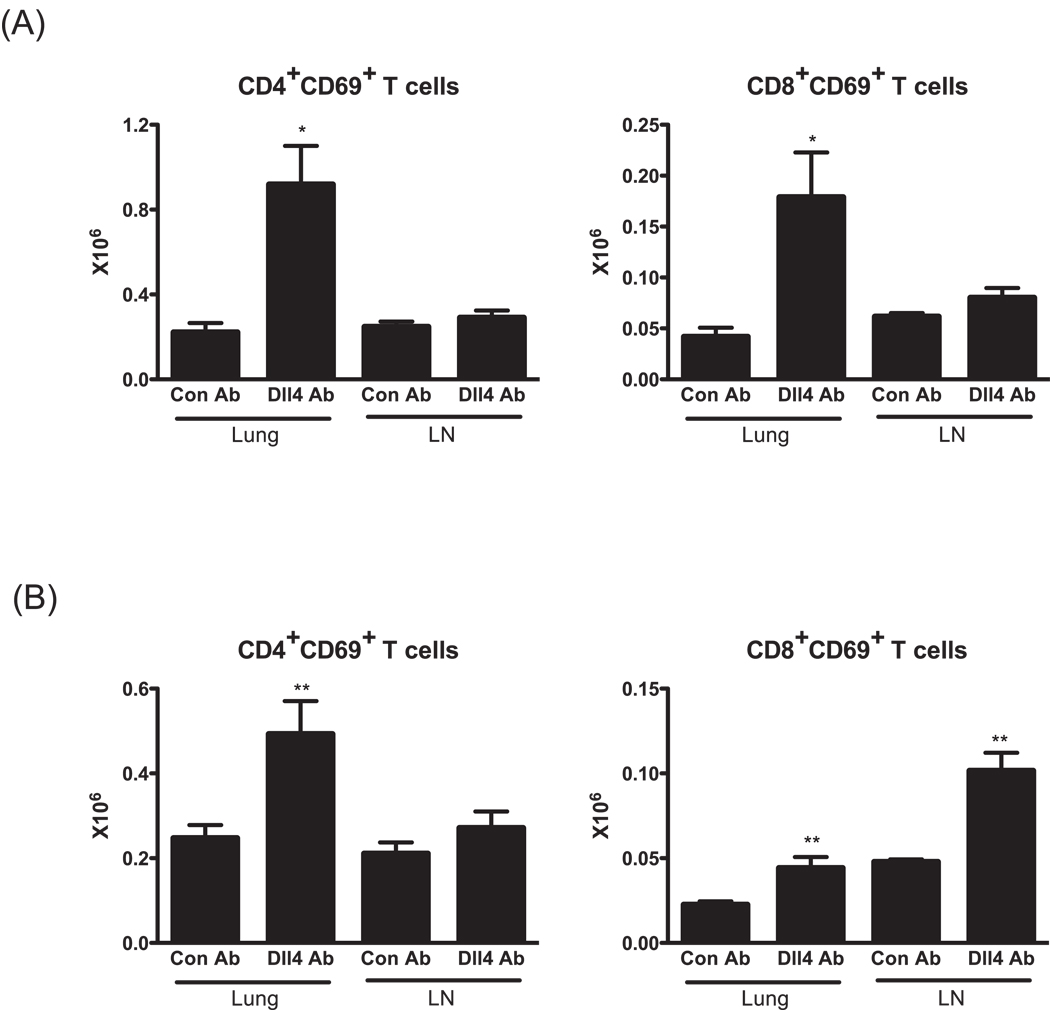
To further characterize the effect of the Dll4 blockade, T cells in lungs and draining lymph nodes of mice 1d after final allergen challenge were analyzed by flow cytometry. Regardless of timing of Dll4 blockade, significantly more CD4+ and CD8+ T cells expressing the early activation marker CD69 were found in the lung from mice treated with anti-Dll4 Abs than those from mice treated with control Abs (Fig. 4). We also quantified the number of T cell subsets in the draining lymph nodes of anti-Dll4 Ab treated mice. Similar to lungs, lymph node FACs data showed that there was a tendency of an increase of activated T cell subsets in anti-Dll4 Ab treated mice compared to the control group, but this increase did not reach statistical significance except with CD8+CD69+ T cells from mice treated with Dll4 during the secondary response (Fig. 4B). These data further imply that anti-Dll4 blockade notably alters the immune environment of the lung and the number of activated lymphocytes.
Figure 4.
The absence of Dll4 alters the number of activated T cells in the lung and lymph nodes of allergen challenged mice. Flow cytometry was performed using lung and lymph node digests taken from 1d after allergen challenge. (A) The total number of CD69+ T cell subsets in the lung and lymph node from mice blocked Dll4 signaling during primary response. (B) The total number of CD69+ T cell subsets in the lung and lymph node from mice blocked Dll4 signaling during secondary response. Data represents mean ± SEM from 5 mice/group. *=P<0.05, **=P<0.01.
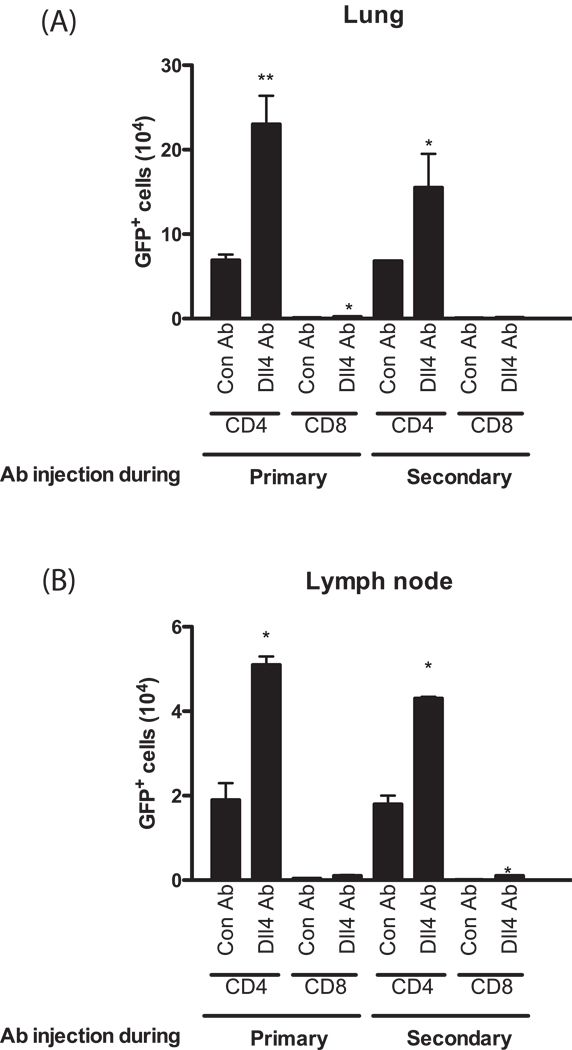
To specifically address whether Dll4 altered Th2 cytokine producing T cell subsets, we utilized 4get mice in which IL-4 mRNA expression is 'reported' by enhanced green fluorescence protein (eGFP) through a bicistronic reporter gene construct. This reporter construct, while not fully representative of IL-4 levels, identifies IL-4 competent cells that are transcriptionally poised for IL-4 and other Th2 cytokine production and therefore can be used as a read out of cells that have transitioned toward Th2 cell differentiation (34). We performed flow cytometry analysis on lung and lymph node cells to investigate IL-4 expressing Th2 cells in the mice treated with either control Abs or anti-Dll4 Abs as indicated by the flow cytometry (Fig. 5). There were significantly more CD4+ T/IL-4 expressing (GFP+) cells in the lungs (A) and lymph nodes (B) of anti-Dll4 Ab treated 4get mice compared to control Ab treated mice regardless of Dll4 blockade timing, primary or secondary (Fig. 5). Very few CD8+/IL-4 positive cells were detected indicating that CD4+ T cells are the main source of IL-4. These data confirm that anti-Dll4 Ab treatment enhances Th2 immunity by increasing the frequency of Th2 type cells during allergic airway hyperresponsiveness.
Figure 5.
Blockade of Dll4 signaling causes more CD4+ T/IL-4 expressing (GFP+) cells in the lungs and lymph nodes compared to control Ab treated mice regardless of Dll4 blockade timing. Lungs (A) and lymph nodes (B) from allergic mice treated with anti-Dll4 at either the primary or secondary stage of sensitization and challenge, as described above, were dispersed with collagenase and single cell suspensions were assessed by flow cytometry for GFP expression. Data represents mean ± SEM from 5 mice/group. *=P<0.05, **=P<0.01.
Dll4 regulates IL-2 production, T cell apoptosis and Th2 cell frequency
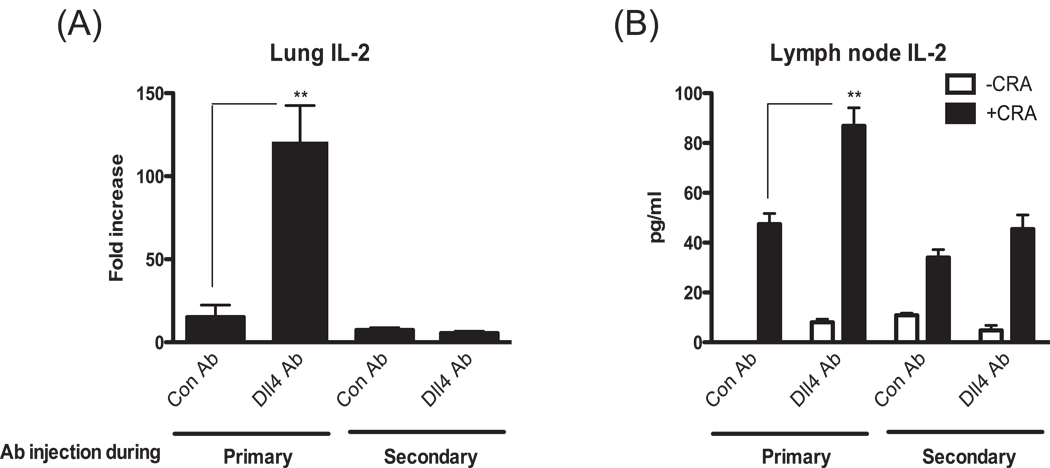
The mechanism of Dll4 function on the frequency of IL-4 producing cells may result from more cell proliferation and/or enhancement of Th2 differentiation. IL-2 has been shown to be a T cell growth factor and more recently demonstrated to directly enhance Th2 differentiation. Interestingly, as shown in Fig. 1, Dll4 suppressed IL-2 production from T cells. Therefore, we analyzed the level of IL-2 in the lung and in restimulated lymph node cells with the hypothesis that Dll4 may be altering the responses through IL-2. Interestingly, we observed an increase of IL-2 levels in the lungs and in restimulated lymph node cells only when Dll4 signaling was blocked during the primary response but not during the secondary response (Fig. 6). To further address the role of increased IL-2 expression in the animals with Dll4 blockade we examined proliferation and apoptosis during the primary response using ex vivo restimulation assays. Mesenteric lymph nodes were isolated from 7-day allergen immunized mice in the presence of control or anti-Dll4 antibody given on day 0 of sensitization. Given the increased number of Th2 type cells in our 4get mouse studies the proliferative effects after allergen sensitization were initially examined using CFSE labeling to monitor cell double by fluorescent dilution in flow cytometry (Figure 6C). The data indicate that a greater percentage of T cells responded by proliferation based upon CFSE dilution in the animals treated with anti-Dll4 compared to those cells taken from control antibody treated animals. We then examined the induction of apoptosis in CD4 T cells 3 or 5 days post re-stimulation with allergen ex vivo using annexin-V staining by flow cytometry (Figure 6D). The data indicate that T cells from anti-Dll4 immunized mice had a significant reduction in apoptosis. Thus, the regulation of Th2 development by Dll4 may be based upon interrelated proliferative and apoptotic mechanisms.
Figure 6.
Treatment of allergic animals at the primary, but not secondary response, with blocking Dll4 antibodies alters IL-2 production. (A) Lung mRNA was isolated from lungs of allergic animals at 8 hrs post-final allergen challenge and analyzed by quantitative PCR for the expression of IL-2. (B) Lymph nodes were isolated from at 24 hrs post-final allergen challenge. Single cell suspensions of the lung draining lymph nodes were analyzed for allergen restimulated IL-2 production by assessing supernatants by ELISA. (C, D) Mice were administrated with Abs 2hrs before cockroach antigen sensitization (i.p.). Lung draining lymph node cells from day 7 immunized mice were ficolled to remove dead cells, and labeled with CFSE (Carboxyfluorescein succinimidyl ester). Live CD4+ T cells (CD4 positive and 7-AAD negative) were cultured with cockroach antigens and analyzed for CFSE dilution by FACs analysis (C). These CFSE labeled cells were analyzed for annexin V staining for apoptotic CD4+ T cells (D). Data represent mean ± SEM from 5 mice/group. **=P<0.01. Histograms are representative Day 5 cells.
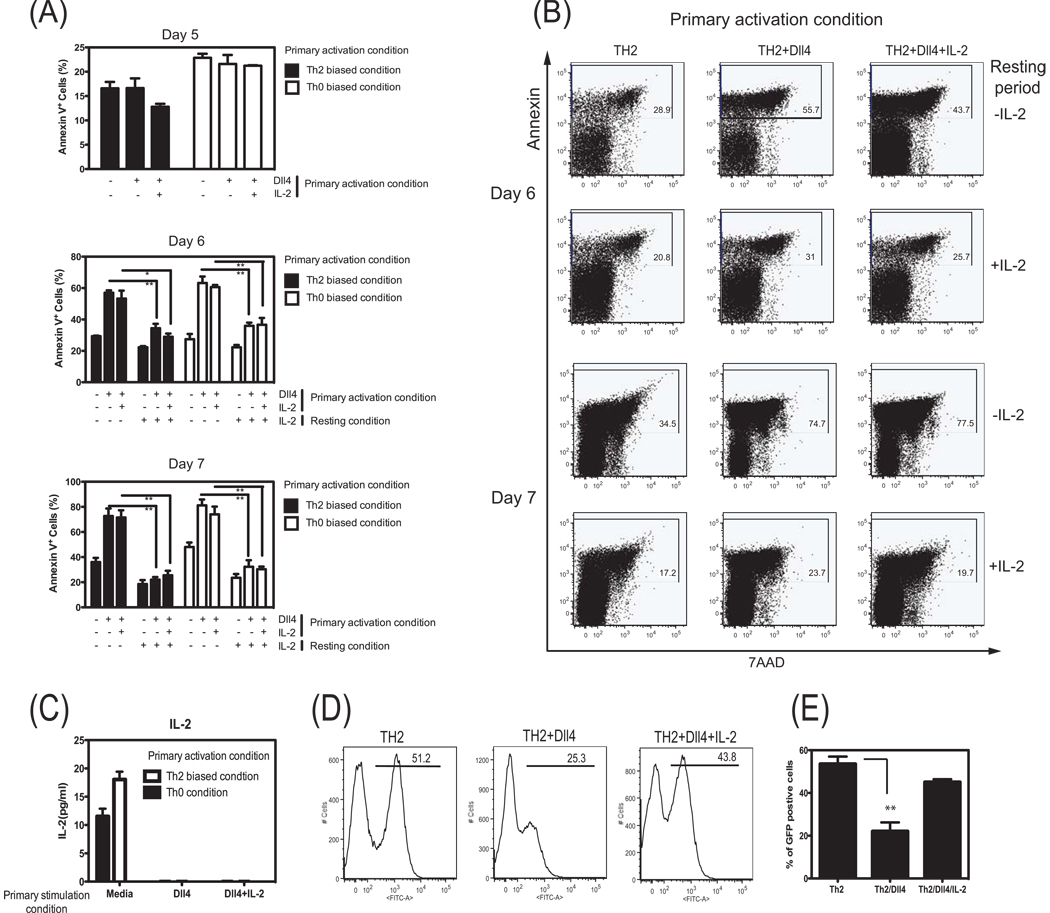
To address the latter mechanism further, we used isolated CD4+ T cells and polyclonally stimulated the cells with anti-CD3/anti-CD28 that allowed examination of a large population of cells. Isolated splenic CD4+ T cells were stimulated through CD3/CD28 (either Th0 or Th2 biased conditions) in the presence or absence of Dll4 ligand. After 5 days incubation, T cell apoptosis was examined by flow cytometry analysis using Annexin-V staining. As shown in Fig. 7A, the percentage of Annexin-V positive cells was not significantly different between Dll4 treated or untreated groups at day 5 of stimulation when a continuous stimulatory signal was being applied. To further examine the apoptosis events during the rest phase of the responses, the day 5 activated T cells were layered on lympholyte-M and centrifugated to remove dead cells and then replated for a rest phase that would represent contraction of the immune response. Flow cytometry analysis data showed that on day 6 and day 7 an increase in apoptosis occurred in T cells that had been previously treated with Dll4 ligand (Fig. 7A,B). To determine whether the Dll4-mediated decrease of IL-2 during primary stimulation leads to T cell apoptosis during the rest phase, rIL-2 was added into cells on day 1 and day 3 that were treated with Dll4. Supplementation of IL-2 during these early stages of activation did not rescue T cell apoptosis during the later resting phase. However, addition of IL-2 during the rest phase after cells were replated without stimulatory signals rescued Dll4 pretreated T cells from apoptosis. This result may suggest that increased apoptosis of Dll4 treated T cells may result from Dll4 mediated IL-2 suppression. In fact, on day 6 Dll4 pretreated T cells produced undetectable IL-2 levels in the supernatant whereas Dll4-untreated T cells continue to secrete excess IL-2 that could be detected (Fig. 7C). These data suggest that one aspect of Dll4 mediated suppression on the capacity of T cells to produce IL-2 is critical for their survival during the contraction/rest phase.
Figure 7.
Dll4-induced activation of CD4+ T cells facilitates increased apoptosis and altered development of IL-4 competent T cells via IL-2 regulation. CD4+ T cells isolated by MACs from Balb/c mice were stimulated for 5 days either Th0 or Th2 biased conditions during a primary skewing culture with or without Dll4 ligand. Cells were ficolled and washed thoroughly after 5 days in culture, then resuspended in fresh media alone or IL-2 (100 U/ml) supplemented media. (A) Summary of FACS analysis data for the percentage of annexin V+ T cells on day 5 (before ficolled), 6, and 7. This is a representative of three independent experiments. (B) Dot plot analysis of CD4+ T cells which were cultured in primary Th2 skewing conditions. Cells were stained with Annexin-V plus 7AAD during resting phase (day 6th and day 7th). Numbers indicate the percentage of total cells in the gate shown. Date shown are the representative flow plots of three independent experiments. (C) CD4+ T cells were stimulated either Th0 or Th2 biased conditions for 5 days with or without Dll4 ligand. On day 5, cells were washed thoroughly, then resuspended in fresh media. After 24 hrs, IL-2 protein in supernatants was determined by ELISA. Data represent the mean ± the SEM from 3 repeat experiments. (D) FACs figures of CD4+ T cells from 4get mice that were cultured for 5 days in Th2 skewing conditions in the presence or absence of Dll4. In a separate group IL-2 was supplemented into the cultured cells treated with Dll4. (E) Summary of FACS analysis of data of IL-4+ (GFP) cells on day 5 of treated cells, representing Mean ± SEM from 5 repeats.**=P<0.01
In a final set of experiments CD4+ T cells isolated from 4get mice were utilized to examine whether the Dll4 induction of diminished IL-2 production observed above altered the development and frequency of IL-4 producing cells. The analysis of IL-4 competent cells was monitored by GFP expression in cells from the 4get animals. The data demonstrate that in the presence of Dll4 during stimulation that there was a significant decrease in the frequency of IL-4 producing cells at day 5 of culture (Figure 7D). Interestingly, even though early rIL-2 given on day 1 and day 3 did not rescue the rest phase associated apoptosis, the frequency of IL-4+ (GFP+) T cells could be reconstituted by addition of rIL-2 during the culture (Figure 7D,E). Thus, these final studies suggest that the defect in Th2 cell development may include a consequence of Dll4 regulation of IL-2 production.
Discussion
While Notch signals have been shown to be important in early T cell progenitor development (35–37), increasing evidence suggests that Notch signaling is required for Th cell activation and differentiation. However, data conflict on the specific role of the Notch system during T cell activation that appears to depend upon the ligand used and the immune conditions being examined (19, 21, 22, 25, 26, 29, 38–40). In vivo or in vitro treatment with an inhibitor of γ-secretase (GSI), an enzyme regulating signaling through all Notch receptors, can lead to inhibition of Th1 response through the blockade of T-bet expression (20). More specific to Th2 biology, the genetic ablation of Notch signaling in T cells results in a reduction in Th2 but not Th1 responses (22–24). In an ovalbumin-induced murine model of allergic lung disease, GSI administration was shown to inhibit asthma-like phenotypes, which was accompanied with an increase of Th1 cytokines and decrease of Th2 cytokines (28), supporting the genetic ablation studies. In contrast, the present research indicated that Dll4 blockade aggravated the pathological features of allergic lung disease, including AHR and mucus production no matter at what phase of disease the blocking antibody was administered. Moreover, lymph node cytokine data showed that Dll4 blockade increased Th2 cytokine production, which is a key factor for development of allergic diseases. Consistent with cytokine data, neutralization of Dll4 signaling during both the primary and secondary response increased the frequency of IL-4+/CD4+ T cells in the lung and lymph nodes of 4get mice, as well as altering IL-4+ T cell development in primary activation using in vitro studies. Thus, these latter data appear to be contrary to previous findings that Notch1/Notch2-deficient T cells and dominant negative MAML transgenic T cells have impairment in Th2 cell differentiation (22, 24). However, due to the complexity of potential Notch receptor/ligand interactions as well as the presence of multiple receptors and ligands on cells, it would be reasonable to hypothesize that notch signaling would result in a different outcome in different settings (38). This may be a consequence of differential Notch ligand signaling and/or use of different receptors (38, 41–44). Alternatively, it may also be possible that Notch can elicit non-canonical pathways that are independent of MAML/RBP-Jк transcriptional regulation (39).
Our data suggest that Dll4 Notch signaling suppresses Th2 immune responses as previously indicated in independent studies (25, 26, 29). Another interesting finding in these studies was that Dll4 affects both naïve T cell differentiation into Th2 cells as well as already differentiated Th2 cell responses. While the exact mechanism by which Dll4 inhibits Th2 cell responses remains to be elucidated, the data from the present set of studies along with previous findings suggest that the regulation results from two possible consequences. The first consequence of Dll4 signaling relates to its ability to regulate IL-2 mRNA and protein production and alter initial expansion of the allergen specific T cells as well as their survival, but would also reduce their development into Th2 cytokine producing effector cells. These latter mechanisms of IL-2-induced Th2 cell differentiation have previously been established and it may be this early event during T cell differentiation that Dll4 is regulating (45–48). However, the observation of decreased IL-2 was not observed by Sun et al. (25) when they used Dll4 transduced DC from IL-12−/− mice to stimulate ova TCR transgenic T cells followed by an ionomycin/PMA rechallenge to examine IL-2 frequency by flow cytometry, whereas the present studies examined IL-2 mRNA and protein. It may be that Dll4 needs to be present upon restimulation in order to maintain the altered IL-2 phenotype since the former study did not provide Dll4 during the subsequent PMA restimulation. The second consequence of Dll4 activation would relate to the ability of Dll4 to alter Th2 cytokine production in previously skewed T cells as a result from a direct effect on important transcription factors such as GATA3, as previously demonstrated using ectopic expression of Dll1 and Dll4 in IL-12−/− DCs (25). The T cells that are already skewed in the challenged mice would be less dependent upon IL-2, which is needed for their development and stabilization of IL-4 gene expression (46), and more dependent upon the regulation of critical transcripton factors, such as GATA3. Interestingly, one target of Notch activation in T cell development is PU.1 (49–51), which has a regulatory role in Th2 cell differentiation via GATA3 regulation (52, 53). More recently Dll4 has been shown to enhance RORγt as well as IL-17 when cells are skewed toward a Th17 phenotype (54). While we did not observe any increase of Foxp3+ cells by flow cytometric analysis in our in vitro analyses (data not shown), it has also been demonstrated that Notch signaling and specific ligands can promote Treg cell development under TGFβ-mediated skewing conditions (55–57). Thus, the resulting immune regulation induced by Dll4 likely depends upon the immune environment that the ligand functions, the state of the activated T cell (primary vs. secondary), and the context of activation.
The expression of Dll proteins by dendritic cells is induced by several stimuli that depend upon MyD88-associated TLR signals, including bacterial, viral, and fungal pathogenic stimuli (25, 26, 54, 58, 59). Interestingly, in contrast to ovalbumin that does not activate DC via a MyD88 pathway, it has been shown that German cockroach (GC) frass contains a TLR2 agonist that regulates the intensity of the immune response (60, 61). Furthermore, TLR2 agonists can directly facilitate induction of the Dll4, but not Jagged 1, on DCs (62, 63). The data in the present studies demonstrate that cockroach antigen induced significant levels of Dll4 mRNA expression in vitro and Dll4 protein on DCs in lungs of allergic mice. The suppressive effect of allergen induced Dll4 on Th2 immunity may be part of a negative feedback regulatory mechanism that limits the exaggerated Th2 immune responses in the host and therefore modulates detrimental immune activation pathways. Interestingly, a recent study found that Treg cells expressed up to 20 fold more Dll4 than do T effector cells and may contribute to the regulation in allergic airway disease (64). Perhaps, the responses observed in the present studies may be related with similar signals induced by Dll4. Subsequent studies will further examine these signaling pathways.
Most novel in these studies, however, is the fact that Dll4 regulates IL-2 and subsequent T cell survival during the activation of T cells leading to T cell apoptosis during the rest or contraction stage. These latter observations appeared to be related to the reduced IL-2 production from Dll4 pretreated T cells, as the cells could be rescued during the contraction phase by addition of exogenous IL-2. This latter mechanism was manifested in the proliferative and apoptotic responses that were altered by the presence and/or neutralization of Dll4, and therefore, the Th2 development may be a reflection of allergen specific T cell expansion and survival. While most studies have identified that Notch-mediated pathways have led to T cell survival especially during thymic development and in transformed T cell populations (16, 65–69), there also appear to be activation of regulatory pathways (70). Examination of the IL-2 promoter in silico did not identify a consensus binding site for conical CSL (RBPJĸ), the transcriptional binding partner for the intracellular domain of Notch, and therefore the regulation is likely indirect. More specifically, studies have shown effects of Notch signaling with up-regulation of GRAIL (gene related to anergy in lymphocytes) in CD4+ T cells, with effects on other regulatory E3 ubiquitin ligases, such as Cbl-b and Itch (71). Furthermore, it was identified that deltex1, a downstream target of Notch activation, leads to development of anergic T cells related to increased expression of Cbl-b via a NFAT-mediated activation pathway (72). As Cbl-b is a known regulator of T cell activation (73–75), it may be these latter pathways that have a most significant role on IL-2 regulation that alters T cell development mediated by Dll4.
This study presents novel data that suggest that Dll4 has a relevant role for Th2 cell regulation and further highlights the complexity of Notch ligand associated activation during development of immune responses. This mechanism may be informative in terms of new therapeutic opportunities that could arise from the manipulation of Notch pathway signaling and/or specific ligand blockade in developing and established allergic diseases.
Acknowledgments
Grant support: NIH grant numbers, AI036302, AI073876. S. Jang was funded by NIH T32 training grant HL007517.
References
- 1.Smith K, Warholak T, Armstrong E, Leib M, Rehfeld R, Malone D. Evaluation of risk factors and health outcomes among persons with asthma. J Asthma. 2009;46:234–237. doi: 10.1080/02770900802627294. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–277. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platts-Mills TA, Erwin E, Heymann P, Woodfolk J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy. 2005;79 60 Suppl:25–31. doi: 10.1111/j.1398-9995.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel S. Mechanisms of severe asthma. Clin Exp Allergy. 2003;33:1622–1628. doi: 10.1111/j.1365-2222.2003.01799.x. [DOI] [PubMed] [Google Scholar]
- 5.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;15 13 Suppl:38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 7.Foster PS, Martinez-Moczygemba M, Huston DP, Corry DB. Interleukins-4, -5, and -13: emerging therapeutic targets in allergic disease. Pharmacol Ther. 2002;94:253–264. doi: 10.1016/s0163-7258(02)00220-6. [DOI] [PubMed] [Google Scholar]
- 8.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 12.Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006 doi: 10.1126/stke.3642006cm7. cm7. [DOI] [PubMed] [Google Scholar]
- 13.Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity. 2003;19:781–791. doi: 10.1016/s1074-7613(03)00325-x. [DOI] [PubMed] [Google Scholar]
- 14.Basson MA, Zamoyska R. The CD4/CD8 lineage decision: integration of signalling pathways. Immunol Today. 2000;21:509–514. doi: 10.1016/s0167-5699(00)01711-4. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson EJ, Jenkinson WE, Rossi SW, Anderson G. The thymus and T-cell commitment: the right niche for Notch? Nat Rev Immunol. 2006;6:551–555. doi: 10.1038/nri1883. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt TM, Zuniga-Pflucker JC. Thymus-derived signals regulate early T-cell development. Crit Rev Immunol. 2005;25:141–159. doi: 10.1615/critrevimmunol.v25.i2.40. [DOI] [PubMed] [Google Scholar]
- 17.Dallman MJ, Champion B, Lamb JR. Notch signalling in the peripheral immune system. Novartis Found Symp. 2003;252:268–276. discussion 276-268. [PubMed] [Google Scholar]
- 18.Hoyne GF, Dallman MJ, Champion BR, Lamb JR. Notch signalling in the regulation of peripheral immunity. Immunol Rev. 2001;182:215–227. doi: 10.1034/j.1600-065x.2001.1820118.x. [DOI] [PubMed] [Google Scholar]
- 19.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 20.Minter LM, Turley DM, Das P, Shin HM, Joshi I, Lawlor RG, Cho OH, Palaga T, Gottipati S, Telfer JC, Kostura L, Fauq AH, Simpson K, Such KA, Miele L, Golde TE, Miller SD, Osborne BA. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol. 2005;6:680–688. [PubMed] [Google Scholar]
- 21.Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 22.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch Directly Regulates Gata3 Expression during T Helper 2 Cell Differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct regulation of gata3 expression determines the T helper differentiation potential of notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol. 2008;180:1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaller MA, Neupane R, Rudd BD, Kunkel SL, Kallal LE, Lincoln P, Lowe JB, Man Y, Lukacs NW. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J Exp Med. 2007;204:2925–2934. doi: 10.1084/jem.20070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto M, Matsuda H, Joetham A, Lucas JJ, Domenico J, Yasutomo K, Takeda K, Gelfand EW. Jagged1 on dendritic cells and Notch on CD4+ T cells initiate lung allergic responsiveness by inducing IL-4 production. J Immunol. 2009;183:2995–3003. doi: 10.4049/jimmunol.0900692. [DOI] [PubMed] [Google Scholar]
- 28.Kang JH, Kim BS, Uhm TG, Lee SH, Lee GR, Park CS, Chung IY. Gamma-secretase inhibitor reduces allergic pulmonary inflammation by modulating Th1 and Th2 responses. Am J Respir Crit Care Med. 2009;179:875–882. doi: 10.1164/rccm.200806-893OC. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima A, Sumi T, Ishida W, Ojima A, Kajisako M, Koyanagi A, Koyama N, Yagita H. Notch ligand Delta-like4 inhibits the development of murine experimental allergic conjunctivitis. Immunol Lett. 2008;121:140–147. doi: 10.1016/j.imlet.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Ray P, Krishnamoorthy N, Ray A. Emerging functions of c-kit and its ligand stem cell factor in dendritic cells: regulators of T cell differentiation. Cell Cycle. 2008;7:2826–2832. doi: 10.4161/cc.7.18.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tekkanat KK, Maassab HF, Cho DS, Lai JJ, John A, Berlin A, Kaplan MH, Lukacs NW. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J Immunol. 2001;166:3542–3548. doi: 10.4049/jimmunol.166.5.3542. [DOI] [PubMed] [Google Scholar]
- 32.Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells [In Process Citation] J Immunol. 1999;163:2160–2167. [PubMed] [Google Scholar]
- 33.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- 34.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zediak VP, Maillard I, Bhandoola A. Closer to the source: notch and the nature of thymus-settling cells. Immunity. 2005;23:245–248. doi: 10.1016/j.immuni.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 37.Robey E. Regulation of T cell fate by Notch. Annu Rev Immunol. 1999;17:283–295. doi: 10.1146/annurev.immunol.17.1.283. [DOI] [PubMed] [Google Scholar]
- 38.Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS One. 2008;3:e2823. doi: 10.1371/journal.pone.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutz S, Mordmuller B, Sakano S, Scheffold A. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol. 2005;35:2443–2451. doi: 10.1002/eji.200526294. [DOI] [PubMed] [Google Scholar]
- 40.Elyaman W, Bradshaw EM, Wang Y, Oukka M, Kivisakk P, Chiba S, Yagita H, Khoury SJ. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5990–5998. doi: 10.4049/jimmunol.179.9.5990. [DOI] [PubMed] [Google Scholar]
- 41.Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol. 2000;2:515–520. doi: 10.1038/35019553. [DOI] [PubMed] [Google Scholar]
- 42.Beck RC, Padival M, Yeh D, Ralston J, Cooke KR, Lowe JB. The Notch ligands Jagged2, Delta1, and Delta4 induce differentiation and expansion of functional human NK cells from CD34(+) cord blood hematopoietic progenitor cells. Biol Blood Marrow Transplant. 2009;15:1026–1037. doi: 10.1016/j.bbmt.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang LT, Nichols JT, Yao C, Manilay JO, Robey EA, Weinmaster G. Fringe glycosyltransferases differentially modulate Notch1 proteolysis induced by Delta1 and Jagged1. Mol Biol Cell. 2005;16:927–942. doi: 10.1091/mbc.E04-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimenez E, Vicente A, Sacedon R, Munoz JJ, Weinmaster G, Zapata AG, Varas A. Distinct mechanisms contribute to generate and change the CD4:CD8 cell ratio during thymus development: a role for the Notch ligand, Jagged1. J Immunol. 2001;166:5898–5908. doi: 10.4049/jimmunol.166.10.5898. [DOI] [PubMed] [Google Scholar]
- 45.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 48.Sun J, Pearce EJ. Suppression of early IL-4 production underlies the failure of CD4 T cells activated by TLR-stimulated dendritic cells to differentiate into Th2 cells. J Immunol. 2007;178:1635–1644. doi: 10.4049/jimmunol.178.3.1635. [DOI] [PubMed] [Google Scholar]
- 49.Georgescu C, Longabaugh WJ, Scripture-Adams DD, David-Fung ES, Yui MA, Zarnegar MA, Bolouri H, Rothenberg EV. A gene regulatory network armature for T lymphocyte specification. Proc Natl Acad Sci U S A. 2008;105:20100–20105. doi: 10.1073/pnas.0806501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothenberg EV, Scripture-Adams DD. Competition and collaboration: GATA-3, PU.1, and Notch signaling in early T-cell fate determination. Semin Immunol. 2008;20:236–246. doi: 10.1016/j.smim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco CB, Scripture-Adams DD, Proekt I, Taghon T, Weiss AH, Yui MA, Adams SL, Diamond RA, Rothenberg EV. Notch/Delta signaling constrains reengineering of pro-T cells by PU.1. Proc Natl Acad Sci U S A. 2006;103:11993–11998. doi: 10.1073/pnas.0601188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang HC, Han L, Jabeen R, Carotta S, Nutt SL, Kaplan MH. PU.1 regulates TCR expression by modulating GATA-3 activity. J Immunol. 2009;183:4887–4894. doi: 10.4049/jimmunol.0900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutton JF, Gargett T, Sadlon TJ, Bresatz S, Brown CY, Zola H, Shannon MF, D’Andrea RJ, Barry SC. Development of CD4+CD25+FoxP3+ regulatory T cells from cord blood hematopoietic progenitor cells. J Leukoc Biol. 2009;85:445–451. doi: 10.1189/jlb.1008620. [DOI] [PubMed] [Google Scholar]
- 56.Ostroukhova M, Qi Z, Oriss TB, Dixon-McCarthy B, Ray P, Ray A. Tregmediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kared H, Adle-Biassette H, Fois E, Masson A, Bach JF, Chatenoud L, Schneider E, Zavala F. Jagged2-expressing hematopoietic progenitors promote regulatory T cell expansion in the periphery through notch signaling. Immunity. 2006;25:823–834. doi: 10.1016/j.immuni.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Ito T, Schaller M, Hogaboam CM, Standiford TJ, Sandor M, Lukacs NW, Chensue SW, Kunkel SL. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. J Clin Invest. 2009;119:33–46. doi: 10.1172/JCI35647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204:1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Page K, Ledford JR, Zhou P, Wills-Karp M. A TLR2 agonist in German cockroach frass activates MMP-9 release and is protective against allergic inflammation in mice. J Immunol. 2009;183:3400–3408. doi: 10.4049/jimmunol.0900838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Page K, Lierl KM, Hughes VS, Zhou P, Ledford JR, Wills-Karp M. TLR2-mediated activation of neutrophils in response to German cockroach frass. J Immunol. 2008;180:6317–6324. doi: 10.4049/jimmunol.180.9.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanhoutte F, Paget C, Breuilh L, Fontaine J, Vendeville C, Goriely S, Ryffel B, Faveeuw C, Trottein F. Toll-like receptor (TLR)2 and TLR3 synergy and cross inhibition in murine myeloid dendritic cells. Immunol Lett. 2008;116:86–94. doi: 10.1016/j.imlet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Palaga T, Buranaruk C, Rengpipat S, Fauq AH, Golde TE, Kaufmann SH, Osborne BA. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol. 2008;38:174–183. doi: 10.1002/eji.200636999. [DOI] [PubMed] [Google Scholar]
- 64.Huang MT, Dai YS, Chou YB, Juan YH, Wang CC, Chiang BL. Regulatory T cells negatively regulate neovasculature of airway remodeling via DLL4-Notch signaling. J Immunol. 2009;183:4745–4754. doi: 10.4049/jimmunol.0804371. [DOI] [PubMed] [Google Scholar]
- 65.Jundt F, Schwarzer R, Dorken B. Notch signaling in leukemias and lymphomas. Curr Mol Med. 2008;8:51–59. doi: 10.2174/156652408783565540. [DOI] [PubMed] [Google Scholar]
- 66.Ciofani M, Zuniga-Pflucker JC. A survival guide to early T cell development. Immunol Res. 2006;34:117–132. doi: 10.1385/IR:34:2:117. [DOI] [PubMed] [Google Scholar]
- 67.Hoyne GF. Notch signaling in the immune system. J Leukoc Biol. 2003;74:971–981. doi: 10.1189/jlb.0303089. [DOI] [PubMed] [Google Scholar]
- 68.Pear WS, Radtke F. Notch signaling in lymphopoiesis. Semin Immunol. 2003;15:69–79. doi: 10.1016/s1044-5323(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 69.von Boehmer H. T-cell development: What does Notch do for T cells? Curr Biol. 1999;9:R186–R188. doi: 10.1016/s0960-9822(99)80110-9. [DOI] [PubMed] [Google Scholar]
- 70.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kostianovsky AM, Maier LM, Baecher-Allan C, Anderson AC, Anderson DE. Up-regulation of gene related to anergy in lymphocytes is associated with Notch-mediated human T cell suppression. J Immunol. 2007;178:6158–6163. doi: 10.4049/jimmunol.178.10.6158. [DOI] [PubMed] [Google Scholar]
- 72.Hsiao HW, Liu WH, Wang CJ, Lo YH, Wu YH, Jiang ST, Lai MZ. Deltex1 is a target of the transcription factor NFAT that promotes T cell anergy. Immunity. 2009;31:72–83. doi: 10.1016/j.immuni.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 73.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 74.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]