Abstract
Zinc-induced aggregation of amyloid-β peptide (Aβ) is a hallmark molecular feature of Alzheimer's disease. Here we provide direct thermodynamic evidence that elucidates the role of the Aβ region 6–14 as the minimal Zn2+ binding site wherein the ion is coordinated by His6, Glu11, His13, and His14. With the help of isothermal titration calorimetry and quantum mechanics/molecular mechanics simulations, the region 11–14 was determined as the primary zinc recognition site and considered an important drug-target candidate to prevent Zn2+-induced aggregation of Aβ.
Alzheimer's disease, a fatal neurodegenerative disorder of the elderly, is characterized by extracellular depositions of amyloid-β peptide (Aβ) saturated with metal ions (1). It is believed that Zn2+ ions play a key role in pathological aggregation of Aβ and therefore affect the pathogenesis of Alzheimer's disease (2–5). The amino acid region 1–16 (Aβ(1–16)) is generally considered as the metal binding domain of Aβ (6). N-acetylated and C-amidated peptide Aβ(1–16) is soluble and stable in the presence of zinc ions under physiological conditions (7), and the three-dimensional structures of this domain in Zn2+-loaded and Zn2+-free states have been solved by NMR (8). In the Zn2+-Aβ(1–16) complex, zinc is tetrahedrally coordinated to His6, His13, and His14 through their N-δ1, N-ɛ2, and N-δ1 atoms, respectively, and to Glu11 through its O-δ atom.
At the same time, several alternative coordination modes of zinc binding to Aβ with nonacetylated N-terminus are discussed (6,9). While there is general agreement regarding the participation of the three histidine residues, the role of Asp1 as fourth zinc chelator instead of Glu11 has been proposed (10). In addition, a pentacoordination of zinc ion by Asp1, His6, Glu11, His13, and His14 has been also suggested (11). However, in contrast to the data published by Zirah et al. (8), none of the alternative complexes has been defined structurally. Thus, the exact mode of zinc coordination by Aβ deserves further investigation.
In this work, we characterize the thermodynamics of the binding of Zn2+ to Aβ fragments and Aβ(1–16) mutants by isothermal titration calorimetry (ITC) in order to determine the minimal Zn2+-binding site of Aβ under physiological conditions. We also use the quantum mechanics/molecular mechanics (QM/MM) method to model Zn2+ recognition by Aβ.
Thermodynamic parameters of Zn2+ binding to Aβ fragments and Aβ(1–16) mutants obtained by ITC (Fig. 1 and Fig. S1 in the Supporting Material) are presented in Table 1. It can be seen that acetylation of N-terminus does not dramatically change Zn2+ binding to Aβ(1–16).
Figure 1.
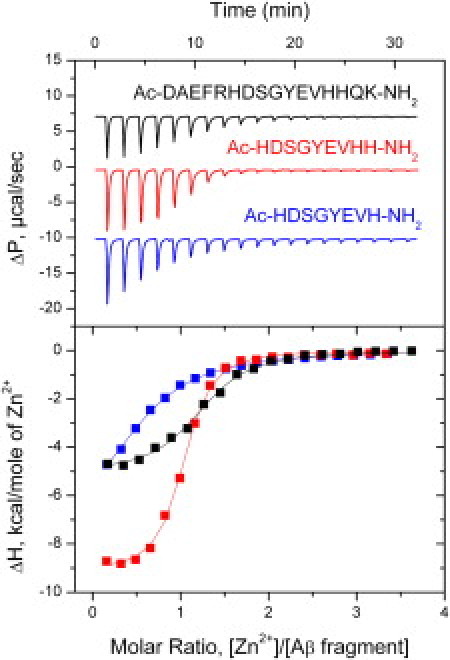
ITC titration curves (upper panel) and binding isotherms (lower panel) for zinc interactions with Aβ(1–16), Aβ(6–14), and Aβ(6–13) (black, red, and blue curves, respectively) at 25°C in 50 mM Tris buffer, pH 7.3.
Table 1.
Thermodynamic parameters of Zn2+ ions binding to Aβ fragments and Aβ(1–16) mutants obtained by ITC at 25°C in 50 mM Tris buffer, pH 7.3
| Peptide∗ | N† |
Ka§ |
ΔH† |
TΔS |
|---|---|---|---|---|
| (M−1 × 104) | (kcal M−1) | (kcal M−1) | ||
| Aβ(1–16) | 1.1 | 1.80 | −4.0 | 1.8 |
| NH2-Aβ(1–16) | 1.1 | 3.90 | −5.4 | 0.9 |
| Aβ(6–14) | 1.0 | 8.60 | −9.2 | −2.5 |
| Aβ(6–13) | 0.6 | 0.34¶ | −8.1 | −3.3 |
| Aβ(7–14) | 0.9 | 0.17 | −7.9 | −3.5 |
| Aβ(11–14) | 0.5 | 0.27¶ | −2.6 | 2.1 |
| Aβ(1–5) | No binding | |||
| Aβ(1–16) H6R | 0.8 | 0.25 | −6.4 | −1.8 |
| Aβ(1–16) E11A | 1.0 | 0.54 | −6.9 | −1.8 |
All experiments were carried out on an iTC200 instrument (MicroCal, Northampton, MA).
All peptides were purchased from Biopeptide (San Diego, CA). The N- and C-termini of each peptide were protected with acetyl and amide, respectively; peptide NH2-Aβ(1–16) was protected at the C-terminus with amide.
Standard deviation did not exceed ±10%.
Standard deviation did not exceed ±20%.
Due to peptide dimerization, Ka dimension is equal to M−2.
The truncation of five amino acids from the N-terminal part and two from the C-terminus of Aβ(1–16) leads to the fivefold increase in its affinity to Zn2+ (Fig. 1, Table 1). This could be explained by the formation of a hydrogen bond between His6 and either Asp1 or Glu3 in Aβ(1–16) in the absence of zinc (Fig. S2). At the same time, titration of the Aβ(1–5) fragment with Zn2+ ions did not produce any heat. It is important to note that angiotensin-converting enzyme proteolytically cleaves this fragment from Aβ (12). Thus, angiotensin-converting enzyme can increase zinc affinity to Aβ and act as a modulator of zinc binding. Visible aggregation of Aβ(6–14) peptide was observed in the calorimetrical cell upon interaction with zinc. However, this aggregation does not affect stoichiometry of Zn2+ binding (Table 1), suggesting that formation of oligomers occurs not via Zn2+ ions, but through interpeptide interactions of Zn2+-loaded Aβ(6–14) in the same manner as for Aβ which first binds to zinc and then is conformationally driven to aggregates (13). Further removing of one of the terminal histidines from Aβ(6–14) results in the remarkable drop in association constant Ka from 8.6 × 104 M−1 to 1.7 × 103 M−1 for peptide Aβ(7–14) and to 3.4 × 103 M−1 for peptide Aβ(6–13).
These data indicate that all Zn2+-chelating amino acids are located within the Aβ(6–14) peptide and that the Aβ (1–5) segment of Aβ is not necessary for Zn2+ binding. Amino acids located between Asp7 and Tyr10 of Aβ do not participate in Zn2+ binding, because their removal (Aβ(7–14) → Aβ(11–14)) only slightly changes the affinity of the peptide to zinc. Substitution of Glu11 to Ala as well as His6 to Arg in Aβ(1–16) causes strong decrease of Zn2+ affinity (Table 1). These data clearly demonstrate that His6, Glu11, and His14 are necessary for Zn2+ coordination, which is in good agreement with NMR structural data (8).
Zinc binding to all studied Aβ fragments and Aβ(1–16) mutants was enthalpy-driven (Table 1). Contrary to the Aβ(1–16) domain, Zn2+ binding to Aβ(6–14), Aβ(7–14), and Aβ(6–13) peptides was entropically unfavorable. This is explained by burying hydrophobic surfaces upon Zn2+ binding to Aβ(1–16), which is in line with the structural data (8). Stoichiometry of Zn2+ binding to Aβ(6–13) and Aβ(11–14) fragments is close to 0.5 (Table 1), indicating dimer formation via Zn2+ ion which is in a good agreement with a recent simulation study (14). High positive entropy of Zn2+ binding to Aβ(11–14) dimer is explained by formation of hydrophobic contacts between the two subunits.
This is also confirmed by an NMR study of the Zn2+-Aβ(11–14) dimer. 1H NMR spectra (Fig. S3) demonstrate that resonance peaks of both valine methyl groups are strongly upshifted upon Zn2+ binding to Aβ(11–14) (from 0.83 and 0.75 to 0.45 and 0.69 ppm), due to the proximity of the zinc ion. The peak that displays the stronger shift undergoes significant broadening, also indicating that the corresponding methyl group is very close to Zn2+. The coordination of Zn2+ by two peptide molecules resulting in the formation of a symmetrical complex is in good agreement with NMR data as well. In such a complex, valine methyl groups within the immediate neighborhood of Zn2+ ion are oriented inward, which means that they are not exposed considerably to the solution.
Thus, we propose that the tetrapeptide Aβ(11–14) containing three Zn2+ chelating amino acids functions as a Zn2+ recognition site. In line with this hypothesis, NMR data demonstrate that this site has a definite structure in the absence of Zn2+ (8). We suppose that Zn2+ capture by the structured EVHH region is the initial step in the formation of the Zn2+-Aβ(1–16) complex.
The dimerization of the Aβ(11–14) peptide upon Zn2+ binding makes it impossible to determine the thermodynamic parameters of binding for the monomeric peptide, i.e., to reveal the role of this fragment in the mechanism of Zn2+ recognition by Aβ in more detail. Therefore, we applied an approach similar to that described by Furlan and La Penna (15). Theoretical calculations of the stability of a transitional EVHH complex with zinc were performed. QM/MM simulations were applied to analyze Zn2+ binding by Aβ(11–14), Aβ(6–14), and Aβ(1–16) peptides. Starting conformation for each peptide complexed with Zn2+ was derived from the Aβ(1–16) structure (PDB ID: 1ZE9).
Simulations showed that both Aβ(6–14) and Aβ(1–16) peptides where Zn2+ was chelated by His6, Glu11, His13, and His14 kept stable tetrahedral coordination of zinc during 8 ps of the QM/MM simulation trajectory. In the case of Aβ(6–14) energy, the QM part (see the Supporting Material for details) was slightly smaller because His13 is better oriented in the complex (Table 2). For Aβ(11–14), fast (1-ps) formation of the tetrahedral Zn2+ coordination environment (Table 2) with a water molecule as the fourth chelator was observed (see Movie S1 in the Supporting Material). In the longer (8-ps) simulation, this water molecule was stable in terms of orientation and position. Summarizing our simulation results, we conclude that initially Zn2+ is recognized and captured by the EVHH region of Aβ and temporarily coordinated by water as the fourth chelator (Fig. 2 A). This state exists until His6 comes close to Zn2+ due to thermal fluctuations and replaces the water molecule in the zinc coordination environment, resulting in the formation of the final complex (Fig. 2 B).
Table 2.
Energy and geometry values of zinc coordination by Aβ(1–16), Aβ(6–14), and Aβ(11–14) peptides obtained by QM/MM simulations
| Aβ(1–16) | Aβ(6–14) | Aβ(11–14) | |
|---|---|---|---|
| Energy of QM subsystem, kJ/mol | |||
| −618571 ± 27 | −618621 ± 64 | NA | |
| Distance, nm | |||
| Zn2+ → His6 (Nδ) | 0.202 ± 0.006 | 0.203 ± 0.005 | 0.206 ± 0.010 |
| Zn2+ → H2O (O) | NA | NA | 0.206 ± 0.010 |
| Zn2+ → Glu11 (Oδ) | 0.220 ± 0.014 | 0.226 ± 0.015 | 0.200 ± 0.007 |
| Zn2+ → His13 (Nɛ) | 0.207 ± 0.006 | 0.207 ± 0.006 | 0.202 ± 0.007 |
| Zn2+ → His14 (Nδ) | 0.209 ± 0.006 | 0.209 ± 0.006 | 0.199 ± 0.006 |
| Angle, degrees | |||
| His6-Zn2+-Glu11 | 96.5 ± 4.6 | 95.6 ± 5.2 | NA |
| His6-Zn2+-His13 | 131.0 ± 3.7 | 134.2 ± 4.2 | NA |
| His6-Zn2+-His14 | 110.8 ± 4.2 | 110.4 ± 4.6 | NA |
| Glu11-Zn2+-His13 | 85.2 ± 4.5 | 83.0 ± 3.2 | 98.1 ± 6.1 |
| Glu11-Zn2+-His14 | 96.3 ± 4.6 | 96.9 ± 5.2 | 112.6 ± 7.0 |
| His13-Zn2+-His14 | 117.0 ± 5.2 | 114.0 ± 4.2 | 116.5 ± 5.7 |
| H2O-Zn2+-Glu11 | NA | NA | 97.5 ± 6.9 |
| H2O-Zn2+-His13 | NA | NA | 111.9 ± 8.0 |
| H2O-Zn2+-His14 | NA | NA | 115.5 ± 7.9 |
NA: not applicable.
Figure 2.
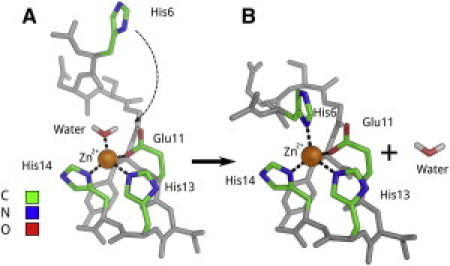
Schema of Zn2+ ion recognition by the Aβ binding site 6–14 according to QM/MM calculations performed with the GROMACS/CPMD package (http://www.tougaloo.edu/research/qmmm/index.htm) based on PDB ID: 1ZE9. (A) structure of the intermediate Zn2+-Aβ complex with a water molecule as the fourth chelator; (B) structure of the final Zn2+-Aβ complex. See also Movie S2 in the Supporting Material.
In summary, we used ITC to characterize the interactions of Zn2+with fragments of native Aβ and Aβ(1–16) mutants. The amino acid region 6–14 of Aβ was determined as the minimal Zn2+-binding site wherein the ion is coordinated by His6, Glu11, His13, and His14. Both ITC and QM/MM showed that three of four residues from the Aβ region 11–14 (EVHH) contribute to zinc binding, and that this tetrapeptide readily forms dimers linked through a zinc ion, similarly to a model proposed for Aβ aggregation in a recent molecular dynamics study (14).
These data allow us to consider Aβ(11–14) tetrapeptide as a primary Zn2+-recognition site of Aβ and an important drug target candidate to prevent Zn2+-induced aggregation of Aβ.
Acknowledgments
Computer resources were provided by the Research Computing Center of Moscow State University. Supercomputer “Chebyshev” was used for all modeling studies.
This work was supported by the Molecular and Cellular Biology Program of the Russian Academy of Sciences, by the Russian Foundation for Basic Research (grant No. 08-04-01465), by the International Centre for Genetic Engineering and Biotechnology (grant No. CRP/RUS08-02), by the Russian Federal Program (contract No. 02.740.11.0776), by the Dmitry Zimin Dynasty Foundation, and by the “Proteomics in Medicine and Biotechnology” Program of the Russian Academy of Medical Sciences.
Contributor Information
Sergey A. Kozin, Email: kozinsa@gmail.com.
Alexander A. Makarov, Email: aamakarov@eimb.ru.
Supporting Material
References and Footnotes
- 1.Goedert M., Spillantini M.G. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 2.Bush A.I. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 3.Cuajungco M.P., Faget K.Y. Zinc takes the center stage: its paradoxical role in Alzheimer's disease. Brain Res. Brain Res. Rev. 2003;41:44–56. doi: 10.1016/s0165-0173(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 4.Maynard C.J., Bush A.I., Li Q.X. Metals and amyloid-β in Alzheimer's disease. Int. J. Exp. Pathol. 2005;86:147–159. doi: 10.1111/j.0959-9673.2005.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsvetkov P.O., Popov I.A., Kozin S.A. Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer's disease amyloid β (1–16) peptide. ChemBioChem. 2008;9:1564–1567. doi: 10.1002/cbic.200700784. [DOI] [PubMed] [Google Scholar]
- 6.Faller P., Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans. 2009:1080–1094. doi: 10.1039/b813398k. [DOI] [PubMed] [Google Scholar]
- 7.Kozin S.A., Zirah S., Debey P. Zinc binding to Alzheimer's Aβ (1–16) peptide results in stable soluble complex. Biochem. Biophys. Res. Commun. 2001;285:959–964. doi: 10.1006/bbrc.2001.5284. [DOI] [PubMed] [Google Scholar]
- 8.Zirah S., Kozin S.A., Rebuffat S. Structural changes of region 1–16 of the Alzheimer disease amyloid β -peptide upon zinc binding and in vitro aging. J. Biol. Chem. 2006;281:2151–2161. doi: 10.1074/jbc.M504454200. [DOI] [PubMed] [Google Scholar]
- 9.Damante C.A., Osz K., Sovago I. Metal loading capacity of Aβ N-terminus: a combined potentiometric and spectroscopic study of zinc(II) complexes with Aβ (1–16), its short or mutated peptide fragments and its polyethylene glycolated analogue. Inorg. Chem. 2009;48:10405–10415. doi: 10.1021/ic9012334. [DOI] [PubMed] [Google Scholar]
- 10.Danielsson J., Pierattelli R., Graslund A. High-resolution NMR studies of the zinc-binding site of the Alzheimer's amyloid β -peptide. FEBS J. 2007;274:46–59. doi: 10.1111/j.1742-4658.2006.05563.x. [DOI] [PubMed] [Google Scholar]
- 11.Gaggelli E., Janicka-Klos A., Wieczerzak E. NMR studies of the Zn2+ interactions with rat and human β-amyloid (1–28) peptides in water-micelle environment. J. Phys. Chem. B. 2008;112:100–109. doi: 10.1021/jp075168m. [DOI] [PubMed] [Google Scholar]
- 12.Toropygin I.Y., Kugaevskaya E.V., Kozin S.A. The N-domain of angiotensin-converting enzyme specifically hydrolyzes the Arg5-His6 bond of Alzheimer's Aβ -(1–16) peptide and its isoAsp7 analogue with different efficiency as evidenced by quantitative matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:231–239. doi: 10.1002/rcm.3357. [DOI] [PubMed] [Google Scholar]
- 13.Talmard C., Guilloreau L., Faller P. Amyloid-β peptide forms monomeric complexes with Cu(II) and Zn(II) prior to aggregation. ChemBioChem. 2007;8:163–165. doi: 10.1002/cbic.200600319. [DOI] [PubMed] [Google Scholar]
- 14.Miller Y., Ma B., Nussinov R. Zinc ions promote Alzheimer Aβ aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. USA. 2010;107:9490–9495. doi: 10.1073/pnas.0913114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlan S., La Penna G. Modeling of the Zn2+ binding in the 1–16 region of the amyloid β peptide involved in Alzheimer's disease. Phys. Chem. Chem. Phys. 2009;11:6468–6481. doi: 10.1039/b822771c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


