Abstract
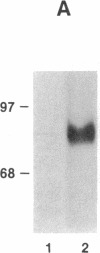
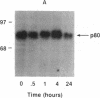
An 80-kDa protein (p80), previously reported to be a major protein kinase C substrate in preneoplastic JB6 mouse epidermal cells, has been shown to be transiently phosphorylated by phorbol 12-O-tetradecanoate 13-acetate. Phosphorylation was maximal at 2 hr of phorbol 12-O-tetradecanoate 13-acetate treatment and returned to basal levels by 24 hr. In contrast, using a p80-specific antibody, we found that phorbol 12-O-tetradecanoate 13-acetate treatment produced no increase in p80 concentration. p80 showed a progressive decrease in JB6 cells during progression from a preneoplastic to neoplastic phenotype. The lack of p80 expression in neoplastic cells was not attributable to lack of protein kinase C; the protein kinase activity and protein concentration were similar in cells of all three phenotypes. When p80 mRNA was analyzed by hybridization to a putative p80 cDNA clone, its relative concentration paralleled that of p80 protein, with high levels present in preneoplastic JB6 cells, and little or no evidence for p80-hybridizing RNA in transformed cells. Thus, p80 appears to be regulated pretranslationally at the level of mRNA concentration during preneoplastic progression in mouse epidermal JB6 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Bishop R., Martinez R., Weber M. J., Blackshear P. J., Beatty S., Lim R., Herschman H. R. Protein phosphorylation in a tetradecanoyl phorbol acetate-nonproliferative variant of 3T3 cells. Mol Cell Biol. 1985 Sep;5(9):2231–2237. doi: 10.1128/mcb.5.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn N. H., Former B. F., Nelson K. A., Yuspa S. H. Tumour promoter induces anchorage independence irreversibly. Nature. 1979 Oct 18;281(5732):589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Koehler B. A., Nelson K. J. A cell culture assay for tumor-promoter-dependent progression toward neoplastic phenotype: detection of tumor promoters and promotion inhibitors. Teratog Carcinog Mutagen. 1980;1(1):87–96. doi: 10.1002/tcm.1770010109. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Lerman M. I., Hegamyer G. A., Gindhart T. D. A transforming activity not detected by DNA transfer to NIH 3T3 cells is detected by JB6 mouse epidermal cells. Mol Cell Biol. 1985 Apr;5(4):890–893. doi: 10.1128/mcb.5.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn N. H., Smith B. M., Wendel E. J., Dowjat W. K., Shimada T. Transfer by pro gene transfection of tumor promoter-sensitive phenotype to promotion-insensitive JB6 cells. Cancer Res. 1988 Mar 1;48(5):1195–1200. [PubMed] [Google Scholar]
- Colburn N. H., Wendel E. J., Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn N. H., Wendel E., Srinivas L. Responses of preneoplastic epidermal cells to tumor promoters and growth factors: use of promoter-resistant variants for mechanism studies. J Cell Biochem. 1982;18(3):261–270. doi: 10.1002/jcb.1982.240180302. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Homologous and heterologous mitogenic desensitization of Swiss 3T3 cells to phorbol esters and vasopressin: role of receptor and postreceptor steps. J Cell Physiol. 1984 Feb;118(2):133–142. doi: 10.1002/jcp.1041180205. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Gindhart T. D., Stevens L., Copley M. P. Transformation and tumor promoter sensitive phosphoproteins in JB-6 mouse epidermal cells: one is also sensitive to heat stress. Carcinogenesis. 1984 Sep;5(9):1115–1121. doi: 10.1093/carcin/5.9.1115. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Teleocidin is not mitogenic for 12-O-tetradecanoylphorbol-13-acetate non-proliferative variants of 3T3 cells. Carcinogenesis. 1983;4(4):489–490. doi: 10.1093/carcin/4.4.489. [DOI] [PubMed] [Google Scholar]
- Kamata T., Sullivan N. F., Wooten M. W. Reduced protein kinase C activity in a ras-resistant cell line derived from Ki-MSV transformed cells. Oncogene. 1987 Mar;1(1):37–46. [PubMed] [Google Scholar]
- Kramer C. M., Sando J. J. Substrates for protein kinase C in cytosol of EL4 mouse thymoma cells. Cancer Res. 1986 Jun;46(6):3040–3045. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerman M. I., Hegamyer G. A., Colburn N. H. Cloning and characterization of putative genes that specify sensitivity to neoplastic transformation by tumor promoters. Int J Cancer. 1986 Feb 15;37(2):293–302. doi: 10.1002/ijc.2910370219. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Automated synthesis of peptides. Science. 1965 Oct 8;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- Patel J., Kligman D. Purification and characterization of an Mr 87,000 protein kinase C substrate from rat brain. J Biol Chem. 1987 Dec 5;262(34):16686–16691. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. M., Colburn N. H. Protein kinase C and its substrates in tumor promoter-sensitive and -resistant cells. J Biol Chem. 1988 May 5;263(13):6424–6431. [PubMed] [Google Scholar]
- Smith B. M., Gindhart T. D., Colburn N. H. Possible involvement of a lanthanide-sensitive protein kinase C substrate in lanthanide promotion of neoplastic transformation. Carcinogenesis. 1986 Dec;7(12):1949–1956. doi: 10.1093/carcin/7.12.1949. [DOI] [PubMed] [Google Scholar]
- Stabel S., Rodriguez-Pena A., Young S., Rozengurt E., Parker P. J. Quantitation of protein kinase C by immunoblot--expression in different cell lines and response to phorbol esters. J Cell Physiol. 1987 Jan;130(1):111–117. doi: 10.1002/jcp.1041300116. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. C., Pardee A. B. Cell cycle and growth factor-dependent phosphoprotein of 78kD differently regulated in normal and transformed mouse fibroblasts. J Cell Physiol. 1987 Nov;133(2):377–382. doi: 10.1002/jcp.1041330224. [DOI] [PubMed] [Google Scholar]