Abstract
The hydrophobic proteins SP-B and SP-C are essential for pulmonary surfactant function, even though they are a relatively minor component (<2% of surfactant dry mass). Despite countless studies, their specific differential action and their possible concerted role to optimize the surface properties of surfactant films have not been completely elucidated. Under conditions kept as physiologically relevant as possible, we tested the surface activity and mechanical stability of several surfactant films of varying protein composition in vitro using a captive bubble surfactometer and a novel (to our knowledge) stability test. We found that in the naturally derived surfactant lipid mixtures, surfactant protein SP-B promoted film formation and reextension to lower surface tensions than SP-C, and in particular played a vital role in sustaining film stability at the most compressed states, whereas SP-C produced no stabilization. Preparations containing both proteins together revealed a slight combined effect in enhancing film formation. These results provide a qualitative and quantitative framework for the development of future synthetic therapeutic surfactants, and illustrate the crucial need to include SP-B or an efficient SP-B analog for optimal function.
Introduction
From the time an infant takes his first breath, pulmonary surfactant forms a continuous film at the liquid-air interface of the alveoli, which is fundamental to breathing. This film, through its surface-tension lowering properties, greatly decreases the work of breathing and imparts a remarkable stability to the alveolar cavities, thus preventing their collapse under the physiological duress of the breathing cycle (1).
The composition of lung surfactant is very complex and includes on the order of 50 different lipids (2) and four surfactant specific proteins (SP-A, SP-B, SP-C, and SP-D) (3). Representative values as obtained from bronchoalveolar lavage give a composition of 85–90% phospholipids, 5–10% neutral lipids, and 6–8% specific surfactant-associated proteins by weight (4,5). Despite this complexity, investigators have elucidated the most critical components for a functional surfactant, which include along with the dominant phospholipid dipalmitoyl phosphatidylcholine (DPPC) the hydrophobic proteins SP-B and SP-C (6). Both proteins enhance surface activity, in particular initial film formation and reextension, which are key for proper dynamic behavior during the breathing cycle (7). However, the proteins are very different structurally and are expected to play distinct functional roles (8). In vitro studies with model surfactant mixtures revealed a certain overlap in protein function, but indicated that SP-C was more effective in promoting the reinsertion of surface-associated lipids (film reextension), whereas SP-B was superior in compression-promoted refining of cycled films (9,10). With respect to the proteins' role in stabilizing surfactant films, the results are somewhat contradictory and appear to depend very much on the conditions employed. Furthermore, the term “stabilization” has been used to describe different effects. In one in vitro study (11), researchers concluded that model surfactant films (DPPC + palmitoyloleoyl phosphatidylglycerol (POPG)) containing SP-C were more mechanically stable than those containing SP-B. However, the experiments in that study were performed with films that were overcompressed to 5% of their original surface area, a situation whose physiological significance is unclear. In another study (12), SP-C knockout mice were raised to maturity with apparently normal surfactant function and only marginal instability, indicating that SP-C is not necessary for alveolar stability. Recently, a study with preterm rabbits indicated that both proteins together are necessary for optimal alveolar stability at end expiration (8).
Numerous studies have examined the surface activities of SP-B and SP-C in different more-or-less simplified surfactant models using a variety of biophysical methods. In this work we sought to clarify these proteins' roles in surfactant film activity and stability by systematically studying their individual and combined actions in vitro with the use of a captive bubble surfactometer (CBS). In addition, we assessed film stability using a novel (to our knowledge) device that evaluates the ability of compressed films to sustain very low tensions during moderately long periods of time, once subjected to mechanical perturbations. In this study we placed a particular emphasis on physiological relevance and tried to mimic the formation of surface films by pulmonary surfactant as it proceeds in vivo. To that end, we formed the surfactant films by directly applying thin layers of aqueous surfactant suspensions of high concentration close to the air-liquid interface. In addition, we paid careful attention to the surfactant material itself. Protein effects were not assayed in simplified model lipid mixtures, but within the more realistic matrix of a full lipid complement carefully derived from whole purified native surfactant.
Materials and Methods
Materials
All surfactant preparations were derived from pig lungs freshly collected at the slaughterhouse. Chloroform and methanol solvents (high-performance liquid chromatography grade) were obtained from Scharlau (Barcelona, Spain). Buffer salts, Tris, and NaCl were obtained from Sigma and Merck, respectively. All of the water used to prepare surfactant suspensions and to test surface function was doubly distilled, with the second distillation performed under permanganate.
Porcine surfactant and its derivatives
Whole natural porcine surfactant was obtained by broncheolar lavage and purified as described previously (13). Stock suspensions were obtained in saline buffer solution (5 mM Tris, 150 mM NaCl, pH 7). Organic extracts were prepared from the purified surfactant using chloroform and methanol. In all cases, the total concentration of phospholipid in the surfactant samples was estimated by phosphorus quantization. The organic extracts were dried under nitrogen and suspended in the appropriate amounts of 5 mM Tris buffer, pH 7, containing 150 mM NaCl. Surfactant proteins SP-B and SP-C were isolated from organic extract by two size-exclusion chromatography steps as described previously (14). Protein was checked for purity by sodium dodecyl sulfate polyacrylamide gel electrophoresis and quantified by amino acid analysis. The isolated proteins were stored in chloroform/methanol 2:1 (v/v) solutions at −20°C. Organic extracts of varying protein composition were prepared by uniting exact amounts from the separated and quantified fractions from the two chromatographic columns. The desired components in solution were mixed, dried under nitrogen, and suspended in saline buffer to obtain aqueous suspensions of surfactant with a phospholipid concentration of 25 mg/mL and protein concentrations ranging from 0.4% to 1.6% w/w with respect to phospholipid. Aqueous suspensions of the united fractions of the first column, i.e., phospholipids + cholesterol + proteins (SP-B + SP-C) with and without proteins, were utilized as positive and negative controls, respectively.
Surface activity evaluation of spread films with CBS
The surface activity of spread films was evaluated with the use of a computer-controlled CBS modified from the original apparatus described by Schürch et al. (15). The CBS chamber was filled with ∼1.5 mL buffer solution (5 mM Tris, 150 mM NaCl, pH 7) containing 10% sucrose (Merck) to significantly increase the density of the buffer solution as compared to the surfactant suspension (∼1.04 g/mL vs. 1.01 g/mL) (16). As a result of the density difference, all surfactant remained at the air-liquid interface instead of sinking to the chamber bottom. Previous studies have demonstrated that the inclusion of sucrose in the subphase does not affect surfactant function and activity (16,17). After the chamber solution was degassed, a small air bubble (2 mm ϕ) was formed that floated against the agarose ceiling. The chamber and bubble were heated to 37°C and left for 10 min to ensure 100% air humidity in the bubble air, as air humidity has been shown to affect surface activity (18). Next, ∼0.1 μL of surfactant (25 mg/mL, enough to guarantee a surface excess of material) was applied directly to the surface of the bubble (see picture in Fig. 1 a) while the bubble shape was monitored over time with a video camera (Pulnix TM 7 CN). The pictures shown in Fig. 1 illustrate how a sample of surfactant, labeled with the fluorescent probe BODIPY-PC, remains surrounding the bubble, forming a layer of concentrated material that somehow mimics the thin layer of surfactant covering the respiratory air-liquid alveolar interface. After 5 min the chamber was sealed and the film-coated bubble was rapidly expanded to a volume of 0.15 mL (diameter ∼6 mm) and imaged for another 5 min. The protocol was continued with four quasistatic film compression/expansion cycles, which consisted of stepwise reductions in chamber volume to compress the film to the minimum surface tension possible before the film collapsed, and then stepwise increases back to the original bubble volume. There was a 4 s delay between each compression or expansion step to allow the film to stabilize, and a 1 min delay between each cycle. Meanwhile, the bubble shape at the beginning and end of each step was imaged and recorded. This was followed by dynamic cycles consisting of continuous compression and expansion over the same volume range determined during the preceding quasistatic cycle. Twenty such cycles were carried out during 1 min, a rate chosen to roughly correspond to respiration rate in the lung. For all imaged bubbles, the volume, interfacial area, and surface tension were calculated by utilizing the height and bubble diameter according to the method of Schoel et al. (19).
Figure 1.
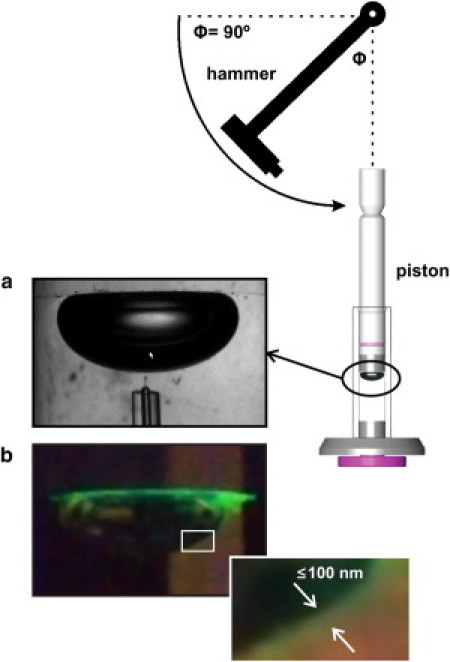
Captive-bubble setup to assess surfactant activity and mechanical stability. (a) Pulmonary surfactant samples are injected onto the surface of a 50 μL air bubble formed into a sucrose-containing subphase, floating against an agarose ceiling. (b) Injection of a surfactant sample labeled with a trace of the fluorescent probe BODIPY-PC shows how, as a result of the sucrose-containing buffer, surfactant forms a layer confined around the bubble. (Inset) Magnified picture illustrating how deposited surfactant forms a continuous layer of material at the surface of the bubble; a homogeneous distribution of the surfactant volume applied around the entire surface of the bubble would produce a layer ∼100 nm thick. The cartoon shows the disposition of the pendulum hammer designed to introduce repetitive mechanical perturbations on the compressed bubble.
Stability evaluation of surfactant films by mechanical perturbation
To assess film stability, we compressed the films (film-coated bubbles) to the minimum surface tension possible without collapsing them, and then perturbed the films by a pendulum hammer that consisted of a uniform steel rod (length: 24 cm) to which was attached a rectangular metal block (weight: 11.5 g) whose small face acted as the striking surface (see Fig. 1). In the experiments described here, the block of the pendulum hammer was released from a position of 90° to the vertical via a latch mechanism. Its striking force was selected so that each mechanical perturbation would cause a marked relaxation/collapse of the film. To assess film stability, typically five perturbations were carried out in succession, as this was judged sufficient for differentiating the samples. The films were also subjected to perturbation until they reached a stable surface tension and area where further perturbation had virtually no effect.
Data reproducibility and statistics
When possible, the figures represent the mean ± SD after averaging data from five independent experiments with at least two completely different batches of surfactant. CBS experiments are illustrated in the figures by overlapping the isotherms from five independent experiments. To analyze the statistical significance of differences in surface behavior, a one-way analysis of variance was used. The Holm-Sidak method was applied for multiple comparisons versus control group, with a significance level of 0.05. To compare two particular groups, the Mann-Whitney rank sum test was applied.
Results
Surface activity: influence of proteins SP-B and SP-C on film formation
In vitro, a sufficient amount of active pulmonary surfactant, when spread or adsorbed at an air-liquid interface (such as the surface of the bubble in the CBS), causes an almost instantaneous drop in surface tension to an equilibrium value of ∼23 mN/m, from which it drops no further without compression. The rate of film formation is shown in Fig. 2 for various surfactant preparations. In each case an excess amount of surfactant was spread (∼3.4 nmol) to ensure equilibrium. Native surfactant (which contains all hydrophilic components, including proteins SP-A and D) and its organic extract were the most active (see Fig. 2, top panels), reaching an equilibrium surface tension of ∼23 mN/m in <3 s. The reassembled organic extract (positive control) containing both hydrophobic proteins B and C in their original relation and concentration was almost as active, needing <20 s to reach equilibrium. The protein-depleted organic extract (negative control) was much less active than all samples containing protein, only reaching surface tensions of ∼48 mN/m. Fig. 2 also displays results for lipid extracts with purified protein added to give protein concentrations of 0.4% and 0.6% w/w for SP-B and SP-C, respectively. These results are illustrative of all protein concentrations tested (see Table S1 in the Supporting Material). All extracts containing only SP-B reached lower surface tensions than those containing only SP-C. However, extracts with both proteins added were more active and reached equilibrium surface tensions comparable to the complete organic extract, albeit somewhat more slowly.
Figure 2.
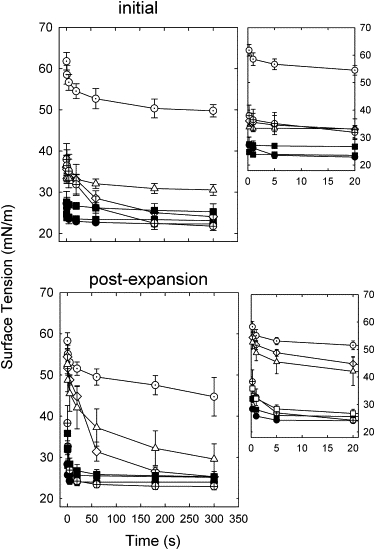
Film formation kinetics of spread surfactant films of varied composition. Shown are the surface tension versus time for spread films of native surfactant (solid circles), its organic extract (solid triangles), reassembled organic extract (open squares), and protein-depleted extracts to which have been added various amounts of protein (no protein, open circles; SP-B 0.4%, open diamonds; SP-C 0.6%, open triangles; or SP-B 0.4% + SP-C 0.6%, cross-hatched circles), all at 37°C. Data are means ± SD for four to six experiments. Upper panel: Initial film formation. Lower panel: Film formation after rapid expansion (∼40% vol.). Insets illustrate kinetics at the shortest times, during the first few seconds.
Five minutes after initial formation of the films, they were rapidly expanded and the resulting surface tension drops were monitored to gauge their ability to incorporate surplus surfactant material from the associated phase or reservoir (10). Adsorption upon expansion can be evaluated with greater accuracy than initial adsorption because this process is not subject to potential experimental artifacts, such as those derived from the proximity of the surfactant capillary to the bubble surface during surfactant deposition. Furthermore, adsorption of surfactant to an expanding surface likely better reflects the adsorption process as it really occurs in the alveoli. All films containing protein showed far better activity than the films without, as evidenced by far greater drops in surface tension after expansion (see Fig. 2, lower panels). Even after 5 min the extracts devoid of protein only reached a surface tension of ∼40–50 mN/m. In contrast, and in similarity to the initial film formation, native and complete organic extracts reached equilibrium very quickly (<5 s) and were superior to all protein-depleted extracts. At all concentrations tested, extracts containing only SP-B were more active than those with only SP-C added, but less active than extracts containing both proteins. Extracts with both proteins added were comparable in activity to the complete organic extracts and native surfactant, attaining equilibrium values of 23 mN/m within 20 s.
Film surface behavior under quasistatic and dynamic compression/expansion cycles
Active films assessed in the CBS reach very low surface tensions (<2 mN/m) when compressed, and exhibit a small area change with little hysteresis, maintaining near-equilibrium surface tensions upon expansion. Quasistatic cycling also provides information about film stability since the film is allowed to relax for several seconds between compression steps.
A look at the first quasistatic compression/expansion isotherm curves (Fig. 3, black symbols and lines) reveals important differences between samples. The extract depleted of proteins showed behavior distinct from that of other extracts, with large relative area changes (>50%), much higher maximum surface tensions (>50 mN/m), and significant hysteresis. In contrast, whole native surfactant and its organic extracts exhibited much better surface activity, with maximum surface tensions that did not exceed 30 mN/m, area changes near 30%, and far less hysteresis. Incorporation of only 0.4% (w/w) of SP-B into the lipid extract results in behavior similar to that of the complete organic extracts, requiring a relatively small area change to reach minimum surface tension, with moderate hysteresis, whereas the sample that contained only SP-C was characterized by 20% more area change on average, significant hysteresis, and higher maximum surface tensions upon expansion. Results obtained at higher protein concentrations show a similar trend and are summarized in Table S2.
Figure 3.
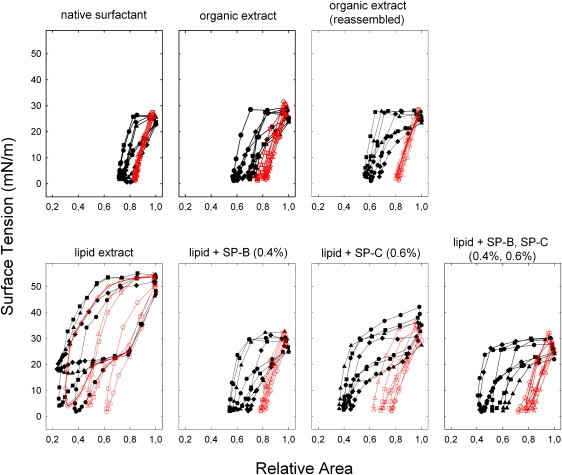
Quasistatic compression/expansion isotherms for spread surfactant films of varied composition. The surface tension-area relation of quasistatic compression-expansion isotherms is shown for various samples as labeled. The isotherms of the first (curves with solid symbols) and fourth (curves with open symbols) cycles are depicted for four independent experiments.
All samples exhibited in this first quasistatic cycle, to varying degrees, a region of less inclined slope, or squeeze-out plateau (20) between 25 and 15 mN/m during the compression phase, from nearly not noticeable in the case of whole native surfactant to prominent for the less active samples (i.e., the lipid extract devoid of protein or with only SP-C added). In all cases, the fourth compression/expansion curves (Fig. 3, red symbols and lines) have a very different shape compared to the first ones, with far smaller area changes and little to no hysteresis, indicative of changed film configuration and/or structure. The extract devoid of protein (negative control) proved to be somewhat of an exception, as it was still defined by large hysteresis, area change, and maximum surface tension values above 40 mN/m. In contrast, the native surfactant and its organic extracts showed almost no hysteresis, area changes of ∼15%, and maximum surface tensions that never exceeded 30 mN/m. The other samples were similar but with slightly larger area changes and more hysteresis, particularly in the case of the extract with only SP-C added.
In Fig. 4 can be seen the dynamic compression/expansion isotherms for several individual experiments centered on the 20th cycle. The curves appear similar to the fourth quasistatic cycles but with less hysteresis and greater maximum surface tensions. The extract depleted of protein is again unique, characterized by far greater maximum tensions and area changes of ∼60 mN/m and 40%, respectively, versus 30 mN/m and <20%, respectively, for the other samples. All samples exhibit a progressive change to less hysteresis with repeated cycling similar to that seen during quasistatic cycling, and by the 20th cycle (Fig. 4) exhibit little or no hysteresis. Curiously, the protein-devoid extract, whose curve uniquely displays a large plateau after four quasistatic cycles, completely lost this feature during dynamic cycling with compression curves of constant slope.
Figure 4.
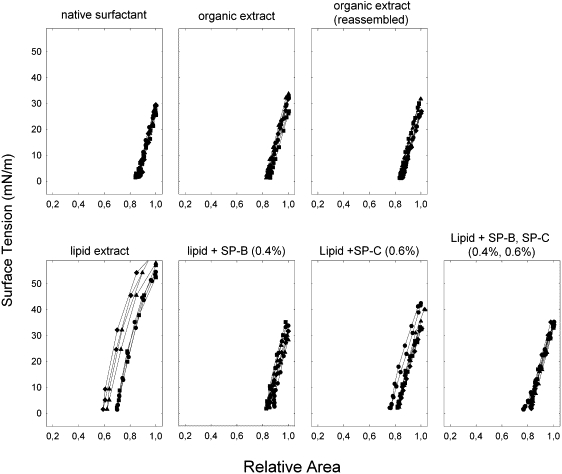
Dynamic compression/expansion isotherms for spread surfactant films of varied composition. The surface tension-area relation of dynamic compression/expansion isotherms is shown for various samples as labeled. Only the 20th cycle is depicted for four individual experiments after compression-expansion at 20 cycles/min.
Proteins and stability
In Fig. 5 the change in surface tension after mechanical perturbation, from the compression minimum, is depicted for all samples. The graph compares the results for perturbation after only one quasistatic compression and after quasistatic compression after the full experimental protocol (four quasistatic + 20 dynamic compressions/expansion cycles). Changes in surface tension were larger for all samples tested after only one compression, particularly for samples containing only partial surfactant composition. The most stable were the native surfactant, its organic extract, and reassembled organic extract. Lipid extracts with only SP-B added were more stable and maintained lower minimum surface tensions than those enriched with only SP-C at all concentrations tested (see Table S2). Furthermore, the samples with only SP-C added did not show significantly different stability compared to films formed from extracts containing no protein (see Fig. 5). Extracts with both proteins added were also significantly more stable than those with only SP-C added. There was, however, a marked concentration dependence, with extracts containing total protein concentrations of ≥2% being markedly less stable. At a total concentration of 1% (0.4% SP-B, 0.6% SP-C; Fig. 5), stability was statistically no different compared to extracts containing only SP-B (0.4%). Organic extracts containing the proteins in their original concentration and ratio (in our hands, ∼0.8% SP-B and ∼1.1% SP-C), i.e., the whole organic extract and its reassembled counterpart, were significantly more stable than extracts containing the two proteins (0.4% SP-B, 0.6% SP-C), especially if the films were perturbed after only one single compression. However, extracts containing up to 1% SP-B as the single protein additive were not distinguishable in terms of stability from films made of whole native surfactant or its organic extract (not shown). Furthermore, films containing 0.4% SP-B, either alone or in combination with SP-C, showed no statistically different stability in comparison with those formed by native surfactant or the full organic extract once the films had been subjected to the full quasistatic and dynamic compression-expansion protocol. Together, these results confirm that SP-B plays a crucial role in sustaining very low tensions in compressed films.
Figure 5.
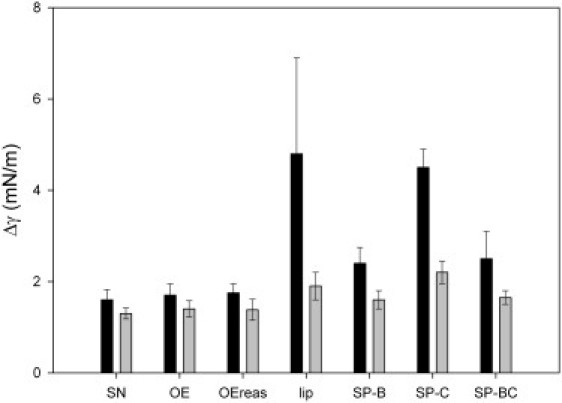
Film stability against mechanical perturbation introduced at a bubble compressed to minimum surface tension. Shown is the change in surface tension from minimum after pendulum hammer perturbation introduced either after one quasistatic compression (black bars) or after four quasistatic and 20 dynamic compression-expansion cycles (gray bars).
The results given above are based on the total change in surface tension resulting from five successive perturbations with the pendulum hammer. Fig. S1 depicts the corresponding surface tension and area change after each consecutive perturbation was introduced into the films compressed to minimum surface tensions. All samples show a steepening curve with successive perturbations, i.e., the area loss lessens significantly with each perturbation relative to the increase in surface tension. In the more-active samples (all containing both proteins or SP-B alone), there appears to be a large area buffer of stability where, upon perturbation, significant area was lost with a relatively small increase in surface tension. For the less-active samples (i.e., the protein-devoid lipid extract and the lipid extract with only SP-C added), this area buffer was smaller (∼10% vs. 20% relative area) and significant changes in surface tension occurred immediately, a behavior similar to the bubble clicks described previously for compressed films (15,21). This is most pronounced in the second lipid curve (Fig. S1, top), which began at a far higher minimum surface tension of >4 mN/m. It is representative of a number of experiments in which the protein-devoid extract exhibited great instability during the first compression and did not reach low surface tensions. This was never observed for samples that contained protein, which always achieved low surface tensions upon the first compression.
In Fig. 6 surface tension is plotted as a function of the number of perturbations introduced into the CBS chamber by the pendulum hammer. Of note are the curve plateaus seen for all samples after several perturbations, which are indicative of stable collapsed structures in which further perturbation effects no change. The trend is clear: the more stable the surfactant, the more perturbation is necessary to reach the stability plateau, and the lower is the stable surface tension. Native surfactant proved most resilient and was characterized by a more linear curve that plateaued at 8 mN/m. The complete organic extracts and the one containing SP-B alone all exhibited similar resilience and plateaued around 9mN/m, whereas the least stable extracts plateaued above 12 mN/m with far fewer perturbations.
Figure 6.
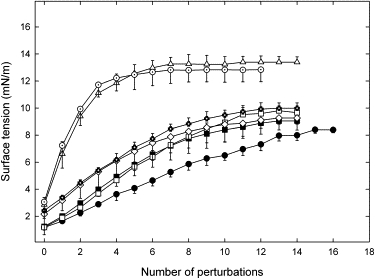
Film stability against mechanical perturbations over the entire range of instability. Surface tension is plotted as a function of the number of perturbations introduced in the compressed bubble by the pendulum hammer. Values are means ± SD based on four independent experiments, and symbols are as in Fig. 2. Surface tension increased with successive perturbations until it became constant; at this stage the films were not perturbed further.
Discussion
Our assessment leaves no doubt as to the superiority of whole native surfactant for all surface activity markers, especially initial film formation and the extent of compression required for the films to reach the lowest surface tensions. However, its organic extract (aqueous suspension) comes remarkably close with respect to many of these surface activity benchmarks. In addition to the complex mixture of lipids, key to the function of these organic extracts are the hydrophobic proteins SP-B and SP-C. In this work, we made every attempt to better distinguish the specific functional properties of the two by studying them in the most physiologically relevant context, with respect to both the method (i.e., applying small amounts of concentrated surfactant at 37° in the CBS) and the surfactant material itself. To our knowledge, this is the first study in which the separate and united effects of both hydrophobic proteins have been investigated in a more physiological context, namely, the intact matrix of lipid extract carefully derived from whole native surfactant. Other groups have also carried out studies on the effect of the whole fraction of hydrophobic surfactant proteins on the structure and surface properties of the full surfactant lipid moiety (22–25), but not to the point (to our knowledge) of analyzing in detail the effect of isolated and combined individual SP-B and SP-C proteins.
Focusing on the proteins individually, our results (summarized in Table 1) point to SP-B as the main protagonist in promoting surface activity and stability. SP-B outperformed SP-C on every activity parameter except for film formation and reextension, where SP-C also exhibited similar enhancement. Our observation that SP-B is more effective than SP-C in reaching near-equilibrium surface tensions ≤25 mN/m suggests that SP-B is more critical for optimal packing of the surface film lipids, which is consistent with results indicating significant interactions between SP-B and condensed phase lipids in monolayers (26,27). However, in tandem with SP-C, equilibrium surface tension was reached more quickly, indicating a slightly combined effect. This potential cooperation was even more pronounced during film formation after rapid expansion, i.e., both proteins together enhanced the incorporation of phospholipids from the surface-associated phase, formed during initial film formation, into the surface film. One can speculate that SP-C improves the transport/mobility of the prepackaged lipid units associated with SP-B to the surface layer, either from the deposited bolus (initial film formation) or from the surfactant reservoir (film formation postexpansion) (10). SP-C is thought to influence the thickness and fluidity of the surrounding lipid reservoir (28), as well as participate in the transfer of phospholipids to interfacial monolayers (29). Thus, it could act as a transport link between the surface film and associated bilayer or multilayer structures, greatly accelerating lipid insertion.
Table 1.
Surface activity and stability performance of protein-containing surfactant preparations
| Sample | Film formation (initial) | Film formation (postexpansion) | Δ Area compression | Compression γmin | Stability γmin |
|---|---|---|---|---|---|
| Lipid extract + SP-B | ++ | + | ++ | ++ | ++ |
| Lipid extract + SP-C | + | + | + | + | 0 |
| Lipid extract + SP-B, SP-C | ++ | +++ | ++ | ++ | ++ |
| Native surfactant | +++ | +++ | +++ | +++ | +++ |
| Organic extract | +++ | +++ | ++ | +++ | +++ |
| Org. extract (reassambled) | ++ | +++ | ++ | +++ | +++ |
0 Poor.
+ Improved behavior (with respect to protein-free lipid preparations).
++ Very good behavior.
+++ Optimal behavior.
The first quasistatic compression/expansion isotherms highlight important differences in the roles of SP-B and SP-C. The substantially smaller area change (∼40% vs. 60%) and lower minimum surface tension for extracts with only SP-B rather than SP-C added indicate that films containing SP-B initially had a more ideal configuration. As a result, a more stable and tightly packed film was formed upon compression with less structural change or squeeze-out. This more ideal configuration does not necessarily mean a film highly enriched in DPPC, as recent results suggest that DPPC enrichment is not necessary to attain the lowest surface tensions (30), and there is no significant selectivity for DPPC incorporation during adsorption (31). Instead, SP-B may optimize and limit two-dimensional to three-dimensional structural transitions, the reversible structural squeeze-out (see the models reviewed in Serrano and Pérez-Gil (7)) in which part of the continuous surface film is excluded from the immediate surface during compression and can be reincorporated during expansion. The most deformable films would show large squeeze-out plateaus and release the work of compression without a full decrease of surface tension (increase in surface pressure). SP-B could contribute to sustain compressed structures, limiting their relaxation to the third dimension upon compression. Apart from this, SP-B could facilitate reincorporation of excluded lipids during expansion. The importance of SP-B in the reincorporation process is underlined by the distinct expansion plateau between 25 and 30 mN/m that is observed in all preparations containing this protein and is largely absent in the SP-C-only sample.
The change in curve shape with successive cycles to lesser area change and hysteresis has often been observed, and has usually been attributed to a supposed DPPC enrichment by various mechanisms (1). We suggest instead a progressive structural refinement of the surface layer and associated phases. Optimal refinement has been proposed to require both proteins (32,33), but our results suggest that SP-B alone is able to refine the films to generate structures that require the lowest compression ratios to attain very low surface tensions. The lack of hysteresis seen in the most surface active films (especially those containing both proteins in their original concentration and relative ratio) likely reflects a stable unchanging configuration, with the film acting as a cohesive unit and three-dimensional, multilayer-associated structures contributing to prevent out-of-plane distortions of the film during cycling. As a result, such films do not behave much differently during dynamic conditions. In contrast, the protein-depleted extract was markedly different during dynamic cycling, showing no hint of a plateau and far less hysteresis. The faster and continuous dynamic compression probably provides the less stable film elements with a transient cohesiveness that is not possible under quasistatic conditions.
Our results show that SP-B is fundamental for promoting low minimum surface tensions and stability, whereas the presence of only SP-C may actually negatively influence both parameters. The previously suggested more ideal film configuration for films containing SP-B, rather than SP-C alone, also translates to a better stability at very low surface tensions reached upon compression. SP-B, which is likely oriented with its α-helices parallel to the plane near the interface, may enhance lateral stability through strong electrostatic interactions between the positively charged regions of the helices and the negatively charged phosphates of the phospholipid heads (7,34). On the other hand, SP-C is thought to adopt a more perpendicular orientation with respect to the surface plane, which maximizes interactions of its α-helix with surface lipid acyl chains (35) while also allowing interactions with associated bilayer structures through the N-terminal segment (36). This configuration may lead to lateral destabilization of the surface film, with SP-C and associated lipids exiting but remaining associated with the interface upon compression. Of interest, the destabilizing effects of SP-C were negated or even reversed in the presence of SP-B, pointing to a possible compensation between the proteins in stabilizing films. This conclusion is consistent with recent in vitro and in vivo results (8,37). At higher concentrations, however, lesser stabilities were observed with both proteins present (see Table S2), as also reported by other authors (25,38). We surmise that an excess of protein leads to discontinuities in the lipid packing, resulting in an overall fluidizing and destabilizing effect on the surface layer. Having the correct total protein concentration and relative ratio appears to be important for surfactant activity and in particular film stability, since the films formed from organic extracts containing both proteins in their original concentration and ratio (i.e., not chromatographically separated) were more active and stable than the depleted extracts with both proteins added, at all concentrations tested.
For all samples, film stability was improved after cycling as compared to after only one quasistatic compression (see Fig. 5), likely due to the aforementioned structural reorganization of the surface active film. In general, the smaller the hysteresis and area change characterizing the compression/expansion isotherm, the more stable was the interfacial film.
The different stability plateau values reached among the samples (see Fig. 6) were largely dependent on the presence or absence of SP-B, and may represent distinct collapse structures. A possible explanation is that SP-B largely dominates film mechanics and packing, and hence films that contain it collapse similarly regardless of the presence or absence of SP-C. The greater resilience of native surfactant, exemplified by its more linear curve shape and lower stable surface tension, is evidence that other factors that are not present in organic extracts also contribute to stability and the formation of a unique collapsed phase. The presence in whole surfactant of the major surfactant protein SP-A (39) or the unique lipid-protein structures assembled through the biogenesis of surfactant in lamellar bodies (40) could be an additional factor that contributes to stability.
With respect to the pendulum hammer method of assessing film stability, our experience shows it to be very quick and effective. Films that were stabilized by repeated cycling, with nearly identical isotherms, were readily distinguished. Furthermore, results not shown here indicated that the method better differentiates stabilities as compared to the traditional in vitro stability measure where the film is compressed to minimum and its relaxation is monitored over time. We believe this is due to the nature of the perturbation induced by the pendulum hammer, which delivers a sharp vibrational shock to the film. Such abrupt forces may also be more similar to some of the stresses a film must endure in a lung due to sudden movement, external impact, coughing, etc. The ability of SP-B-containing films to support strong mechanical perturbations without leading to significant relaxation of the lowest surface tensions may be important to provide the respiratory surface with enough stability during the periods of time required for the lung to complete expiration.
The possible existence of a cooperative action or synergy between the two hydrophobic surfactant proteins to sustain pulmonary surfactant performance is a relevant issue, and has been addressed in previous work. An earlier captive-bubble study found a modest synergy between both proteins in enhancing surface activity (41). In contrast, other studies using far different conditions (i.e., surfactant spread as organic solutions on a Langmuir/Wilhelmy balance) found no interdependency or synergy between the proteins (24,33). One could consider SP-B and SP-C to exhibit a truly cooperative behavior if the functional properties of samples containing both proteins simultaneously were improved with respect to the behavior of samples containing only one of them. In Fig. 7 we compare different parameters that define the quality of the surface performance of native surfactant and samples containing SP-B and SP-C (0.4 + 0.6 protein to lipid by weight, respectively) with samples containing 1% (w/w) of only SP-B or SP-C. Generally, samples containing 1% of only SP-B showed surface behavior that was as good as that observed for samples containing both proteins. In contrast, samples containing 1% of SP-C as the only protein additive were inferior to SP-B-containing preparations. This confirms the prevalent role of SP-B as the most essential protein in surfactant. Still, the fact that samples containing only 0.4% SP-B plus SP-C had surface activity that was fully comparable to that of samples containing a whole 1% SP-B could be an indication, in our opinion, of a somehow potentiated effect of the combined action of the two proteins under limited conditions. We are aware that these protein percentages are below the reported physiological amounts of SP-B and SP-C in surfactant. However, we came to these values as the minimal amounts that produce surface behaviors comparable to those of native surfactant and its extract. Higher protein concentrations could mask subtle differences in the performance in vitro of the two proteins, either alone or combined. Such subtle differences might well be relevant under defined pathophysiological constraints. The amount of SP-B and SP-C present in the different clinical surfactant preparations currently in use is substantially reduced with respect to native surfactant complexes (4). We think that our results define a threshold for the minimum protein composition required to produce surfactant preparations with optimal surface activity.
Figure 7.
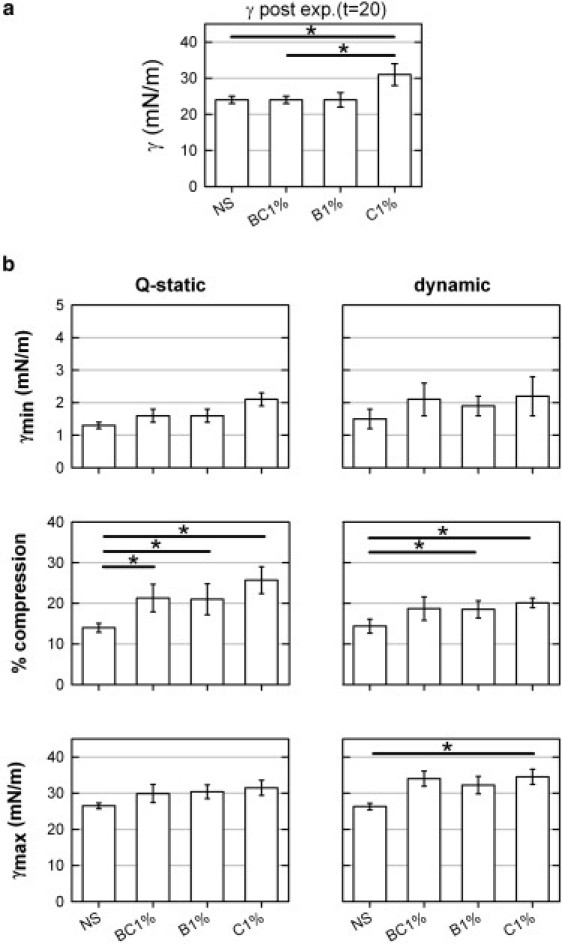
Combined effect of the presence of surfactant proteins SP-B and SP-C on interfacial adsorption and compression-expansion dynamics. (a) Minimal surface tension reached by adsorption upon bubble expansion. (b) Parameters that define surface activity under quasistatic (left panels) or dynamic (right panels) compression-expansion cycling are compared for native surfactant and for samples reconstituted from the full lipid extract supplemented with SP-B and SP-C (BC1%; 0.4 and 0.6% protein to lipid w/w, respectively), only SP-B (B1%; 1% w/w), or only SP-C (C1%; 1% w/w). Samples reconstituted from purified proteins were all compared with native surfactant, and the behavior of the sample containing SP-B plus SP-C was compared with that of the samples containing only SP-B or SP-C. Horizontal bars with asterisk indicate which of these comparisons were statistically significant (p = 0.05).
Acknowledgments
This work was funded by grants from Spanish Ministry of Science (BIO2009-09694, CSD2007-00010) and the Community of Madrid (P-MAT-000283-0505) and Marie Curie Network Pulmonet (RTN-512229) in conjunction with the European Commission. O.L.O. received financial support from Pontificia Universidad Javeriana (Bogotá, Colombia) and Fundación Carolina (Madrid, Spain).
Footnotes
The authors dedicate this article to Dr. Samuel Schürch. His dedication during so many years and his seminal work in the field of pulmonary surfactant biophysics have been for these modest scientists—and for many others—an invaluable source of motivation and inspiration.
Supporting Material
References
- 1.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim. Biophys. Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 2.Berggren P., Curstedt T., Robertson B. Physiological activity of pulmonary surfactant with low protein content: effect of enrichment with synthetic phospholipids. Exp. Lung Res. 1985;8:29–51. doi: 10.3109/01902148509069678. [DOI] [PubMed] [Google Scholar]
- 3.Johansson J., Curstedt T. Molecular structures and interactions of pulmonary surfactant components. Eur. J. Biochem. 1997;244:675–693. doi: 10.1111/j.1432-1033.1997.00675.x. [DOI] [PubMed] [Google Scholar]
- 4.Blanco O., Pérez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat respiratory distress syndrome: role of the different components in an efficient pulmonary surfactant. Eur. J. Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Veldhuizen R., Nag K., Possmayer F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 6.Nogee L.M. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu. Rev. Physiol. 2004;66:601–623. doi: 10.1146/annurev.physiol.66.032102.134711. [DOI] [PubMed] [Google Scholar]
- 7.Serrano A.G., Pérez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem. Phys. Lipids. 2006;141:105–118. doi: 10.1016/j.chemphyslip.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Almlén A., Stichtenoth G., Curstedt T. Surfactant proteins B and C are both necessary for alveolar stability at end expiration in premature rabbits with respiratory distress syndrome. J. Appl. Physiol. 2008;104:1101–1108. doi: 10.1152/japplphysiol.00865.2007. [DOI] [PubMed] [Google Scholar]
- 9.Possmayer F., Nag K., Schürch S. Surface activity in vitro: role of surfactant proteins. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;129:209–220. doi: 10.1016/s1095-6433(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 10.Schürch S., Green F.H., Bachofen H. Formation and structure of surface films: captive bubble surfactometry. Biochim. Biophys. Acta. 1998;1408:180–202. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 11.Qanbar R., Cheng S., Schürch S. Role of the palmitoylation of surfactant-associated protein C in surfactant film formation and stability. Am. J. Physiol. 1996;271:L572–L580. doi: 10.1152/ajplung.1996.271.4.L572. [DOI] [PubMed] [Google Scholar]
- 12.Glasser S.W., Burhans M.S., Whitsett J.A. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc. Natl. Acad. Sci. USA. 2001;98:6366–6371. doi: 10.1073/pnas.101500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taeusch H.W., Bernardino de la Serna J., Zasadzinski J.A. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys. J. 2005;89:1769–1779. doi: 10.1529/biophysj.105.062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Gil J., Cruz A., Casals C. Solubility of hydrophobic surfactant proteins in organic solvent/water mixtures. Structural studies on SP-B and SP-C in aqueous organic solvents and lipids. Biochim. Biophys. Acta. 1993;1168:261–270. doi: 10.1016/0005-2760(93)90181-8. [DOI] [PubMed] [Google Scholar]
- 15.Schürch S., Bachofen H., Possmayer F. A captive bubble method reproduces the in situ behavior of lung surfactant monolayers. J. Appl. Physiol. 1989;67:2389–2396. doi: 10.1152/jappl.1989.67.6.2389. [DOI] [PubMed] [Google Scholar]
- 16.Gunasekara L., Schürch S., Amrein M. Pulmonary surfactant function is abolished by an elevated proportion of cholesterol. Biochim. Biophys. Acta. 2005;1737:27–35. doi: 10.1016/j.bbalip.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Codd J.R., Schürch S., Orgeig S. Torpor-associated fluctuations in surfactant activity in Gould's wattled bat. Biochim. Biophys. Acta. 2002;1580:57–66. doi: 10.1016/s1388-1981(01)00185-8. [DOI] [PubMed] [Google Scholar]
- 18.Zuo Y.Y., Acosta E., Neumann A.W. Effect of humidity on the stability of lung surfactant films adsorbed at air-water interfaces. Biochim. Biophys. Acta. 2006;1758:1609–1620. doi: 10.1016/j.bbamem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Schoel W.M., Schürch S., Goerke J. The captive bubble method for the evaluation of pulmonary surfactant: surface tension, area, and volume calculations. Biochim. Biophys. Acta. 1994;1200:281–290. doi: 10.1016/0304-4165(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 20.Schürch S., Schürch D., Robertson B. Surface activity of lipid extract surfactant in relation to film area compression and collapse. J. Appl. Physiol. 1994;77:974–986. doi: 10.1152/jappl.1994.77.2.974. [DOI] [PubMed] [Google Scholar]
- 21.Schürch S., Bachofen H., Green F. Surface properties of rat pulmonary surfactant studied with the captive bubble method: adsorption, hysteresis, stability. Biochim. Biophys. Acta. 1992;1103:127–136. doi: 10.1016/0005-2736(92)90066-u. [DOI] [PubMed] [Google Scholar]
- 22.Schram V., Hall S.B. Thermodynamic effects of the hydrophobic surfactant proteins on the early adsorption of pulmonary surfactant. Biophys. J. 2001;81:1536–1546. doi: 10.1016/S0006-3495(01)75807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schram V., Hall S.B. SP-B and SP-C alter diffusion in bilayers of pulmonary surfactant. Biophys. J. 2004;86:3734–3743. doi: 10.1529/biophysj.10x.037630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z., Gurel O., Notter R.H. Differential activity and lack of synergy of lung surfactant proteins SP-B and SP-C in interactions with phospholipids. J. Lipid Res. 1996;37:1749–1760. [PubMed] [Google Scholar]
- 25.Wang Z., Hall S.B., Notter R.H. Dynamic surface activity of films of lung surfactant phospholipids, hydrophobic proteins, and neutral lipids. J. Lipid Res. 1995;36:1283–1293. [PubMed] [Google Scholar]
- 26.Bringezu F., Ding J., Zasadzinski J.A. Influence of pulmonary surfactant protein B on model lung surfactant monolayers. Langmuir. 2002;18:2319–2325. [Google Scholar]
- 27.Cruz A., Vázquez L., Pérez-Gil J. Effect of pulmonary surfactant protein SP-B on the micro- and nanostructure of phospholipid films. Biophys. J. 2004;86:308–320. doi: 10.1016/S0006-3495(04)74106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmblad M., Johansson J., Curstedt T. Biophysical activity of an artificial surfactant containing an analogue of surfactant protein (SP)-C and native SP-B. Biochem. J. 1999;339:381–386. [PMC free article] [PubMed] [Google Scholar]
- 29.Oosterlaken-Dijksterhuis M.A., Haagsman H.P., Demel R.A. Characterization of lipid insertion into monomolecular layers mediated by lung surfactant proteins SP-B and SP-C. Biochemistry. 1991;30:10965–10971. doi: 10.1021/bi00109a022. [DOI] [PubMed] [Google Scholar]
- 30.Piknova B., Schief W.R., Hall S.B. Discrepancy between phase behavior of lung surfactant phospholipids and the classical model of surfactant function. Biophys. J. 2001;81:2172–2180. doi: 10.1016/S0006-3495(01)75865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S.H., Possmayer F. Lipid compositional analysis of pulmonary surfactant monolayers and monolayer-associated reservoirs. J. Lipid Res. 2003;44:621–629. doi: 10.1194/jlr.M200380-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson M., Palmblad M., Schürch S. Palmitoylation of a pulmonary surfactant protein C analogue affects the surface associated lipid reservoir and film stability. Biochim. Biophys. Acta. 2000;1466:169–178. doi: 10.1016/s0005-2736(00)00198-x. [DOI] [PubMed] [Google Scholar]
- 33.Krol S., Ross M., Janshoff A. Formation of three-dimensional protein-lipid aggregates in monolayer films induced by surfactant protein B. Biophys. J. 2000;79:904–918. doi: 10.1016/S0006-3495(00)76346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochrane C.G. Pulmonary surfactant in allergic inflammation: new insights into the molecular mechanisms of surfactant function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L608–L609. doi: 10.1152/ajplung.00434.2004. [DOI] [PubMed] [Google Scholar]
- 35.Johansson J. Structure and properties of surfactant protein C. Biochim. Biophys. Acta. 1998;1408:161–172. doi: 10.1016/s0925-4439(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 36.Plasencia I., Keough K.M., Perez-Gil J. Interaction of the N-terminal segment of pulmonary surfactant protein SP-C with interfacial phospholipid films. Biochim. Biophys. Acta. 2005;1713:118–128. doi: 10.1016/j.bbamem.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Gil L., Schürch D., Pérez-Gil J. Pulmonary surfactant protein SP-C counteracts the deleterious effects of cholesterol on the activity of surfactant films under physiologically relevant compression-expansion dynamics. Biophys. J. 2009;97:2736–2745. doi: 10.1016/j.bpj.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Post A., Nahmen A.V., Galla H.J. Pulmonary surfactant protein C containing lipid films at the air-water interface as a model for the surface of lung alveoli. Mol. Membr. Biol. 1995;12:93–99. doi: 10.3109/09687689509038502. [DOI] [PubMed] [Google Scholar]
- 39.McCormack F.X. Structure, processing and properties of surfactant protein A. Biochim. Biophys. Acta. 1998;1408:109–131. doi: 10.1016/s0925-4439(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim. Biophys. Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Capote K., Nag K., Possmayer F. Surfactant protein interactions with neutral and acidic phospholipid films. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L231–L242. doi: 10.1152/ajplung.2001.281.1.L231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


