Abstract
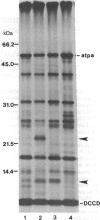
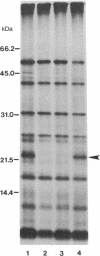
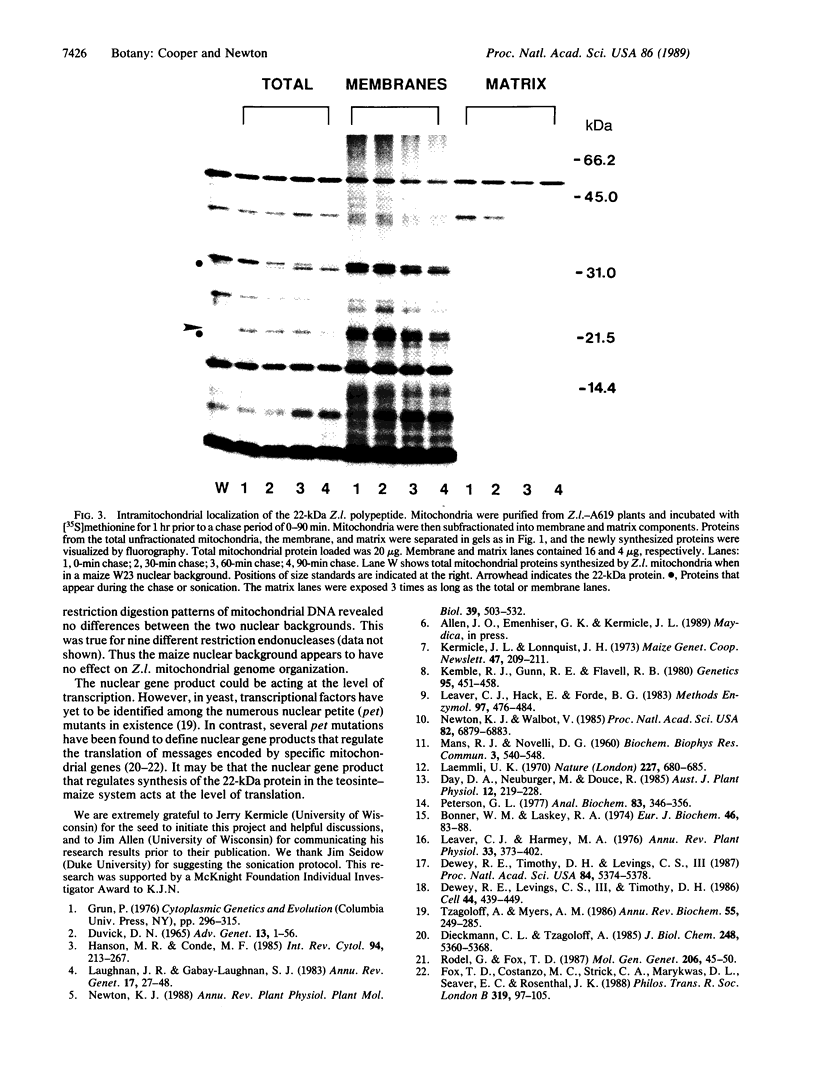
When cytoplasm of the teosinte Zea luxurians is introduced into certain maize inbred nuclear backgrounds, the pattern of protein synthesis in the teosinte mitochondria is altered. Teosinte mitochondria purified from plants possessing a maize A619 nuclear background (Z.l.—A619 plants) synthesize a novel 22-kDa polypeptide that is associated exclusively with the membrane fraction of the organelle. Mitochondria from plants possessing a W23 nuclear background do not synthesize this protein. The F1 hybrids Z.l.—A619 × W23 and Z.l.—W23 × A619 do not synthesize the protein. However, synthesis of the polypeptide was observed in 14 of 21 individual progeny from the backcross of the F1 hybrid Z.l.—A619 × W23 to the pollen parent A619. These data suggest that a single nuclear gene controls the synthesis of the 22-kDa protein in mitochondria, with the recessive allele of the gene allowing expression of the polypeptide. Mitochondria from the F1 hybrid Z.l.—A619 × Mo17 synthesize the 22-kDa protein, whereas mitochondria from Z.l.—A619 × B73 do not. Data from these outcrosses demonstrate that other maize lines also possess nuclear genes capable of regulating the synthesis of the 22-kDa Zea luxurians mitochondrial protein.
Keywords: nuclear-cytoplasmic incompatibility, mitochondrial protein synthesis, teosinte, Zea mays
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5374–5378. doi: 10.1073/pnas.84.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Costanzo M. C., Strick C. A., Marykwas D. L., Seaver E. C., Rosenthal J. K. Translational regulation of mitochondrial gene expression by nuclear genes of Saccharomyces cerevisiae. Philos Trans R Soc Lond B Biol Sci. 1988 May 31;319(1193):97–105. doi: 10.1098/rstb.1988.0034. [DOI] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughnan J. R., Gabay-Laughnan S. Cytoplasmic male sterility in maize. Annu Rev Genet. 1983;17:27–48. doi: 10.1146/annurev.ge.17.120183.000331. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Hack E., Forde B. G. Protein synthesis by isolated plant mitochondria. Methods Enzymol. 1983;97:476–484. doi: 10.1016/0076-6879(83)97156-2. [DOI] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- Newton K. J., Walbot V. Maize mitochondria synthesize organ-specific polypeptides. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6879–6883. doi: 10.1073/pnas.82.20.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rödel G., Fox T. D. The yeast nuclear gene CBS1 is required for translation of mitochondrial mRNAs bearing the cob 5' untranslated leader. Mol Gen Genet. 1987 Jan;206(1):45–50. doi: 10.1007/BF00326534. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Myers A. M. Genetics of mitochondrial biogenesis. Annu Rev Biochem. 1986;55:249–285. doi: 10.1146/annurev.bi.55.070186.001341. [DOI] [PubMed] [Google Scholar]