Abstract
Muscular dystrophy results in the progressive wasting and necrosis of skeletal muscle. Glucocorticoids such as prednisone have emerged as a front-line treatment for many forms of this disease. Recently, Debio-025, a cyclophilin inhibitor that desensitizes the mitochondrial permeability pore and subsequent cellular necrosis, was shown to improve pathology in 3 different mouse models of muscular dystrophy. However it is not known if Debio-025 can work in conjunction with prednisone, or how it compares against prednisone in mitigating disease in dystrophic mouse models. Here we show that Debio-025 reduced the variations in myofiber cross-sectional areas, decreased fibrosis, and decreased infiltration of activated macrophages more efficiently than prednisone. However the use of prednisone and Debio-025 together had no additional effect on these histopathological indexes. Orally administered Debio-025 also reduced creatine kinase blood levels and improved grip-strength in mdx mice after 6 weeks of treatment, and the combination of Debio-025 with prednisone increased muscle function slightly better than prednisone alone. Thus, our results suggest that Debio-025 is as effective or slightly better than prednisone in mitigating muscular dystrophy in the mdx mouse model of disease.
Introduction
The muscular dystrophies are a group of inherited genetic diseases characterized by progressive muscle weakness and wasting [1]. The most common of these diseases is Duchenne muscular dystrophy, an X-linked disorder that occurs in 1 in 3500 male births [1]. Individuals affected by Duchenne muscular dystrophy lose the ability to walk between ages 7 to 13, and typically do not survive past their twenties due to extensive loss of muscle and/or cardiomyopathy [1–3]. Duchenne muscular dystrophy is caused by mutations in the dystrophin gene, a structural protein involved in the dystrophin glycoprotein complex (DGC) that connects the underlying contractile elements to the membrane anchored glycoproteins that themselves are affixed to the extracellular matrix [1,4].
The loss of dystrophin causes destabilization of the sarcolemma leading to contraction induced microtears and the influx of ions such as Ca2+, as well as the loss of selected proteins from the myofibers [4–8]. Although an area of ongoing controversy, an increase in intracellular Ca2+ concentration is thought be a primary effect leading to myofiber necrosis and the secondary replacement with fibrotic and adipose tissue [5–10]. Ca2+ overload is known to cause cellular necrosis by directly inducing the opening of the mitochondrial permeability transition pore (MPTP) [11,12]. The MPTP spans the inner and outer membranes of the mitochondria, and when opened for prolonged periods of time, leads to loss of ATP generation, swelling, rupture, and induction of cell death [11–12]. Importantly, MPTP formation can be desensitized by cyclosporine A (CsA) through inhibition of cyclophilin D (CypD), a matrix prolyl cis-trans isomerase that facilitates pore formation due to Ca2+ overload or increased reactive oxygen species generation [11–14]. Indeed, it was recently shown that Debio-025, a cyclosporine analog that is more potent than CsA in blocking cyclophilin D [15], was capable of reducing muscle pathology in three different mouse models of muscular dystrophy [16–18]. Although controversial, CsA treatment itself can have protective effects on muscle pathology in dystrophic animal models and humans [19–22]. However, CsA is not an ideal agent for treating muscular dystrophy since it suppresses the immune system and inhibits calcineurin signaling, which secondarily reduces myotube differentiation and muscle regeneration [23–24]. Thus, non-immunosuppressant analogs of CsA that do not inhibit calcineurin, such as Debio-025, are likely to be far more effective in treating muscular dystrophy.
The corticosteroids prednisone or deflazacort are currently the standard of care for treatment of Duchenne muscular dystrophy, which appear to prolong muscle strength and have other minor benefits in reducing disease severity [25–27]. However, corticosteroids cause excessive weight gain, stunted growth, cataracts, osteoporosis, and hypertension [25–28]. Novel and more effective pharmacologic treatment strategies are desperately needed. Here we investigated the possibility that Debio-025 and prednisone would function more effectively in combination to reduce the overall muscular dystrophy phenotype and pathology in mdx mice. We show that Debio-025 reduced central nucleation, fibrosis, activated macrophage infiltration, and the percentage of the smallest myofibers better than prednisone. However, prednisone and Debio-025 together did not reduce disease better than Debio-025 alone in the mdx mouse model.
Materials and Methods
Mice
Male mdx mice (C57BL/10) and male control mice (C57BL/10) were obtained from The Jackson Laboratory (Bar Harbor, ME). mdx and control mice were divided into four treatment groups: vehicle, prednisone, Debio-025 (Debiopharm, Lausanne, Switzerland), and prednisone/Debio-025. We treated mdx and control mice beginning at 6 weeks of age. Debio-025 was administered at 80 mg/kg/day for gavage or 50 mg/kg/day for s.c. injection. Prednisone was given at 1 mg/kg once per day by gavage or subcutaneous injection. All studies lasted for 6 weeks. At the end of the treatment period all mice were sacrificed and muscle weights were recorded. Blood was collected at the end of all studies for analysis of creatine kinase (CK) levels using a standard assay within the clinical laboratory at Cincinnati Children’s Hospital. All mouse experimentation was approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital.
Histology
Muscles were fixed in 10% formalin buffered with phosphate overnight, and stored in 70% EtOH until processing. Processed muscles were cut longitudinally at the middle of the muscle and embedded into paraffin. Histological sections (7 μm) were stained with Masson’s trichrome or H&E. Approximately 600 fibers per mouse per muscle were quantified in a blinded manner for central nucleation, myofiber areas, and fibrosis with ImageJ and MetaMorph 6.1 software [29].
Immunohistochemistry
Paraffin embedded muscle tissue was cut in cross-section at the mid-belly of the muscle (7 μm thickness). Deparafinization of the slides was done following a series of incubation times in xylene, 100% EtOH, 95% EtOH, 70% EtOH, water, and 1X PBS. Antigen retrieval was done by microwave oven treatment in 1X Antigen Retrieval Citra solution (BioGenex, San Franscisco, CA). Sections were subsequently blocked in 1% BSA, 0.1% cold water fish gelatin, 0.1% Tween-20, 0.05% sodium azide in 1X PBS. Primary antibody for Mac-3 (BD Pharmingen, Franklin Lakes, NJ) was added at a dilution of 1:50 and incubated overnight in a humidified box at 4ºC. Goat anti-Rat green AlexaFlor secondary antibody (Invitrogen, Tåstrup, Denmark) was added at a dilution of 1:100 and incubated for 1 hour at room temperature. Slides were also stained with wheat germ agglutinin-TRITC (Sigma) (1:500) and TO-PRO-3 (Molecular Probes, Carlsbad, CA) (1:1000).
Grip strength functional assessment
Mice were assessed for average muscle performance by using a grip strength meter (Columbus Instruments, serial number 04513). Each mouse was measured at baseline (time 0) or after 6 weeks of treatment. Mice were allowed to grab a the metal grate that is attached to a force transducer (Digital Force Gauge, DF152) with their front paws only and pulled away from the bar by the tail. Five consecutive pulls were performed for each mouse with a few seconds of rest between each pull. Normalized grip strength (Kg) for each mouse was calculated by dividing absolute peak-force generated by the body weight (Kg), averaged across the five pulls.
Statistical Analysis
The results are presented as means ± s.e.m. We used a two way ANOVA for comparisons of all treatment groups for mdx and Wt mice with the Newman-Keuls post-hoc test (SigmaPlot). We considered values as significant when P < 0.05.
Results
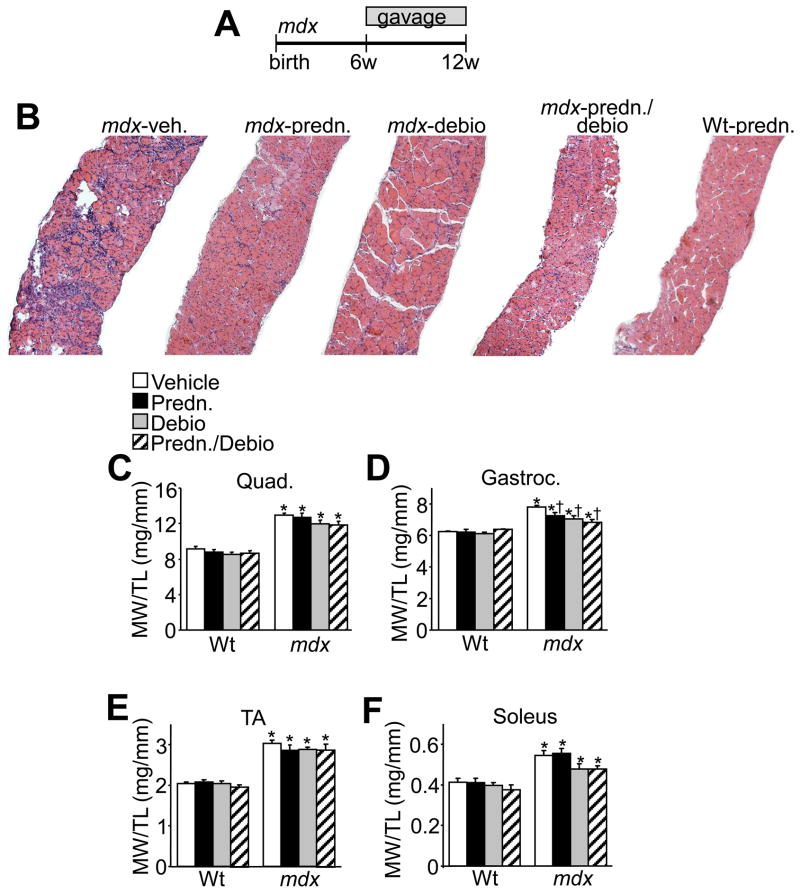
While we have previously shown that Debio-025 can reduce muscle pathology in mdx and Scgd−/− mice by s.c. injection [16], a number of critical questions remain to be addressed. For example, Debio-025 might have to be administered in combination with prednisone (current standard of care), and it is not clear how these agents would perform together or if one would be superior to the other, nor is it known if oral administration would be equally effective. Here we treated 6 week-old mdx mice for 6 weeks with Debio-025 by oral gavage, either alone or in combination with prednisone (Fig. 1A). At the end of the study, mice were sacrificed and the diaphragm, quadriceps, gastrocnemius, tibialis anterior, and soleus were isolated, weighed, and a full histological analysis was performed. As previously reported, mdx mice show pseudohypertrophy with increased muscle weights compared to wildtype (Wt) controls (Fig. 1C, D, E and F). Treatment of mdx mice with Debio-025, or the combination of prednisone/Debio-025 significantly reduced the weight of the gastrocnemius, although reductions in the weights of the quadriceps, tibialis anterior, and soleus only showed a trend (Fig. 1C, D, E and F). Prednisone alone also decreased the weight of the gastrocnemius, but none of the other 3 muscles examined (Fig. 1C, D, E and F). These results suggest that Debio-025 lessened the pseudohypertrophy in the gastrocnemius of 3-month old mdx mice but that co-treatment with prednisone was of no additive benefit (see discussion).
Fig. 1.
Treatment of mdx mice with Debio-025 improves pathology more than prednisone. (A) Schematic diagram of the experimental design for gavage administration of Debio-025 (80 mg/kg/day) and/or prednisone (1 mg/kg/day) to mdx and wildtype (Wt) control mice. (B) H&E staining of histological sections of diaphragm from mdx or Wt mice. (C) Muscle weights normalized to tibia length for quadriceps (Quad), (D) gastrocnemius (Gastroc.), (E) tibialis anterior (TA), (F) and soleus. *P < 0.05 versus Wt vehicle; †P < 0.05 vs mdx vehicle. The key above C applies to C–F. Error bars represent s.e.m. Six mdx and 4 Wt mice were analyzed for each panel.
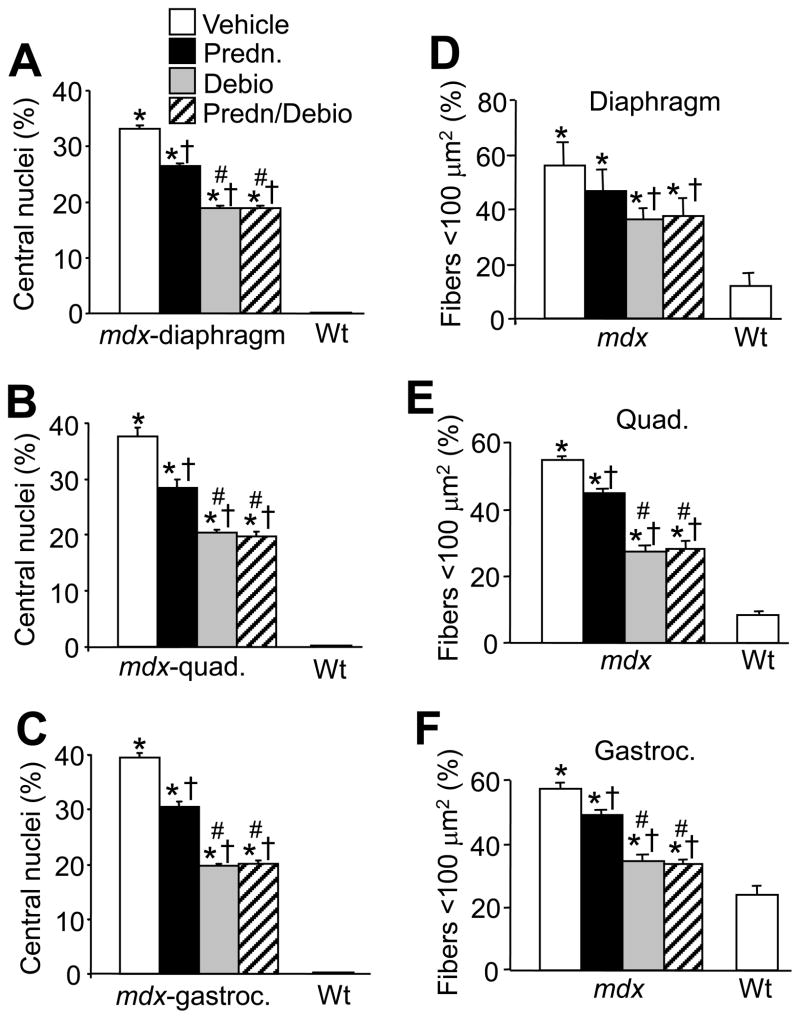
Extensive histological analysis of the diaphragm revealed substantial improvement in pathology due to Debio-025, prednisone, or Debio-025/prednisone together (Fig. 1B). The histological improvement in disease with these agents was rigorously quantified using multiple endpoints. For example, vehicle treated mdx muscle showed extensive centrally located nuclei in the diaphragm, quadriceps, and gastrocnemius, a hallmark feature of ongoing degeneration and regeneration of muscle fibers. Treatment of mdx mice with prednisone, Debio-025, and prednisone/Debio-025 together significantly decreased the amount of centrally located nuclei compared with vehicle treated mdx mice. (Fig. 2A, B and C). More importantly, Debio-025 significantly decreased the percentage of central nuclei compared with prednisone alone in mdx mice, although the combination of prednisone with Debio-025 was not significantly better than Debio-025 alone (Fig. 2A, B and C).
Fig. 2.
Debio-025 reduces pathology in mdx mice better than prednisone. (A, B, C) Percentage of myofibers containing centrally located nuclei from diaphragm, quadriceps, and gastrocnemius. (D–F) Percentage of myofibers with a cross-sectional area <100 μm2 from diaphragm, quadriceps, and gastrocnemius. The key in A also applies to B–F. *P < 0.05 vs Wt vehicle; †P < 0.05 vs mdx vehicle; #P < 0.05 vs mdx prednisone. Error bars represents s.e.m. Six mdx and 4 Wt mice were analyzed for each panel.
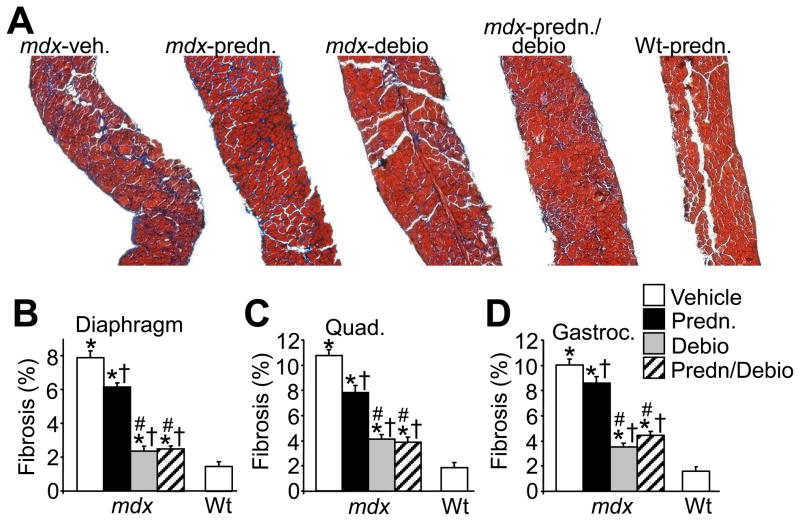
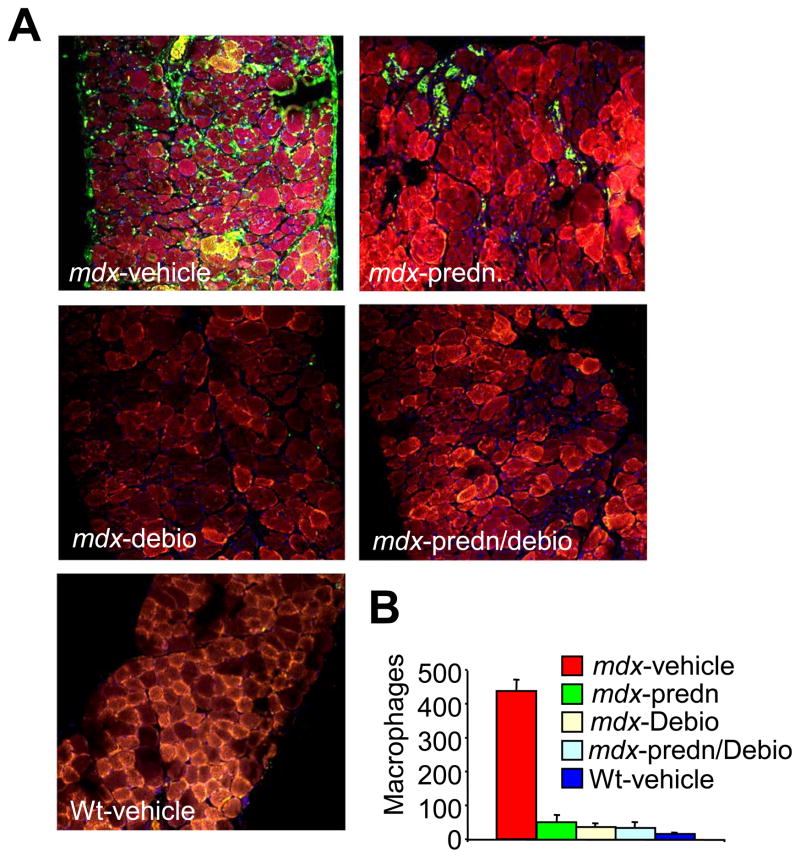
Another index of degenerating and regenerating muscle fibers is an increase in the percentage of smaller myofibers within a given muscle. For example, compared with Wt mice, vehicle treated mdx mice showed an increase in total fiber numbers with areas less than 100 μm2. (Fig. 2D, E and F). Treatment with prednisone, Debio-025, and prednisone/Debio-025 together reduced this effect, and once again, Debio-025 was significantly more effective than prednisone in all three muscles analyzed, although prednisone alone did not significantly affect the diaphragm (Fig. 2D, E and F). Consistent with these results, Debio-025, prednisone, and Debio-025/prednisone together significantly reduced the extent of fibrosis in the diaphragm, quadriceps, and gastrocnemius over the 6-week treatment period in mdx mice (Fig. 3A, B, C and D). Debio-025 was also superior to prednisone in reducing the extent of muscular fibrosis in histological sections stained with Masson’s trichrome (Fig. 3A, B, C and D). These results are also consistent with analysis of macrophage infiltration in dystrophic muscle, which is another hallmark of ongoing muscular degeneration and necrosis. A large significant influx of activated macrophages was observed in Mac-3 antibody-stained histological muscle sections in vehicle treated mdx mice, compared with essentially none in Wt control muscle (Fig. 4A and B). Treatment of mdx mice with prednisone showed a marked decrease in macrophage content, which was also significantly reduced with Debio-025 and prednisone/Debio-025 co-treatments (Fig. 4A and B). The same effect was observed with an antibody against CD-45, a marker for neutrophils (Data not shown). Taken together, these data indicate that while prednisone benefits pathology in skeletal muscle of mdx mice, Debio-025 treatment is significantly better. However, supplementing Debio-025 with prednisone did not provide additional benefit over Debio-025 alone in reducing skeletal muscle pathology in the mdx mouse.
Fig. 3.
Debio-025 reduces the amount of interstitial fibrosis in mdx mice better than prednisone. (A) Representative histological sections of diaphragm stained with Masson’s trichrome from mdx mice treated with vehicle, prednisone, Debio-025, or prednisone/Debio-025, as well as from prednisone treated Wt mice. (B, C, D) Quantification of fibrotic area from trichrome-stained sections of diaphragm, quadriceps, and gastrocnemius. *P < 0.05 vs Wt vehicle; †P < 0.05 vs mdx vehicle; #P < 0.05 vs mdx prednisone. The key in D also applies to B and C. Six mdx and 4 Wt mice were analyzed for each panel.
Fig. 4.
Debio-025 decreases inflammatory cell infiltration in mdx mice. (A) Mac-3 antibody staining (green) of mdx diaphragm treated with vehicle, prednisone (predn.), Debio-025 (debio), or prednisone/Debio-025 together, as well as vehicle treated Wt diaphragm. The outline of the fibers is shown in red with wheat germ agglutinin-TRITC while nuclei are shown in blue with TO-PRO-3. (B) Quantitation of macrophages per unit area from 3 different fields each for 6 mdx mice and 4 Wt mice.
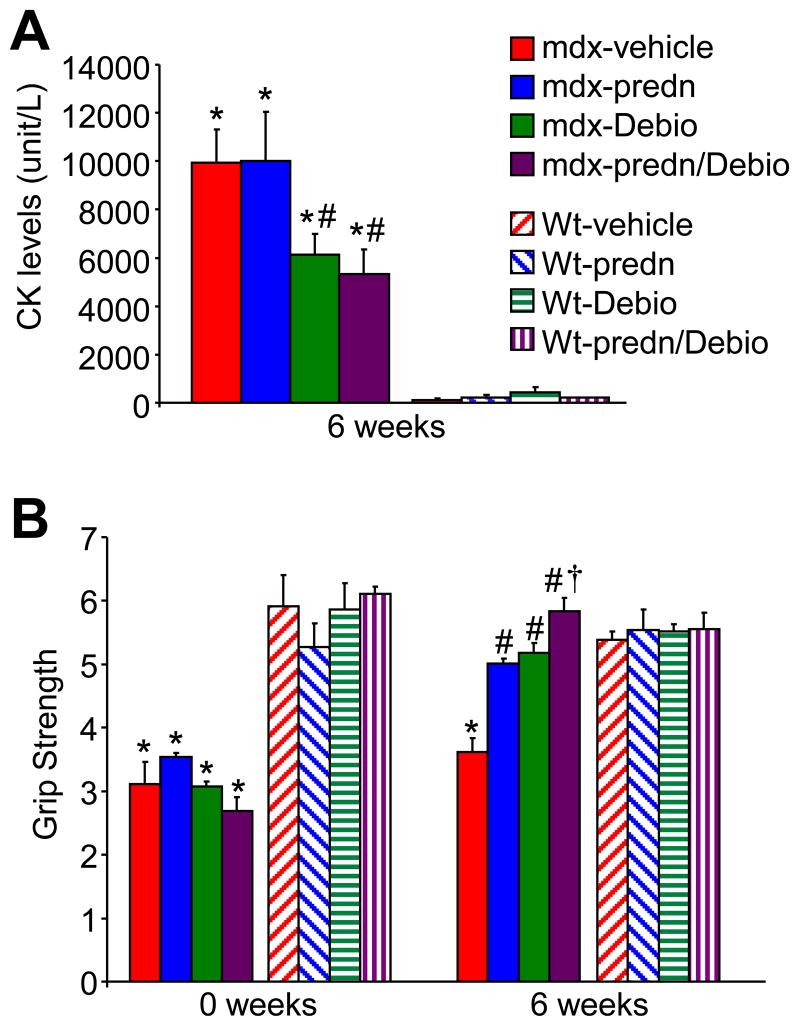
We also investigated other indexes of muscular dystrophy that are more routinely analyzed in the clinic. Blood was collected at the termination of the gavage treatment protocol for assessment of total CK release, which is suggestive of the extent of ongoing muscle degeneration and necrosis. Vehicle and prednisone treated mice showed extremely high blood CK levels compared to wildtype controls (Fig. 5A). However, Debio-025 and prednisone/Debio-025 combination treated mice each showed a significant reduction in total blood CK levels (Fig. 5A). We also assessed skeletal muscle function using a grip-strength assessment assay, which can reveal the extent of disease in the forelimb musculature. Six-week old wildtype and mdx mice were parsed into the 4 treatment groups and measured for grip strength before treatment, then again after 6 weeks of drug treatment (Fig. 5B). All mdx treatment groups showed significantly compromised forelimb grip strength compared to wildtype mice at the beginning of the study. While vehicle-treated mdx mice continued to show compromised grip strength after 6 additional weeks, the three drug treatment groups all showed a significant improvement in function, comparable to wildtype mice (Fig. 5B). Interestingly, and in contrast to all the histological data, co-treatment with prednisone/Debio-025 slightly but significantly increased grip strength better than Debio-025 alone or prednisone alone. These results suggest that prednisone and Debio-025 each improved muscle function in the mdx mice over 6 weeks of treatment.
Fig. 5.
Debio-025 improves muscle function and reduces CK release in mdx mice. (A) Serum creatine kinase (CK) levels in the blood of mdx and Wt treatment groups at the end of the 6-week treatment period. (B) Comparison of mdx and Wt grip-strength measurements between time point 0 and the end of the treatment period (6 weeks). *P < 0.05 vs Wt vehicle; #P < 0.05 vs mdx vehicle; † P < 0.05 vs mdx prednisone or Debio.
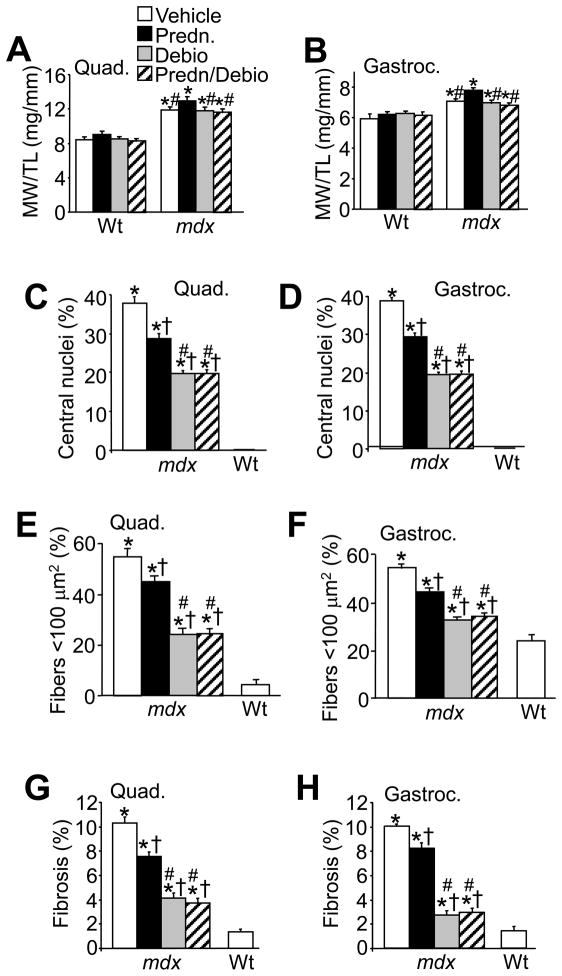
We felt it important to compare the efficacy of gavage versus s.c. injection. Thus, the entire study was repeated in mdx mice beginning at 6 weeks of age by s.c. injection for 6 weeks with vehicle, prednisone, Debio-025, and prednisone/Debio-025 together. Once again, we quantified muscle weights for pseudohypertrophy, central nucleation in histological muscle sections, fiber area variation, and extent of fibrosis (Fig. 6A, B, C, D, E, F, G and H). The results showed that s.c. injection with Debio-025 was equally effective to oral administration in reducing muscle pathology in mdx mice. While prednisone also significantly reduced these same indexes of skeletal muscle pathology, Debio-025 was significantly more effective than prednisone, and once again, the combination of prednisone/Debio-025 together was identical to Debio-025 alone (Fig. 6A, B, C, D, E, F, G and H). Thus, Debio-025 reduced muscle pathology to a greater extent than prednisone in mdx mice within an entirely independent study performed by s.c. injection.
Fig. 6.
Subcutaneous injection of Debio-025 reduces pathology in mdx mice better than prednisone. (A, B) Muscle weight (MW) normalized to tibia length (TL) for quadriceps and gastrocnemius treated with vehicle, prednisone (1 mg/kg/day), Debio-025 (50 mg/kg/day), or prednisone/Debio-025. (C, D) Percentage of fibers containing centrally located nuclei from quadriceps and gastrocnemius in the indicated treatment groups. (E, F) Percentage of myofibers with surfaces areas <100 μm2 from quadriceps and gastrocnemius in the indicated treatment groups. (G, H) Quantification of fibrotic area from trichrome stained sections of quadriceps and gastrocnemius in the indicated treatment groups. *P < 0.05 vs Wt vehicle; †P < 0.05 vs mdx vehicle; #P < 0.05 vs mdx prednisone. The key in A also applies to B–H. The error bars represent s.e.m. Five mdx and 3 Wt mice were assessed here for each panel.
Discussion
The current treatment of choice in the United States for Duchenne muscular dystrophy at most specialized care centers is early dosing with prednisone [26,27]. However, corticosteroids are only mildly effective and at the same time cause considerable side effects such as weight gain, osteoporosis, cataracts, hypertension and stunted growth [25–28]. Thus, more effective and less toxic pharmacologic agents are desperately needed for treating this disease. We recently identified Debio-025 as an alternative pharmacologic agent in reducing disease severity in 2 different mouse models of muscular dystrophy [16]. In the current study we extend our previous observations with Debio-025 in 3 important ways. First, we demonstrated that oral delivery is equally effective to s.c. injection in mitigating skeletal muscle pathology in mdx mice. Second, we showed that Debio-025 could be given in combination with prednisone without untoward effect. Third, we showed that Debio-025 is quantitatively more effective than prednisone in reducing skeletal muscle pathology in mdx mice.
Unfortunately, we did not observe a synergistic or an additive effect of prednisone/Debio-025 co-treatment in reducing skeletal muscle pathology. We were hopeful that these 2 agents might act at different molecular levels, so that combinatorial treatment would be even more effective than either one alone in attenuating disease. The exact mechanism of action for corticosteroids in reducing muscular dystrophy disease severity is currently a matter of debate. Prednisone and deflazacort are thought to function primarily as anti-inflammatory agents by reducing immune cell activity and secondary muscle wasting. However, corticosteriods are also known to enhance plasma membrane stability in dystrophic skeletal muscle fibers. This effect could reduce the extent of myofiber degeneration and necrosis, secondarily attenuating the ongoing inflammatory response in skeletal muscle given less cellular debris from dying myofibers [30–32]. This later mechanism of action for corticosteroids could explain why we failed to observe an additive effect on disease reduction between prednisone and Debio-025 together, as we have shown that Debio-025 also functions to reduce myofiber necrosis, resulting in less inflammatory cell recruitment [16]. Debio-025 desensitizes the mitochondria to permeability transition downstream of increases in Ca2+ associated with membrane instability and microtears [11,12]. This desensitization of the MPTP secondarily reduces the propensity of myofibers to undergo necrosis [16]. If prednisone indeed stabilizes the plasma membrane of dystrophic fibers it would lead to less Ca2+ influx and less induction of MPTP as a consequence. If true, these results further suggest that combinatorial treatment with prednisone and Debio-025 would not be more protective than either agent alone. However, other drugs that function at a different molecular level could still provide additive benefit with Debio-025, such as antifibrotic agents, agents that enhance regeneration, and agents that enhance muscle size and strength.
In the current study, mdx mice were treated once daily from 6 to 12 weeks of age. We selected this early time span given the mechanism of action of Debio-025, which simply reduces the initiation rate of myofiber necrosis [16]. We reasoned that such a mechanism of action would be most effective in a preventative manner, rather than employed late in the course of disease when many of the myofibers are lost and replaced with fatty and fibrotic tissue. Thus, agents like Debio-025 are likely to be most effective when employed early, such that disease is prevented rather than reversed. While we could certainly attempt to evaluate this hypothesis more directly by treating much older mdx mice with Debio-025, some support is offered by our previous study in 4-week old mdx mice. Millay et al. treated even younger mdx mice with Debio-025 for 6 weeks, demonstrating a pronounced prevention of pseudohypertrophy in all skeletal muscles examined [16]. Here we began treatment at 6-weeks of age when the pseudohypertrophy response had already begun, such that this disease manifestation was only significantly reduced in the gastrocnemius, but no other muscles examined. Reutenauer et al. also showed that Debio-025 partially improved the dystrophic phenotype in muscle from 3-week old mdx mice even with only 2-weeks of treatment [17]. These results suggest that Debio-025 might not be overly effective in reversing disease in dystrophic muscle once it begins in earnest, and that treatment should begin as early as possible.
Debio-025 has also shown a beneficial effect in other mouse models of muscular dystrophy. Millay et al. also treated Scgd−/− mice with Debio-025 and reported a similar decrease in dystrophic muscle pathology to that observed in mdx treated mice. Recently a new study using Col6a1−/− mice, a model of human Ullrich congenital muscular dystrophy and Bethlem myopathy, also showed that Debio-025 was effective and could prevent muscle cell death and histopathology [18]. Collectively, these studies suggest a much wider role for Debio-025 in the treatment of Duchenne, limb-girdle, and congenital muscular dystrophies, further supporting the hypothesis that a mitochondrial-dependent necrotic process is common to diverse dystrophies. A potential next step with Debio-025 would be to employ the Golden Retriever dog model of Duchenne muscular dystrophy or human clinical trials.
Acknowledgments
Supported in part by grants from the National Institutes of Health (J.D.M., J.R.), an award from the Jain Foundation (J.D.M.), and the Howard Hughes Medical Institute (J.D.M.). D.P.M. was supported through the Jain Foundation. E.R.W. was supported by a NIH training grant (#T32 HL007752).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 2.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biggar WD, Klamut HJ, Demacio PC, Stevens DJ, Ray PN. Duchenne muscular dystrophy: current knowledge, treatment, and future prospects. Clin Orthop Relat Res. 2002;401:88–106. doi: 10.1097/00003086-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- 5.Hopf FW, Turner PR, Steinhardt RA. Calcium misregulation and the pathogenesis of muscular dystrophy. Subcell Biochem. 2007;45:429–464. doi: 10.1007/978-1-4020-6191-2_16. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006;33:657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 7.Turner PR, Westwood T, Regen CM, Steinhardt RA. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988;335:735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- 8.Mallouk N, Jacquemond V, Allard B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc Natl Acad Sci U S A. 2000;97:4950–4955. doi: 10.1073/pnas.97.9.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Head SI. Membrane potential, resting Ca2+ and Ca2+ transients in isolated muscle fibres from normal and dystrophic mice. J Physiol. 1993;469:11–19. doi: 10.1113/jphysiol.1993.sp019801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gailly P, Boland B, Himpens B, Casteels R, Gillis JM. Critical evaluation of cytosolic Ca2+ determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium. 1993;14:473–483. doi: 10.1016/0143-4160(93)90006-r. [DOI] [PubMed] [Google Scholar]
- 11.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 12.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuinness O, Yafei N, Costi A, Crompton M. The presence of two classes of high-affinity cyclosporin A binding sites in mitochondria. Evidence that the minor component is involved in the opening of an inner-membrane Ca(2+)-dependent pore. Eur J Biochem. 1990;194:671–679. doi: 10.1111/j.1432-1033.1990.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 15.Hansson MJ, Mattiasson G, Månsson R, et al. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J Bioenerg Biomembr. 2004;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- 16.Millay DP, Sargent MA, Osinska H, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reutenauer J, Dorchies OM, Patthey-Vuadens O, Vuagniaux G, Ruegg UT. Investigation of Debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. Br J Pharmacol. 2008;155:574–584. doi: 10.1038/bjp.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiepolo T, Angelin A, Palma E, et al. The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1(−/−) myopathic mice. Br J Pharmacol. 2009;157:1045–1052. doi: 10.1111/j.1476-5381.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Luca A, Nico B, Liantonio A, et al. A multidisciplinary evaluation of the effectiveness of cyclosporine a in dystrophic mdx mice. Am J Pathol. 2005;166:477–489. doi: 10.1016/S0002-9440(10)62270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merlini L, Angelin A, Tiepolo T, et al. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc Natl Acad Sci U S A. 2008;105:5225–5229. doi: 10.1073/pnas.0800962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicks D, Lampe AK, Laval SH, et al. Cyclosporine A treatment for Ullrich congenital muscular dystrophy: a cellular study of mitochondrial dysfunction and its rescue. Brain. 2009;132:147–155. doi: 10.1093/brain/awn289. [DOI] [PubMed] [Google Scholar]
- 22.Sharma KR, Mynhier MA, Miller RG. Cyclosporine increases muscular force generation in Duchenne muscular dystrophy. Neurology. 1993;43:527–532. doi: 10.1212/wnl.43.3_part_1.527. [DOI] [PubMed] [Google Scholar]
- 23.Stupka N, Gregorevic P, Plant DR, Lynch GS. The calcineurin signal transduction pathway is essential for successful muscle regeneration in mdx dystrophic mice. Acta Neuropathol (Berl) 2004;107:299–310. doi: 10.1007/s00401-003-0807-x. [DOI] [PubMed] [Google Scholar]
- 24.Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149:657–66. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muntoni F, Fisher I, Morgan JE, Abraham D. Steroids in Duchenne muscular dystrophy: from clinical trials to genomic research. Neuromuscul Disord. 2002;12 (Suppl 1):S162–5. doi: 10.1016/s0960-8966(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 26.Angelini C. The role of corticosteroids in muscular dystrophy: a critical appraisal. Muscle Nerve. 2007;36:424–435. doi: 10.1002/mus.20812. [DOI] [PubMed] [Google Scholar]
- 27.Balaban B, Matthews DJ, Clayton GH, Carry T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil. 2005;84:843–850. doi: 10.1097/01.phm.0000184156.98671.d0. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi ML, Mazzanti A, Galbiati E, et al. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporos Int. 2003;14:761–767. doi: 10.1007/s00198-003-1443-y. [DOI] [PubMed] [Google Scholar]
- 29.Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol. 2006;168:1975–1985. doi: 10.2353/ajpath.2006.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs SC, Bootsma AL, Willems PW, Bär PR, Wokke JH. Prednisone can protect against exercise-induced muscle damage. J Neurol. 1996;243:410–416. doi: 10.1007/BF00869001. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JE, Weber M, Vargas C. Deflazacort increases laminin expression and myogenic repair, and induces early persistent functional gain in mdx mouse muscular dystrophy. Cell Transplant. 2000;9:551–564. doi: 10.1177/096368970000900411. [DOI] [PubMed] [Google Scholar]
- 32.St-Pierre SJ, Chakkalakal JV, Kolodziejczyk SM, Knudson JC, Jasmin BJ, Megeney LA. Glucocorticoid treatment alleviates dystrophic myofiber pathology by activation of the calcineurin/NF-AT pathway. FASEB J. 2004;18:1937–1939. doi: 10.1096/fj.04-1859fje. [DOI] [PubMed] [Google Scholar]