Abstract
Background
Aging-associated nitro-oxidative stress causes tissue injury and activates proinflammatory pathways that play an important role in the pathogenesis of aging-associated cardiovascular dysfunction. It has been recently reported, that the copper(II)–aspirinate complex (CuAsp) exerts not only the well-known anti-inflammatory and platelet antiaggregating effects of aspirin, but, due to its superoxide dismutase mimetic activity, it acts as a potent antioxidant as well. In this study we investigated the effects of CuAsp on aging-associated myocardial and endothelial dysfunction.
Methods and Results
Aging and young rats were treated for 3 weeks with vehicle, or with CuAsp (200 mg/kg per day per os). Left ventricular pressure–volume relations were measured by using a microtip pressure–volume conductance catheter, and indexes of contractility (e.g., slope of end-systolic pressure–volume relationships [ESPVR] [Ees], and dP/dtmax – end-diastolic volume [EDV]) were calculated. In organ bath experiments for isometric tension with isolated aortic rings, endothelium-dependent and -independent vasorelaxation were investigated by using acetylcholine and sodium nitroprusside. When compared to the young controls, aging rats showed impaired left ventricular contractility (Ees, 0.51 ± 0.04 vs. 2.16 ± 0.28 mmHg/μL; dP/dtmax – EDV, 10.71 ± 2.02 vs. 37.23 ± 4.18 mmHg/sec per μL; p < 0.05) and a marked endothelial dysfunction (maximal relaxation to acetylcholine: 66.66 ± 1.30 vs. 87.09 ± 1.35%; p < 0.05). Treatment with CuAsp resulted in reduced nitro-oxidative stress, improved cardiac function (Ees, 1.21 ± 0.17 vs. 0.51 ± 0.04 mmHg/μL; dP/dtmax – EDV, 23.40 ± 3.34 vs. 10.71 ± 2.02 mmHg/sec per μL; p < 0.05) and higher vasorelaxation to acetylcholine in aging animals (94.83 ± 0.73 vs. 66.66 ± 1.30%; p < 0.05). The treatment did not influence the cardiovascular functions of young rats.
Conclusions
Our results demonstrate that oxidative stress and inflammatory pathways contribute to the pathogenesis of cardiovascular dysfunction in the aging organism, which can be reversed by CuAsp.
Introduction
There is evidence that aging is a principal risk factor in the development of ischemic heart disease. This may be due to an age-associated increase in coronary vascular resistance, leading to reduced myocardial blood supply and flow reserve.1 Several studies have suggested that aging is associated with impaired endothelial function in laboratory animals2 and humans,3 and this endothelial dysfunction predisposes the aging organism to cardiovascular complications. Recent studies demonstrate that aging-associated cardiovascular dysfunction is related to the local formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS)4–7 and chronic inflammatory processes8 in the myocardium and coronary vasculature.
According to the “oxidative stress theory of aging,” age-related loss of physiological function and aging is caused by the deleterious effects of progressive and irreversible accumulation of nitro-oxidative damage. Aging organisms are exposed to continuous oxidative injury, due to the higher rate of superoxide production from the mitochondrial electron-transport chain.9,10 Nitro-oxidative stress (NiOxStr) (increases in ROS/RNS) at old age can elicit nitrosative-oxidative modifications of various cell components, such as lipids, proteins, and particularly DNA.11–13 A potent oxidant, peroxynitrite, is a major contributor to NiOxStr. It is formed by the reaction of superoxide and nitric oxide (NO) and has been shown to trigger DNA breakage, leading to the activation of the nuclear enzyme poly(ADP-ribose) polymerase (PARP). Excessive PARP activation results in rapid depletion of intracellular adenosine triphosphate (ATP) pools, leading to cellular dysfunction and death.14
The “molecular inflammation hypothesis of aging” illustrates that aging-associated increased NiOxStr results in the activation of redox-sensitive transcription factors (like nuclear factor-κB [NF-κB]) involved in inflammatory processes, thereby leading to a chronic low-grade proinflammatory state at the molecular level in the aging organism.15–17 NF-κB is known to regulate the expression of proinflammatory cytokines and enzymes, such as inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2). At old age, increased expression of iNOS results in unregulated generation of large amounts of NO, and thus enhanced peroxynitrite-formation,15 whereas elevated COX-2 levels trigger inflammatory cascades and contribute to ROS overproduction.18 These processes evoke a vicious cycle that aggravates NiOxStr and tissue injury associated with advanced aging.
Pharmacological attempts against NiOxStr using classic antioxidants (vitamin E, C, or glutathione) have resulted in conflicting results in experimental models of aging. On the basis of results of recent cardiovascular studies, pharmacological treatment with synthetic superoxide dismutase (SOD) mimetics,19 peroxynitrite decomposition catalysts,7 or PARP inhibitors20,21 emerge as novel antioxidant therapeutic possibilities for aging-associated cardiovascular dysfunction. Most recently, chronic aspirin supplementation has been shown to reduce aging-associated “molecular inflammation” and NiOxStr and improve vascular dysfunction at old age.22,23 It has been reported that coordination of aspirin to copper (synthesis of the copper(II)-aspirinate complex [CuAsp]) amplifies both the well-known anti-inflammatory24 and platelet antiaggregating effects25 of aspirin. Furthermore, CuAsp was observed to be a potent antioxidative compound in vitro and in vivo, presumably mainly due to its SOD-mimetic activity.26,27
To examine whether cardiovascular dysfunction at old age can be beneficially affected by simultaneously targeting both ROS and inflammatory pathways, this study investigated the effects of CuAsp on NiOxStr, inflammatory markers, left ventricular (LV) performance, and the vascular function of aortic rings in a rat model of aging-associated cardiovascular dysfunction.
Materials and Methods
Animals and treatment protocols
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). All procedures and handling of animals during the investigations were reviewed and approved by the local Ethical Committee for Animal Experimentation.
Young adult (3 months old, 200–250 grams) and aging (22 months old, 460–580 grams) Lewis rats (Charles River, Sulzfeld, Germany) were housed in a room at a constant temperature of 22 ± 2°C with 12-h light/dark cycles and fed a standard laboratory rat diet and water ad libitum.
Aging rats were treated for 3 weeks with vehicle (aging control group, n = 6) or with CuAsp-suspension per os as a daily gavage (200 mg/kg per day, a dose found to be effective in previous studies26) (aging treatment group, n = 7). Young rats treated for the same time with vehicle (young control group, n = 8), or with same dosed CuAsp-dosed (young treatment group, n = 8) rats were used as controls.
Hemodynamic measurements
Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (3 mg/kg) intraperitoneally (i.p.) and then tracheotomized and intubated to facilitate breathing. The animals were placed on controlled heating pads, and their core temperature was measured via a rectal probe and was maintained at 37°C. A polyethylene catheter was inserted into the left external jugular vein for fluid administration. A 2F microtip pressure–volume catheter (SPR-838, Millar Instruments, Houston, TX) was inserted into the right carotid artery and advanced into the ascending aorta. After stabilization, arterial blood pressure was recorded. After that, the catheter was advanced into the LV under pressure control, and LV pressure–volume analysis was performed as described previously.28 Briefly, after stabilization, the signals were recorded continuously at a sampling rate of 1000/sec using a pressure–volume conductance system (MPVS-400, Millar Instruments), stored, and displayed on a computer by the PowerLab Chart5 Software (ADInstruments, Colorado Springs, CO). With the help of a pressure–volume analysis program (PVAN, Millar Instruments) mean arterial pressure (MAP), maximal LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), maximal slope of systolic pressure increment (dP/dtmax), and time constant of LV pressure decay (Tau) were computed and calculated. LV pressure–volume relations were measured by transiently compressing the inferior vena cava under the liver using a cotton-tipped applicator. As load-independent indexes of LV contractility, the slope (Ees) of the curvilinear LV end-systolic pressure-volume relationships (ESPVR), preload recruitable stroke work (PRSW), and maximal slope of systolic pressure increment-end-diastolic volume relation (dP/dtmax – EDV) were calculated as described previously.28–30
At the end of each experiment, 100 μL of hypertonic saline was injected intravenously, and, from the shift of pressure–volume relations, parallel conductance volume was calculated by the software and used for correction for the cardiac mass volume. The volume calibration of the conductance system was performed as described previously.28
In vitro assessment of vascular function
After the hemodynamic measurements, the descending thoracic aorta was removed and placed in cold (+4°C) Krebs-Henseleit solution (KHS, 118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 1.77 mmol/L CaCl2, 25 mmol/L NaHCO3, 11.4 mmol/L glucose; pH 7.4). The aortae were prepared and cleaned from periadventitial tissues and cut transversely into 4-mm-width rings (n = 3 or 4 from each animal) using an operation microscope.
Isolated aortic rings were mounted on special hooks in individual organ baths (Radnoti Glass Technology, Monrovia, CA), containing 25 mL of KHS at 37°C and aerated with 95%O2 and 5%CO2. Special attention was paid during the preparation to avoid damaging the endothelium.
Isometric contractions were recorded using isometric force transducers (Radnoti Glass Technology), digitized, stored, and displayed with the IOX Software (EMKA Technologies, Paris, France).
The aortic rings were placed under a resting tension of 2 grams and equilibrated for 60 min. During this period, tension was periodically adjusted to the desired level and the KHS was changed every 30 min. Maximal contraction forces to potassium chloride (KCl; 100 mmol/L) were determined, and aortic rings were washed until resting tension was again obtained. Phenylephrine (PE; 10−6 mol/L) was used to precontract the rings until a stable plateau was reached. Relaxation responses were examined by adding cumulative concentrations of endothelium-dependent dilator acetylcholine (ACh; 10−9 to 10−4 mol/L) and endothelium-independent dilator sodium nitroprusside (SNP; 10−10 to 10−5 mol/L). Maximal forces of contraction (after subtraction of resting tension) are expressed as grams of tension; relaxation is expressed as percent of contraction induced by PE.
Immunohistochemistry
Myocardial and aortic wall sections of the rats were removed for immunohistochemical processing immediately after completing the LV pressure–volume analysis. The tissue samples were fixed in buffered paraformaldehyde solution (4%) and embedded in paraffin. Three adjacent sections were processed for each of the following types of immunohistochemical labeling. According to the methods previously described,31 we performed immunohistochemical staining for nitrotyrosine (NT) and for poly(ADP-ribose) (PAR) as markers of NiOxStr. Primary antibodies used for the stainings were polyclonal sheep anti-nitrotyrosine antibody (OXIS, Portland, OR) and mouse monoclonal anti-PAR antibody (Calbiochem, San Diego, CA).
On the basis of the intensity and distribution of labeling, semiquantitative histomorphological assessment of myocardial sections was performed by experimentators blinded to the treatment groups using conventional microscopy and the COLIM software (Pictron, Budapest, Hungary). On the basis of the staining intensity, specimens were coupled with intensity scores as follows: 0 = no positive staining, 1–2 = increasing degrees of intermediate staining, and 3 = extensive staining. According to the amount of positive stained cells, an area score was assigned (1 = < 10% positive cells, 2 = 11–50% positive cells, 3 = 51–80% positive cells, 4 = > 80% positive cells). Finally an average score (0–12) for the whole picture was calculated (intensity score × area score).
Myocardial RNA isolation and cDNA preparation
Frozen myocardial tissue samples (approximately 100 mg) were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA), and total RNA was isolated according to the protocol provided by the manufacturer. Briefly, insoluble material was removed after homogenization by centrifuging the samples at 12,000 × g for 10 min and an additional phenol-chloroform extraction step was introduced after the initial phase separation. RNA was precipitated from the aqueous phase using 0.8 volume of isopropanol and the nucleic acid pellet was repeatedly washed with 75% ethanol. RNA was dissolved in diethyl pyrocarbonate- (DEPC; Biomol, Hamburg, Germany) treated water and stored at −80°C. RNA was quantitated using Quant-IT RNA fluorometric assay and Qubit fluorometer (Invitrogen). RNA integrity was checked using formaldehyde agarose gel electrophoresis.
In a 100-μL reaction volume, 10 μg of total RNA was used for the reverse transcription reaction using a High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions. The reaction 100 U RNase inhibitor was included in the cDNA sysnthesis reaction to minimize RNA degradation. Reverse transcription reaction was run at 37°C for 120 min on an iCycler Thermal Cycler (Biorad, Hercules, CA), and cDNA samples were stored at −20°C.
Quantitation of transcipts with real-time PCR
TaqMan assays were performed to quantitate mRNA levels of COX-2, interleukin-1β (IL-1β), iNOS, transforming growth factor-β1 (TGF-β1). An internal control reaction targeting the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was run in multiplex with each reaction and used to normalize results for transcripts. Primers and Taq-Man probes for rat GAPDH (TaqMan Rodent GAPDH Control Reagents) were purchased from Applied Biosystems. All other primers and probes were purchased from Metabion International, (Martinsried, Germany). TaqMan probes were synthesized with a fluorescent 5′ reporter dye (FAM:6-carboxy-fluorescein) and a 3′ quencher dye (TAMRA:6-carboxy-tetramethyl-rhodamine). The primer and probe sequences are summarized in Table 1.
Table 1.
Primer and Probe Sequences Used in Real-Time PCR
| mRNA transcript | Forward primer | Reverse primer | TaqMan probe |
|---|---|---|---|
| COX-2 | 5′-AGT CTC TCA ATG AGT ACC GC-3′ | 5′-GCA GCC ATT TCT TTC TCT CC-3′ | 5′-AAC GAT GTG TAA GGT TTC AGG GAG AAG CG-3′ |
| IL-1β | 5′-TCA CAC ACT AGC AGG TCG TC-3′ | 5′-ACC CAA GCA CCT TCT TTT CC-3′ | 5′-TGA AGA AGA GCC CGT CCT CTG TGA CTC-3′ |
| iNOS | 5′-AAC TCG GGC ATA CCT TCA GG-3′ | 5′-TCG ATG TCA TGA GCA AAG GC-3′ | 5′-TAC ATG CTG GAG CCC AGG CCA AAT AC-3′ |
| TGF-β1 | 5′-CCC TGG ATA CCA ACT ACT GC-3′ | 5′-CCC AGG TCC TTC CTA AAG TC-3′ | 5′-GCT GCC GTA CAC AGC AGT TCT TCT CTG-3′ |
The PCR was carried out in duplicates in a reaction volume of 25 μL that consisted of a cDNA sample representing 200 ng of input total RNA, 0.625 U AmpliTaq Gold, 5.5 mmol/L MgCl2, 0.25 mmol/L each dNTP, 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), 0.01 mmol/L EDTA, 600 nmol/L passive reference dye (ROX) (TaqMan Buffer A, Applied Biosystems), 2.5 pmol of each of forward and reverse primers, and 5 pmol of each TaqMan probes. PCR amplification was performed with the following cycling conditions: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 58°C for 1 min on an Mx3005P real-time PCR system (Stratagene, La Jolla, CA). Amplification efficiency was determined using serial dilutions of pooled cDNA samples and used to calculate relative quantity of transcripts using MxPro Analysis software (Stratagene). The mRNA level for each transcript was determined by normalizing the signal for GAPDH level and averaging duplicate measurements.
iNOS immunoblotting
Frozen heart samples were homogenized (homogenization buffer: 42.5 mmol/L Tris [pH 7.4], 112.5 mmol/L NaCl, 1 mol/L urea, 1.5% sodium dodecyl sulfate [SDS], 0.75% NP-40, 0.4% sodium deoxycholate, 1 mmol/L phenylmethane-sulfonylfluoride, protease inhibitor cocktail [Complete Mini EDTA-free, Roche]). The homogenate was sonicated, and the lysate was cleared by centrifuging (14,000 rpm, 10 min). Protein concentration was determined by DC Protein Assay (BioRad), and samples normalized to total protein content were mixed with 4 × LDS sample buffer and dithiothreitol (Invitrogen) and heated to 70°C for 10 min. 20 μg of total protein was resolved on 4–12% NuPage Bis-Tris acrylamide gels (Invitrogen) and transferred to nitrocellulose. Membranes were blocked in 5% nonfat dried milk and probed overnight with anti-iNOS antibody (1:500, Millipore, Billerica, MA). Anti-rabbit-horseradish peroxidase conjugate (HRP; 1:500, Cell Signaling) and enhanced chemiluminescent substrate (ECL; Pierce) were used to detect the chemiluminescent signal in a CCD camera-based chemiluminescence detection system (Genegnome HR, Syngene). To normalize signals, membranes were stripped and reprobed with an antibody against α-tubulin (1:4000, Sigma). After the application of anti-mouse-HRP conjugate (1:4000, Cell Signaling) and ECL, chemiluminescence was detected with the same imaging system. The iNOS-signal (∼130 kDa) and the α-tubulin signal (∼50 kDa) were quantitated with Genetools software and their ratio was expressed as relative iNOS expression.
Statistical analysis
All data are expressed as means ± standard error of the mean (SEM). Intergroup comparisons were performed by using one-way analysis of variance (ANOVA) followed by a Student's unpaired t-test with Bonferroni correction for multiple comparisons. Differences were considered significant when p < 0.05.
Drugs
CuAsp (tetrakis[acetylsalicylato]-μ-dicopper[II], Cu2[asp]4) was provided by Prof. Hiromu Sakurai (Kyoto Pharmaceutical University, Kyoto, Japan). It was prepared as previously described26 and was suspended in 0.5% carboxymethyl cellulose solution vehicle. PE, ACh, and SNP (Sigma, Germany) were dissolved in normal saline.
Results
Cardiac function
In the aging control group, we found significantly decreased LVSP, dP/dtmax, and increased Tau when compared to the young controls. Aging rats treated with CuAsp showed a significant improvement of the systolic functional parameter dP/dtmax and a tendency toward higher LVSP and improved diastolic index Tau, without reaching the level of statistical significance. LVEDP did not differ in any groups studied (Fig. 1).
FIG. 1.
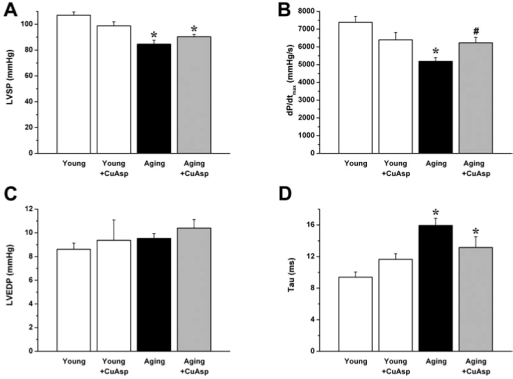
The effect of aging and CuAsp on systolic and diastolic cardiac function. LVSP (A), dP/dtmax (B), LVEDP (C), and Tau (D) are shown in young adult, young treated with CuAsp, aging, and aging treated with CuAsp rats. Values are mean ± SEM of six to eight experiments in each group. (*) p < 0.05 versus young control; (#) p < 0.05 vs. aging control.
When compared to the young control group, aging in rats was associated with significantly decreased LV contractility. The load-independent, pressure–volume loop derived contractility indexes (Ees, PRSW, dP/dtmax – EDV) showed a marked reduction in aging animals. After treatment with CuAsp, we observed a significant increase in these parameters, indicating the improvement of LV contractility (Fig. 2). CuAsp treatment in young rats had no substantial effect on any of the hemodynamic parameters studied (Figs. 1 and 2).
FIG. 2.
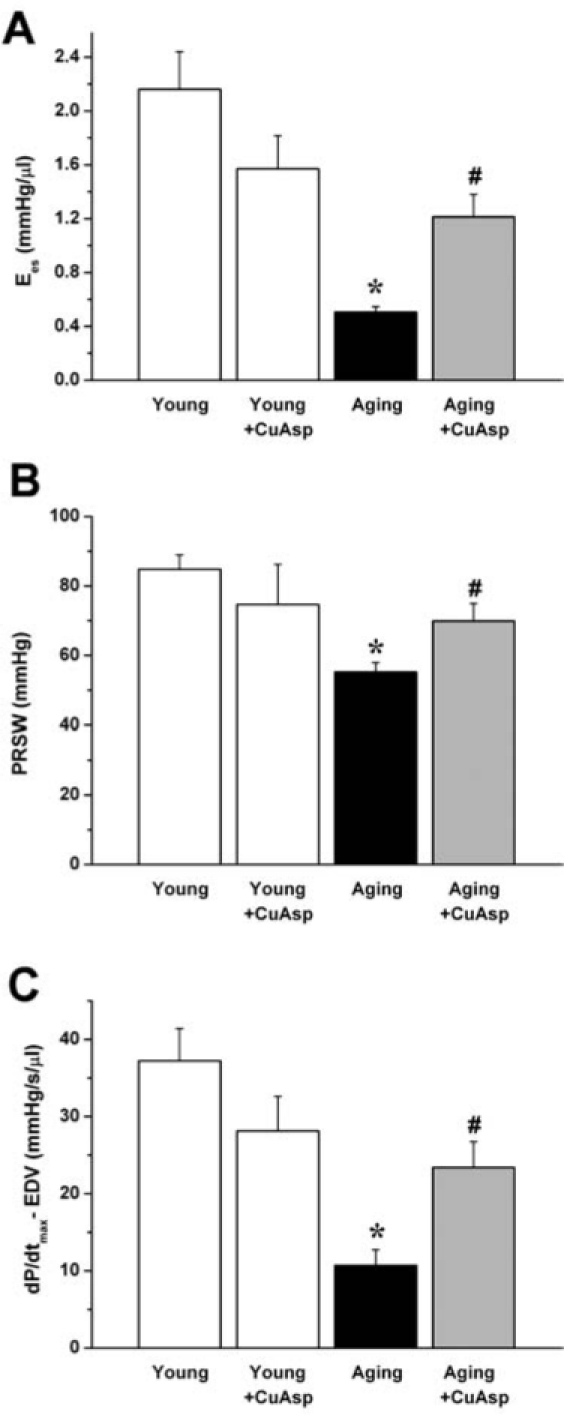
The effect of aging and CuAsp on left ventricular contractility. The slope (Ees) of the left ventricular ESPVR (A), PRSW (B), and dP/dtmax – EDV (C) are shown in young adult, young treated with CuAsp, aging, and aging treated with CuAsp rats. Values are mean ± SEM of six to eight experiments in each group. (*) p < 0.05 vs. young control; (#) p < 0.05 vs. aging control.
MAP was decreased in aging animals (55.5 ± 3.7 mmHg aging control vs. 77.2 ± 2.2 mmHg young control; p < 0.05), and it was not significantly altered by CuAsp treatment (60.4 ± 3.6 mmHg aging + CuAsp, 66.3 ± 4.1 mmHg young + CuAsp).
Vascular function
A marked impairment of endothelial function in aging rats was demonstrated in our in vitro organ bath experiments. The aging-associated endothelial dysfunction was indicated by the reduced relaxation of aortic rings to ACh (at ACh 10−4 mol/L, 64.2 ± 1.7% aging control vs. 86.4 ± 1.4% young control, p < 0.05). CuAsp treatment significantly improved the ACh-induced, endothelium-dependent, NO-mediated vasorelaxation in aging animals (at ACh 10−4 mol/L, 94.7 ± 0.8% aging treatment group vs. 64.2 ± 1.7% aging control, p < 0.05). The same treatment had no significant effect in young rats (Fig. 3).
FIG. 3.
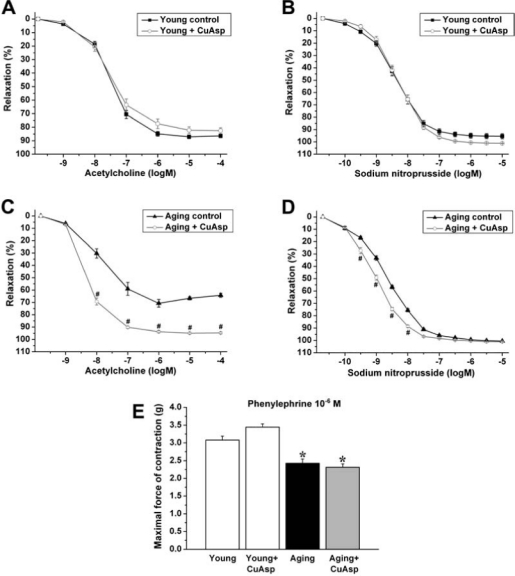
The effect of aging and CuAsp on vascular function of rat aortic rings. ACh-induced endothelium-dependent relaxation is shown in young adult, young treated with CuAsp (A), aging, and aging treated with CuAsp (C) rats. SNP-induced endothelium-independent relaxation is shown in young adult, young treated with CuAsp (B), aging, and aging treated with CuAsp (D) rats. Isometric contraction forces induced by PE (10−6 mol/L) are shown in each group (E). Each point of the curves and each column represent mean ± SEM of 21–32 experiments with thoracic aortic rings in all groups. (*) p < 0.05 vs. young control; (#) p < 0.05 vs. aging control.
The endothelium-independent vascular smooth muscle function indicated by the vasorelaxation to SNP was not impaired in aging rats and was only slightly enhanced by CuAsp treatment. The same treatment had no effect in young rats (Fig. 3).
Maximal isometric forces produced by aortic rings precontracted by PE (10−6 mol/L) were significantly lower in the aging control group as compared with young animals, which was not influenced by CuAsp treatment (Fig. 3).
Immunohistochemistry
Immunohistochemical staining showed increased immunoreactivity for NT and PAR, indicating increased NiOxStr, in the LV myocardium (Fig. 4) and aortic wall (Fig. 5) of aging rats, as evidenced also by higher myocardial NT and PAR scores, when compared with young animals (Fig. 4).
FIG. 4.
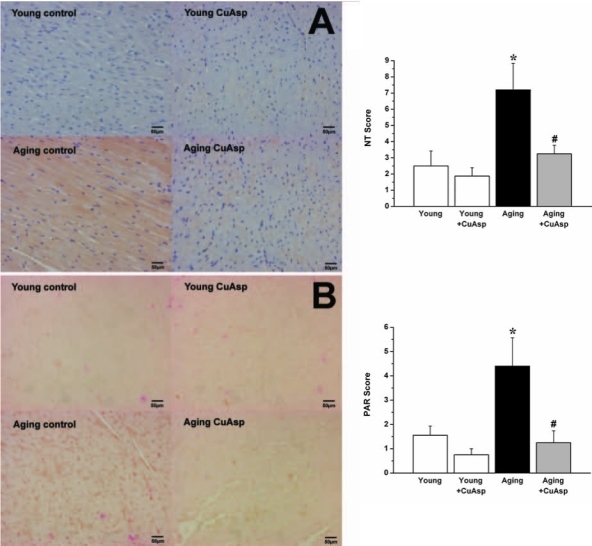
Photomicrographs and scoring of myocardial NT and PAR immunohistochemistry. Representative immunohistochemical stainings (left) and immunohistochemical scores (right) for NT (brown staining) (A) and for PAR (brown/black staining mainly in cell nuclei) (B) in the left ventricular myocardium of young adult, young treated with CuAsp, aging, and aging treated with CuAsp rats. Magnification, 400 × ; scale bar, 50 μm. Values are shown as mean ± SEM of six to eight experiments (each performed in three adjacent sections) in each group. (*) p < 0.05 versus young control; (#) p < 0.05 versus aging control.
FIG. 5.
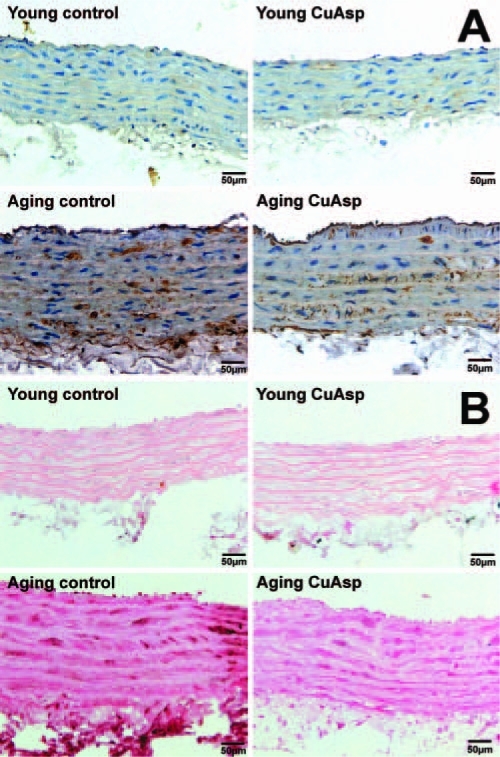
Photomicrographs of aortic NT and PAR immunohistochemistry. Representative immunohistochemical stainings for NT (brown staining) (A) and for PAR (dark brown/black staining mainly in cell nuclei) (B) in the thoracic aortic walls of young adult, young treated with CuAsp, aging, and aging treated with CuAsp rats. Magnification, 400×; scale bar, 50 μm.
Treatment with CuAsp in aging rats markedly reduced myocardial and aortic NT immunoreactivity and PAR formation, but did not have any effects in young rats (Figs. 4 and 5).
Quantitative real-time PCR
Quantitative real-time PCR from LV myocardial RNA extracts revealed that mRNA expression for COX-2 was increased in aging control rats over young controls. CuAsp treatment resulted in an increase in COX-2 mRNA expression both in young and aging rats (Fig. 6A). iNOS mRNA expression was slightly increased in the aging myocardium and showed a marked suppression after CuAsp treatment (Fig. 6B). mRNA expression for IL-1β and TGF-β1 showed no significant differences among the groups studied (data not shown).
FIG. 6.
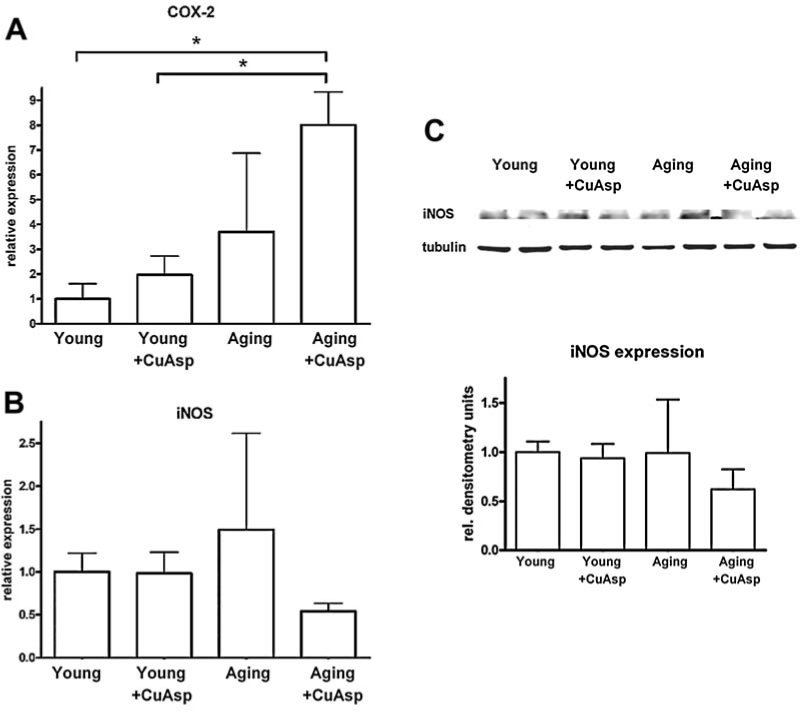
The effect of aging and CuAsp on the mRNA-expression for COX-2 and iNOS and on protein-expression for iNOS. Relative expression of mRNA for COX-2 (A) and iNOS (B) in the left ventricular myocardium of young adult, young treated with CuAsp, aging, and aging treated with CuAsp rats. Representative blot image (C, upper panel) of iNOS protein expression (detected at 130 kDa) and normalization signal α-tubulin (50 kDa) in the left ventricular myocardium of young adult, young treated with CuAsp, aging, and aging treated with CuAsp rats. Normalized densitometry units are shown as relative expression of iNOS (C, lower panel). Values are shown as mean ± SEM of four to eight experiments in each group. (*) p < 0.05.
iNOS immunoblotting
iNOS protein expression showed a suppression after CuAsp treatment in aging animals (Fig. 6C).
Discussion
In the present study, we have demonstrated that pharmacological treatment with the COX inhibitor and SOD-mimetic compound CuAsp remarkably reduces NiOxStr, improves LV myocardial contractility, and enhances endothelium-dependent vasorelaxation in a rat model of aging-associated cardiovascular dysfunction.
Recent studies have elucidated numerous cellular/molecular mechanisms responsible for the functional decline of the cardiovascular system at old age.13 The “oxidative stress hypothesis of aging” (“free radical theory,” as it was originally proposed)32 is one of the most favored explanations for how aging leads to progressive cellular damage at the biochemical level. According to this theory, age-related loss of physiological function and aging is caused by the deleterious effects of progressive and irreversible accumulation of oxidative damage. Previous studies have demonstrated that aging organisms have a higher rate of free-radical production (superoxide and H2O2) due to the incomplete terminal oxidation in the mitochondria at an older age.9,10,33 Large amounts of superoxide produced in aging tissues interact with the vascular mediator NO (derived from endothelial [e]NOS/iNOS), forming the potent oxidant peroxynitrite. Along with other NiOxStr-related free radicals and oxidants, peroxynitrite attacks biomolecules and cellular structures, thereby inducing lipid peroxidation, inactivating functionally important receptors and enzymes,5 and causing DNA injury. Peroxynitrite formation strongly reduces NO bioavailability by scavenging the vasorelaxant NO, whereas nitration of tyrosine by peroxynitrite inactivates among others the prostacyclin synthase, the manganese SOD (thereby aggravating NiOxStr), src kinases, mitochondrial complex I, and sarcoplasmic reticular Ca-ATPase, all resulting in cardiovascular dysregulation.5
DNA strand breaks induced by NiOxStr trigger the activation of PARP, which mediates the cellular response to DNA damage. Severe DNA injury causes excessive PARP activation that initiates intensive PAR formation in the nucleus. This process results in rapid depletion of intracellular ATP pools (energetic crisis) and cellular dysfunction, then to cell necrosis. In the aging cardiovascular system, these processes cause a functional impairment of contractile function at the cellular level and reduced ability of endothelial cells to produce NO when stimulated by endothelium-dependent relaxant agonists, like ACh. Impairment of endothelial function in the coronary arteries may lead to regional/global myocardial ischemia, which secondarily impairs cardiac performance.14,20,21
In this study, we demonstrated increased NT immunore-activity (evidence for the nitrating effect of peroxynitrite and NiOxStr) and for PAR in the LV myocardium and aortic wall of aging rats (Figs. 4 and 5), which confirm the intensive NiOxStr and the activation of the PARP pathway at old age and are consistent with previous studies5,13 and with the “oxidative stress theory” of aging discussed above. Oral treatment with the SOD-mimetic CuAsp in aging rats resulted in markedly reduced NiOxStr, as reflected by substantially lower NT and PAR immunoreactivity (Figs. 4 and 5). These results are in line with previous studies with other SOD-mimetic compounds in rat models of physiological aging.34
Recently, Chung et al. proposed the “molecular inflammation theory of aging,” showing that an age-related imbalance to the redox state due to NiOxStr activates redox-sensitive transcription factors involved in inflammatory processes. These transcription factors, such as NF-κB, are key regulators of activation of inflammatory genes (among others COX-2, iNOS, TGF-β1, IL-1β) during aging. Their enhanced activation leads to a chronic low-grade proinflammatory state on the molecular level in the aging organism.15,16,35 Correspondingly, we detected a tendency toward higher mRNA expression of COX-2 and iNOS in aging control rats (Fig. 6A,B). Whereas endothelial NO constitutively produced by eNOS appears to offer protective and beneficial effects in the vascular system, enhanced iNOS expression and the subsequent unregulated excessive NO production are associated with chronic inflammation and tissue injury. Most of the deleterious effects of iNOS-derived NO depend on its ability to react with superoxide and to form the strong oxidant peroxynitrite.15 This theory has been confirmed by the detected intensive immunoreactivity for NT, indicating increased peroxynitrite-formation in aging rats (Figs. 4 and 5). Although the main sources of ROS in aging tissues are supposed to be mitochondria, increased COX-2 expression at old age (as demonstrated in the present study) may contribute to the aging-associated ROS overproduction by oxygen radical generation during conversion of prostaglandin G2 to H2.18
CuAsp has a similar antiinflammatory spectrum, but more potent antiinflammatory activity than aspirin.24 Our results showing reduced NiOxStr (Figs. 4 and 5) and suppression of iNOS mRNA and protein expression in aging hearts after 3 weeks of CuAsp treatment (Fig. 6) presumably reflect the antiinflammatory effects of CuAsp; nevertheless, the same treatment resulted in a strong upregulation of COX-2 expression. The latter data might represent a strong compensatory response to chronic inhibition of prostanoid synthesis by CuAsp, similar to that described in the case of aspirin treatment.36
Endothelial dysfunction associated with advanced aging is a well-known phenomenon and can be explained by the reduced NO production of endothelial cells or by the increased NO inactivation by superoxide (peroxynitrite formation), resulting in altered NO bioavailability.5 The underlying intracellular pathways and molecular mechanisms have been subject of intensive investigations recent years.5,7,19,20,23 In accordance with these studies, we report here impaired endothelium-dependent ACh-induced relaxation of isolated aortic rings of aging rats (Fig. 3). The endothelium-independent relaxation induced by the exogenously administered NO donor SNP was not impaired by aging, indicating the normal dilative capacity of the vascular smooth muscle. In contrast with a previous work using epinephrine for precontraction,37 we found a significant decrease in contraction forces induced by PE in aging animals that was in line with our previous studies7,21 and may be due to alterations in receptor density and/or receptor/effector coupling. Similar to the effects of aspirin,23 the SOD-mimetic tempol,19 or catalytic peroxynitrite decomposition,7 CuAsp treatment significantly enhanced the endothelium-dependent vasorelaxations (i.e., improved the endothelial function) in rats with advanced aging, whereas endothelium-independent vasorelaxation was only slightly increased. Considering these functional changes and the observed reduced NT immunreactivity, we hypothesize that reduced NiOxStr and increased NO bioavailibility can be the main mechanism underlying these effects of CuAsp; however, alternative explanations are also theoretically possible. The vascular function of young rats remained unaffected by the complex (Fig. 3).
Previous studies performing invasive hemodynamic measurements in aging rats report decreased cardiac performance and development of progressive heart failure after the age of 20 months.20,28,38 A recent study provided detailed echocardiographic evidence of a progressive decrement in multiple aspects of systolic and diastolic LV function in aging rats.39 Consistent with these results, we demonstrated that aging is associated with impaired cardiac relaxation (as reflected by prolonged Tau) and a marked depression of systolic pressure development (as indicated by decreased MAP, LVSP, and depressed contractility index dP/dtmax). Although dP/dtmax has been used as a cardiac contractile parameter, it is well recognized that it is load dependent, especially on changes regarding preload.29 That is why other pressure–volume loop-derived indexes of LV contractility have been determined in our study. Ees, PRSW, and dP/dtmax – EDV are widely used as sensitive cardiac contractile parameters, because they are independent on changes in loading conditions and therefore especially informative in assessing cardiac contractility in models, where preload/afterload are altered.20,28 All these load-independent indexes of LV contractility, similar to the baseline index dP/dtmax, were significantly decreased in aging control rats, indicating severe contractile dysfunction (Figs. 1 and 2).
CuAsp treatment only moderately ameliorated the load-dependent contractility index dP/dtmax, whereas it improved all load-independent parameters of myocardial contractility remarkably (Figs. 1 and 2), indicating improved contractile function. LV diastolic dysfunction in aging animals, as indicated by Tau, has been slightly (not significantly) improved by CuAsp. Our cardiac functional data are also consistent with results of recent studies investigating the beneficial effects of the PARP inhibitors or peroxynitrite decomposition catalysts in age-related cardiac dysfunction.7,20,21 We found that CuAsp did not affect cardiovascular functions of young rats. Thus, the improved cardiovascular function seen in the aging treatment group is a specific phenomenon, reflecting a reversal of aging-associated suppressed cardiovascular performance rather than the consequence of nonspecific direct cardiac/vascular effects of CuAsp.
This is the first study reporting reduced NiOxStr and improvement of cardiovascular dysfunction associated with advanced aging by the SOD-mimetic and antiinflammatory complex CuAsp. The current findings indicate the importance of NiOxStr and inflammatory pathways in the pathogenesis of myocardial and endothelial dysfunction at old age. Our work further supports the concept that CuAsp, or other antiinflammatory compounds and potent SOD mimetics, may represent a potential therapy approach to improve cardiovascular dysfunction associated with aging.
Limitations
Because CuAsp contains the heavy metal ion copper, the possible toxicity of this compound (especially by chronic treatment) emerges as a valid danger. Although a previous study reported the high accumulation of copper in the livers of rats accompanied by reduction in the concentration of iron in different organs after 22 days of treatment with high-dose CuAsp,40 the preliminary chronic toxicity study of CuAsp41 found no remarkable histopathological changes in various rat tissues after 3 months of CuAsp treatment, with the exception of an increase of phagocytic Kupffer cells in the liver. Thus, further detailed toxicological investigations are needed.
With respect to human cardiovascular aging, the major limitation of our rodent model is that MAP, as reported also by other recent studies,7,20,21,28 falls with age, the opposite of what generally happens in humans. This phenomenon should be kept in mind, and further preclinical studies are necessary to elucidate the possible clinical benefit of this novel therapeutical approach.
Acknowledgments
This work was supported by a grant from the German Research Foundation (SFB 414) to G.S. and by the Hungarian Research Fund (T49488) and the National Institutes of Health (R01GM060915) to C.S.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hachamovitch R. Wicker P. Capasso JM. Anversa P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am J Physiol. 1989;256:H66–H73. doi: 10.1152/ajpheart.1989.256.1.H66. [DOI] [PubMed] [Google Scholar]
- 2.Tschudi MR. Barton M. Bersinger NA. Moreau P. Cosentino F. Noll G. Malinski T. Lüscher TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egashira K. Inou T. Hirooka Y. Kai H. Sugimachi M. Suzuki S. Kuga T. Urabe Y. Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Bejma J. Ramires P. Ji LL. Free radical generation and oxidative stress with aging and exercise: differential effects in the myocardium and liver. Acta Physiol Scand. 2000;169:343–351. doi: 10.1046/j.1365-201x.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 5.van der Loo B. Labugger R. Skepper JN. Bachschmid M. Kilo J. Powell JM. Palacios-Callender M. Erusalimsky JD. Quaschning T. Malinski T. Gygi D. Ullrich V. Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csiszar A. Ungvari Z. Edwards JG. Kaminski P. Wolin MS. Koller A. Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 7.Radovits T. Seres L. Gero D. Lin LN. Beller CJ. Chen SH. Zotkina J. Berger I. Groves JT. Szabó C. Szabó G. The peroxynitrite decomposition catalyst FP15 improves ageing-associated cardiac and vascular dysfunction. Mech Ageing Dev. 2007;128:173–181. doi: 10.1016/j.mad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Chung HY. Sung B. Jung KJ. Zou Y. Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 9.Sawada M. Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech Ageing Dev. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 10.Sohal RS. Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Aging Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- 11.Balaban RS. Nemoto S. Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Ungvári Z. Gupte SA. Recchia FA. Bátkai S. Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csiszar A. Pacher P. Kaley G. Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3:285–291. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virág L. Szabó E. Gergely P. Szabó C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140-141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- 15.Chung HY. Kim HJ. Kim KW. Choi JS. Yu BP. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech. 2002;59:264–272. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- 16.Chung HY. Kim HJ. Kim JW. Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann NY Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- 17.Vasto S. Candore G. Balistreri CR. Caruso M. Colonna-Romano G. Grimaldi MP. Listi F. Nuzzo D. Lio D. Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW. Baek BS. Kim YK. Herlihy JT. Ikeno Y. Yu BP. Chung HY. Gene expression of cyclooxygenase in the aging heart. J Gerontol A Biol Sci Med Sci. 2001;56:B350–B355. doi: 10.1093/gerona/56.8.b350. [DOI] [PubMed] [Google Scholar]
- 19.Tatchum-Talom R. Martin DS. Tempol improves vascular function in the mesenteric vascular bed of senescent rats. Can J Physiol Pharmacol. 2004;82:200–207. doi: 10.1139/y04-010. [DOI] [PubMed] [Google Scholar]
- 20.Pacher P. Vaslin A. Benko R. Mabley JG. Liaudet L. Haskó G. Marton A. Bátkai S. Kollai M. Szabó C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radovits T. Seres L. Gero D. Berger I. Szabó C. Karck M. Szabo G. Single dose treatment with PARP-inhibitor INO-1001 improves aging-associated cardiac and vascular dysfunction. Exp Gerontol. 2007;42:676–685. doi: 10.1016/j.exger.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung KJ. Kim JY. Zou Y. Kim YJ. Yu BP. Chung HY. Effect of short-term, low dose aspirin supplementation on the activation of pro-inflammatory NF-kappaB in aged rats. Mech Ageing Dev. 2006;127:223–230. doi: 10.1016/j.mad.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Bulckaen HG. Prevost G. Boulanger E. Robitaille G. Roquet V. Gaxatte C. Garcon G. Corman B. Gosset P. Shirali P. Creusy C. Puisieux F. Low-dose aspirin prevents age-related endothelial dysfunction in a mouse model of physiological aging. Am J Physiol Heart Circ Physiol. 2008;294:H1562–H1570. doi: 10.1152/ajpheart.00241.2007. [DOI] [PubMed] [Google Scholar]
- 24.Zhiqiang S. Lei WY. Li L. Chen ZH. Liu WP. Coordination of copper with aspirin improves its anti-inflammatory activity. Inflammopharmacology. 1998;6:357–362. doi: 10.1007/s10787-998-0018-0. [DOI] [PubMed] [Google Scholar]
- 25.Shen ZQ. Liang Y. Chen ZH. Liu WP. Duan L. Effects of copper-aspirin complex on platelet aggregation and thrombosis in rabbits and mice. J Pharm Pharmacol. 1998;50:1275–1279. doi: 10.1111/j.2042-7158.1998.tb03345.x. [DOI] [PubMed] [Google Scholar]
- 26.Fujimori T. Yamada S. Yasui H. Sakurai H. In Y. Ishida T. Orally active antioxidative copper(II) aspirinate: synthesis, structure characterization, superoxide scavenging activity, and in vitro and in vivo antioxidative evaluations. J Biol Inorg Chem. 2005;10:831–841. doi: 10.1007/s00775-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 27.Fujimori T. Yasui H. Hiromura M. Sakurai H. Suppressive effect of orally administered copper(II)-aspirinate (Cu2(asp)4) complex on the generation of reactive oxygen species in the skin of animals subjected to UVA exposure. Exp Dermatol. 2007;16:746–752. doi: 10.1111/j.1600-0625.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 28.Pacher P. Mabley JG. Liaudet L. Evgenov OV. Marton A. Haskó G. Kollai M. Szabó C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2132–H2137. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kass DA. Maughan WL. Guo ZM. Kono A. Sunagawa K. Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- 30.Sato T. Shishido T. Kawada T. Miyano H. Miyashita H. Inagaki M. Sugimachi M. Sunagawa K. ESPVR of in situ rat left ventricle shows contractility-dependent curvilinearity. Am J Physiol. 1998;274:H1429–H1434. doi: 10.1152/ajpheart.1998.274.5.H1429. [DOI] [PubMed] [Google Scholar]
- 31.Liaudet L. Soriano FG. Szabó E. Virag L. Mabley JG. Salzman AL. Szabó C. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc Natl Acad Sci USA. 2000;97:10203–10208. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 33.Kim JD. McCarter RJ. Yu BP. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging (Milano) 1996;8:123–129. doi: 10.1007/BF03339566. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y. Armstrong SJ. Arenas IA. Pehowich DJ. Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol. 2004;287:H165–H171. doi: 10.1152/ajpheart.00037.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ungvari Z. Orosz Z. Labinskyy N. Rivera A. Xiangmin Z. Smith K. Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 36.Davies NM. Sharkey KA. Asfaha S. Macnaughton WK. Wallace JL. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment Pharmacol Ther. 1997;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 37.Pacher P. Mabley JG. Soriano FG. Liaudet L. Komjáti K. Szabó C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anversa P. Puntillo E. Nikitin P. Olivetti G. Capasso JM. Sonnenblick EH. Effects of age on mechanical and structural properties of myocardium of Fischer 344 rats. Am J Physiol. 1989;256:H1440–H1449. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- 39.Boluyt MO. Converso K. Hwang HS. Mikkor A. Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 40.Kishore V. Effects of copper aspirinate and aspirin on tissue copper, zinc, and iron concentrations following chronic oral treatment in the adjuvant arthritic rat. Biol Trace Elem Res. 1990;25:123–135. doi: 10.1007/BF02990273. [DOI] [PubMed] [Google Scholar]
- 41.Sorenson RJ. Rolniak MT. Preliminary chronic toxicity study of copper aspirinate. Inorganica Chim Acta. 1984;91:L31–L34. [Google Scholar]


