Abstract
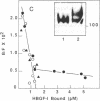
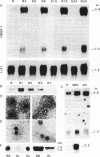
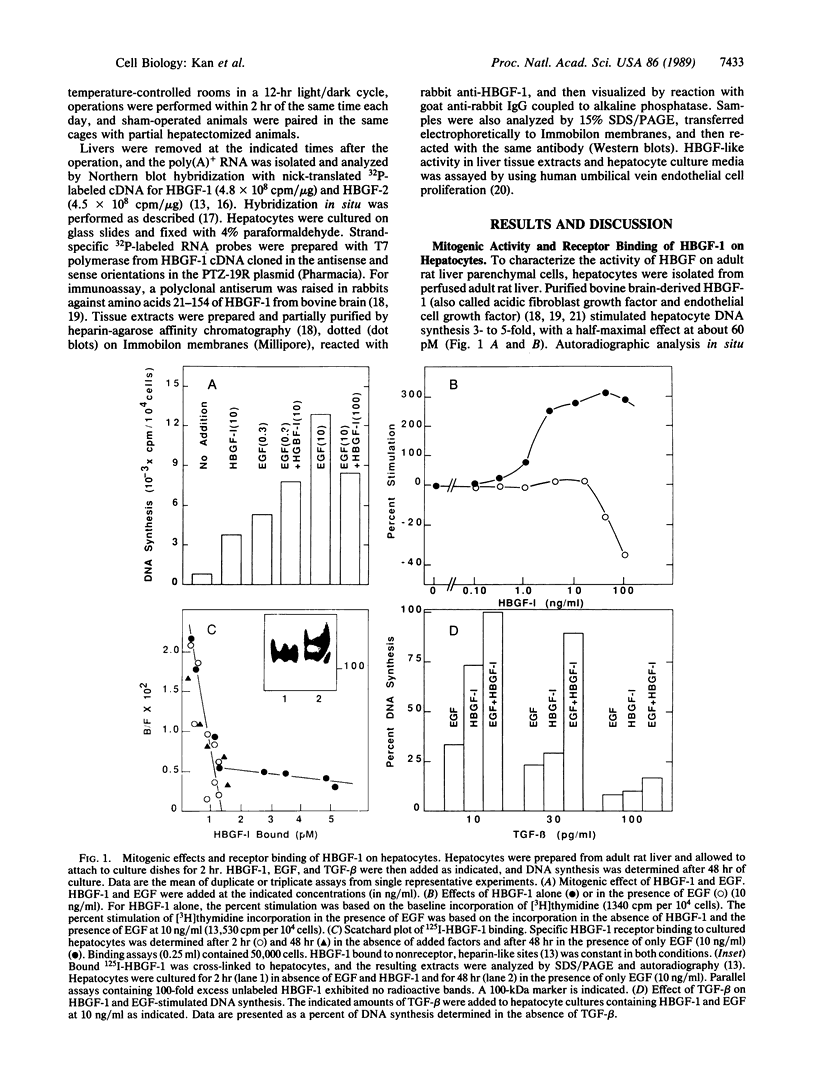
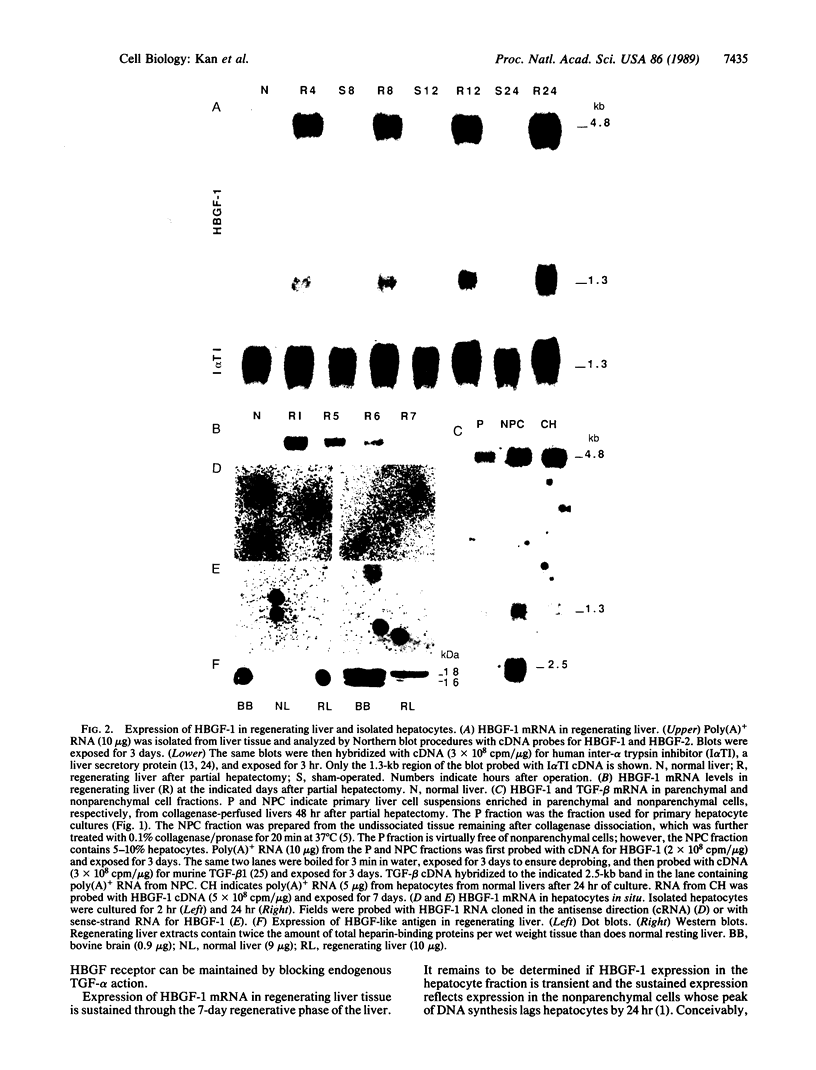
Heparin-binding growth factor type 1 (HBGF-1; sometimes termed acidic fibroblast growth factor) is potentially an important factor in liver regeneration. HBGF-1 alone (half-maximal effect at 60 pM) stimulated hepatocyte DNA synthesis and bound to a high-affinity receptor (Kd = 62 pM; 5000 per cell). Epidermal growth factor (EGF) neutralized or masked the mitogenic effect of HBGF-1 concurrent with appearance of low-affinity HBGF-1 binding sites. HBGF-1 reduced the inhibitory effect of transforming growth factor type beta (TGF-beta) on the EGF stimulus. Nanomolar levels of HBGF-1 decreased the EGF stimulus. An increase in hepatic HBGF-1 gene expression after partial hepatectomy precedes increases in expression of the EGF homolog, TGF-alpha, and nonparenchymal-cell-derived TGF-beta in the regenerating liver. Expression of HBGF-1 mRNA occurs in both hepatocytes and nonparenchymal cells and persists for 7 days in liver tissue after partial hepatectomy. HBGF-1 acting through a high-affinity receptor is a candidate for the early autocrine stimulus that drives hepatocyte DNA synthesis prior to or concurrent with the EGF/TGF-alpha stimulus. It may allow hepatocyte proliferation to proceed in the presence of low levels of TGF-beta. An EGF/TGF-alpha-dependent change in HBGF-1 receptor phenotype and increasing levels of nonparenchymal-cell-derived HBGF-1 and TGF-beta may serve to limit hepatocyte proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr B. I., Hayashi I., Branum E. L., Moses H. L. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986 May;46(5):2330–2334. [PubMed] [Google Scholar]
- Carr B. I., Huang T. H., Itakura K., Noël M., Marceau N. TGF beta gene transcription in normal and neoplastic liver growth. J Cell Biochem. 1989 Apr;39(4):477–487. doi: 10.1002/jcb.240390413. [DOI] [PubMed] [Google Scholar]
- Crabb J. W., Armes L. G., Carr S. A., Johnson C. M., Roberts G. D., Bordoli R. S., McKeehan W. L. Complete primary structure of prostatropin, a prostate epithelial cell growth factor. Biochemistry. 1986 Sep 9;25(18):4988–4993. doi: 10.1021/bi00366a003. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [PubMed] [Google Scholar]
- Fausto N., Mead J. E., Braun L., Thompson N. L., Panzica M., Goyette M., Bell G. I., Shank P. R. Proto-oncogene expression and growth factors during liver regeneration. Symp Fundam Cancer Res. 1986;39:69–86. [PubMed] [Google Scholar]
- GRISHAM J. W. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962 Aug;22:842–849. [PubMed] [Google Scholar]
- Hoshi H., Kan M., Chen J. K., Mckeehan W. L. Comparative endocrinology-paracrinology-autocrinology of human adult large vessel endothelial and smooth muscle cells. In Vitro Cell Dev Biol. 1988 Apr;24(4):309–320. doi: 10.1007/BF02628833. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Kan M., DiSorbo D., Hou J. Z., Hoshi H., Mansson P. E., McKeehan W. L. High and low affinity binding of heparin-binding growth factor to a 130-kDa receptor correlates with stimulation and inhibition of growth of a differentiated human hepatoma cell. J Biol Chem. 1988 Aug 15;263(23):11306–11313. [PubMed] [Google Scholar]
- Koch K. S., Shapiro P., Skelly H., Leffert H. L. Rat hepatocyte proliferation is stimulated by insulin-like peptides in defined medium. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1054–1060. doi: 10.1016/0006-291x(82)92046-0. [DOI] [PubMed] [Google Scholar]
- Mansson P. E., Adams P., Kan M., McKeehan W. L. Heparin-binding growth factor gene expression and receptor characteristics in normal rat prostate and two transplantable rat prostate tumors. Cancer Res. 1989 May 1;49(9):2485–2494. [PubMed] [Google Scholar]
- McKeehan W. L., Adams P. S. Heparin-binding growth factor/prostatropin attenuates inhibition of rat prostate tumor epithelial cell growth by transforming growth factor type beta. In Vitro Cell Dev Biol. 1988 Mar;24(3):243–246. doi: 10.1007/BF02623554. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Crabb J. W. Isolation and characterization of different molecular and chromatographic forms of heparin-binding growth factor 1 from bovine brain. Anal Biochem. 1987 Aug 1;164(2):563–569. doi: 10.1016/0003-2697(87)90534-3. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A. Calcium, magnesium, and serum factors in multiplication of normal and transformed human lung fibroblasts. In Vitro. 1980 Jun;16(6):475–485. doi: 10.1007/BF02626460. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Sakagami Y., Hoshi H., McKeehan K. A. Two apparent human endothelial cell growth factors from human hepatoma cells are tumor-associated proteinase inhibitors. J Biol Chem. 1986 Apr 25;261(12):5378–5383. [PubMed] [Google Scholar]
- McMahon J. B., Richards W. L., del Campo A. A., Song M. K., Thorgeirsson S. S. Differential effects of transforming growth factor-beta on proliferation of normal and malignant rat liver epithelial cells in culture. Cancer Res. 1986 Sep;46(9):4665–4671. [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Hirai R., Yamaoka K., Kaji K., Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- Richman R. A., Claus T. H., Pilkis S. J., Friedman D. L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. E., Coffey R. J., Jr, Ouellette A. J., Moses H. L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. E. Transforming growth factor beta (TGF-beta) inhibits hepatocyte DNA synthesis independently of EGF binding and EGF receptor autophosphorylation. J Cell Physiol. 1988 May;135(2):253–261. doi: 10.1002/jcp.1041350212. [DOI] [PubMed] [Google Scholar]