Abstract
Since osteoclasts are terminally differentiated cells without proliferating activity, efficient and stable gene expression into these cells remains a difficulty. In the current study, we investigate gene transduction into human preosteoclasts by a replication defective lentivirus-based vector containing a modified HIV-1 genome. Human preosteoclasts (differentiating osteoclasts) were transduced with lentiviruses bearing an enhanced green fluorescent protein (EFGP) reporter gene. Transduction efficiencies were measured by flow cytometry for EGFP protein expression. Sorted human transduced preosteoclasts were replated and differentiated under human macrophage colony-stimulating factor and human receptor activator of NF-κB ligand. Mature osteoclasts were then analyzed by the cell viability assay, TRACP assay, and pit formation assay. Efficient gene transduction was obtained at multiplicity of infection of 10, and gene expression lasted for over 4 weeks using our protocol. Lentiviral transduction did not affect osteoclast survival, formation, or function. These results establish an efficient method for gene transduction into human preosteoclasts using a lentiviral vector. Importantly, these transduced preosteoclasts could differentiate into mature osteoclasts without a negative impact from the lentiviruses. This protocol provides a new tool for studies of osteoclast biology. Further work in this area may open new avenues for the study of osteoclast gene signaling and gene therapy of disorders of osteoclast function.
Introduction
Bone remodeling is a dynamic and lifelong process to maintain skeletal strength and integrity. It is a balance between old bone resorbed from the skeleton by osteoclasts and new bone formed by osteoblasts. Osteoclasts are formed by fusion of promonocytic precursors, which are large, multinucleated, and motile. Since osteoclasts are terminally differentiated cells without proliferating activity, efficient and stable gene expression into these cells remains an obstacle to advances in osteoclast biology. Osteoclasts are virtually resistant to conventional nonviral transfection methods because they are fragile and undergo apoptosis rapidly when challenged chemically or mechanically (Laitala-Leinonen, 2005). Although a recent study (Taylor et al., 2007) has shown that nucleofector technology (modified electroporation method) could be a useful gene transfection method, the cell mortality is very high (over 50% from Taylor et al., 2007, and over 80% from our unpublished data). In addition, adenovirus-mediated gene expression has efficiently transduced foreign genes into osteoclasts (Tanaka et al., 1998; Fukuda et al., 2005; Kobayashi et al., 2005); however, it remains difficult to achieve long-term stable expression. This is mainly because adenovirus does not integrate into genomic DNA, but stays as an episome in the nucleus. Retrovirus-mediated gene transduction has been successfully used in osteoclasts (Shin et al., 2005; Humphrey et al., 2006). HIV-1–based lentiviral vectors provide particularly attractive vehicles for gene delivery to osteoclasts since they efficiently transduce both dividing and nondividing cells, and mediate sustained transgene expression (Naldini et al., 1996a). Thus, this delivery system is an important tool in the study of osteoclast biology and may also be useful in preclinical development of a range of potential gene therapies in the bone field.
In the current study, we investigate gene transduction into human preosteoclasts by a replication defective lentivirus-based vector containing a modified HIV-1 genome. We sought to determine if this vector could efficiently introduce genes into human preosteoclasts with sustained gene expression. We also studied whether gene expression delivered by lentiviruses affects mature osteoclast viability, differentiation, or function.
Materials and Methods
Human osteoclast cell culture
Human osteoclasts were generated from human peripheral blood. The Indiana University Institutional Review Board approved the research protocol. All subjects were given written informed consent prior to participating in the study. Methods for differentiating osteoclast cell culture were described previously (Chu et al., 2006). In brief, 50 mL of peripheral blood was obtained by venepuncture from each subject. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (GE Healthcare/Amersham, Piscataway, NJ) sedimentation. Monocytes were enriched using the Human Monocyte Isolation Kit II (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instruction. Monocytes were washed in PBS and plated on six-well plates at a density of 3 × 106 per well at 37°C in DMEM, supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 25 ng/mL of hM-CSF (human macrophage colony-stimulating factor), and 25 ng/mL of hRANKL (human receptor activator of NF-κB ligand). According to the previous investigation (Chu et al., 2006), the various doses of hRANKL did not affect osteoclast formation. After 6 days of treatment with hM-CSF and hRANKL, monocytes start to differentiate to preosteoclasts with monocyte-like morphology, which are easily detached by 0.25% Trypsin-EDTA. In our hands, both preosteoclasts and mature osteoclasts can be treated with trypsin and detached without physical damage. The trypsinized preosteoclasts can then be replated and continue further differentiation. The mature osteoclasts can reattach to both dentine and plastic well surface.
Lentiviral vector production and transduction
A self-inactivating lentiviral transfer vector, pcDNA-CS-CGW (Fig. 1), expressing the enhanced green fluorescent protein (EGFP), was kindly provided by Dr. Philip Zoltick (Children's Hospital of Pennsylvania, Philadelphia, PA). The third-generation lentiviral packaging system (Dull et al., 1998) includes pMDL for expression of gag-pol genes, pMDG for expression of VSV-G envelope glycoprotein, and pRSV-Rev for expression of viral rev gene, and was provided by Cell Genesys (South San Francisco, CA). Lentiviral particles were produced by transient transfection of 293T cells using the calcium phosphate transfection method. In brief, on day 0, 293T cells were plated at 5×106 per T75 flask. On day 1, the cell culture medium was changed. Four hours later, calcium phosphate transfection was performed according to the manufacturer's instructions (Promega, Madison, WI). On day 2, about 15 h after transfection, cell culture medium was replaced with the fresh medium. Vector-containing supernatants were harvested 48 h after transfection, filtered through a 0.45 μm syringe filter, and then stored at −80°C. Infection titers were determined as previously described (Kahl et al., 2004).
FIG. 1.
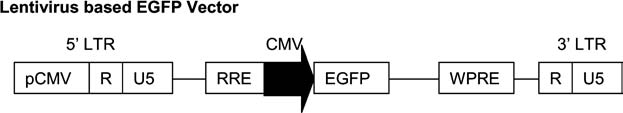
Schematic drawing of the fluorescence lentivirus-based EGFP vector pcDNA-CS-CGW. EGFP: enhanced green fluorescent protein; LTR: long terminal repeat; RRE: rev-responsive element; WPRE: woodchuck postregulatory element.
Gene transduction and flow cytometry analysis
Gene transduction was performed on preosteoclasts, which were differentiating under hRANKL and hM-CSF for 6 days. The cells were then washed with PBS to remove serum and subsequently incubated with EGFP viral supernatant supplemented with 8 μg/mL Polybrene® at 37°C. After 4h, the medium was replaced by fresh medium containing hRANKL and hM-CSF.
To analyze the percentage of cells expressing EGFP, the preosteoclast cells or mature osteoclasts were lifted from plates by 0.25% Trypsin-EDTA (at this stage, preosteoclasts are still monocyte-like) and fixed overnight in 1% para-formaldehyde/PBS. Then fixed cells were subjected to flow cytometry on a FACStarPlus or FACS Vantage SE (Becton Dickinson Immunocytometry Systems, San Jose, CA) by the flow cytometry facility at Indiana University School of Medicine.
To sort EGFP-positive cells, the transduced preosteoclasts were detached and washed with PBS. About 1 × 106 per mL cells in 10% BSA/PBS medium were subjected to flow cytometry. Five thousand EGFP-positive osteoclasts were sorted and collected in a single well of 96-well plate with or without dentine slices in triplicate.
Cell viability assay
Cell viability was evaluated using the Cell-Titer assay from Promega (#G5421) according to the manufacturer's instructions. This is a colorimetric method for determining the number of viable cells. It measures dehydrogenase activity, a marker of cell metabolic activity. In brief, 100 μL of PMS solution was mixed with 2.0 mL of MTS solution. Twenty μL of the combined MTS/PMS solution was pipetted into each well of the 96-well assay plate containing 100 μL of cells in culture medium. The plate was incubated for 2 h at 37°C in a humidified, 5% carbon dioxide atmosphere. Then the absorbance at 490 nm was recorded using ELISA plate reader.
TRACP assay
TRACP assays were performed as previously described (Chu et al., 2006). In brief, osteoclasts were fixed in 4% (wt/vol) glutaraldehyde in PBS and stained using the Leukocyte Acid Phosphatase 387-A Kit (Sigma Chemical, St Louis, MO) as recommended by the manufacturer. TRACP-positive cells were visualized by light microscopy with a Zeiss Axiovert 40 microscope (Oberkochen, Germany), and the images were taken with a Zeiss AxioCam MRc5. The number of TRACP-positive cells was scored by the light microscopic visual inspection, and TRACP-positive cells with three or more nuclei were scored as positive.
Pit formation assay
The pit formation assay has been described previously (Chu et al., 2006). In brief, osteoclasts on dentine slices were removed by bleach. Dentine slices were fixed with 4% glutaraldehyde in 0.2 M sodium cacodylate solution for 30min, followed by staining with 1% toluidine blue in 0.5% sodium tetraborate solution for 3 min. Resorption lacunae were identified by light microscopy. The area of the resorption, defined as regions of contiguous resorption pits, was assessed by AvioVision AC software (Zeiss).
Statistical analysis
Data were analyzed using the unpaired Student's t-test for analysis of two groups. Results were expressed as mean ± SD. p < 0.05 was considered statistically significant.
Results
Lentivirus delivery system induced effective and long-term gene expression
Human osteoclasts were differentiated from monocytes, which are isolated from the peripheral blood, under the stimulation of hM-CSF and hRANKL (Chu et al., 2006). We tested an HIV-1-based lentiviral vector for its ability to transduce preosteoclasts (differentiating osteoclasts) in culture at multiplicities of infection (ratio of virus to cell) of 0, 5, 10, and 15. On day 6, preosteoclasts were infected with EGFP-expressing lentiviruses. The percentage of EGFP-expressing cells was determined at 3 and 14 days posttransduction using flow cytometry. As shown in Table 1, EGFP started to be expressed in human preosteoclasts as early as day 3 posttransduction at a multiplicity of infection as low as 5. The percentage of EGFP-expressing cells increased as multiplicity of infection was increased, and was sustained for 14 days.
Table 1.
Lentivirus Infection: Percentage of GFP Expression
| Multiplicity of infection (MOI) | 0 | 5 | 10 | 15 |
|---|---|---|---|---|
| 3 days (%) | 0 | 25.2 | 31.2 | 30.4 |
| 14 days (%) | 2.2 | 25.1 | 24.6 | 33.8 |
To further enhance gene transduction into human preosteoclasts, we established and tested a repeated-transduction protocol. We hypothesized that initial efficiency of gene transduction of the human preosteoclasts would be significantly elevated by repeated virus infection. Therefore, we infected preosteoclasts at both days 6 and 7 at multiplicity of infection of 10 by using a single transduction at day 6 as control. Strikingly, a 67% transduction rate was achieved by this protocol compared to a single transduction (45% transduction rate). Moreover, we also evaluated EGFP expression by fluorescence microscopy at 26 days posttransduction. As shown in Figure 2, the mature osteoclasts strongly expressed EGFP at almost 4 weeks posttransduction, demonstrating that lentivirus-mediated gene expression in human osteoclasts is both efficient and stable.
FIG. 2.
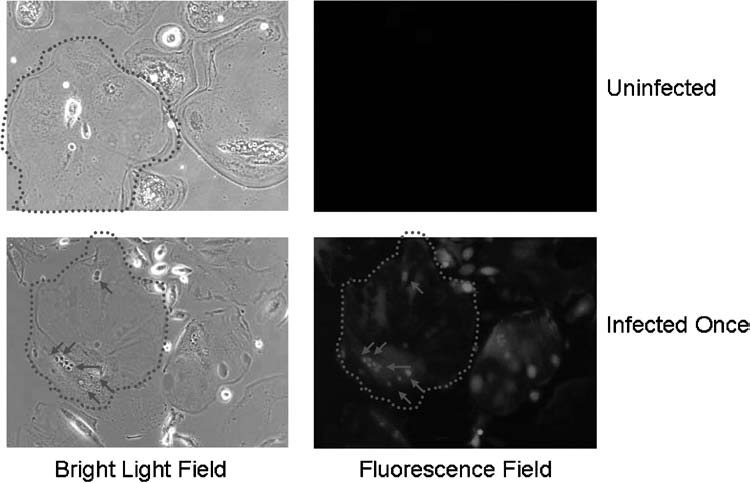
Lentiviruses mediated gene expression in human osteoclasts. Human osteoclasts were uninfected/infected by EGFP lentiviruses on day 6. Images were taken on 26 days posttransduction under bright light field and green fluorescence field. Dotted lines outline the matured osteoclasts. Arrows point to the nuclei.
Lentiviral vector–mediated gene transduction has no effect on osteoclast viability, differentiation, or activity
To determine if lentivirus gene transduction affects human osteoclast viability, we assayed cell viability by Cell-Titer assay for dehydrogenase activity. The preosteoclasts were infected by EGFP lentiviruses on only day 6 or both days 6 and 7. Ten days posttransduction, 5000 EGFP-expressing preosteoclasts were sorted in a single well of a 96-well plate, and the Cell-Titer assays were performed 21 days post-transduction when osteoclasts matured. There was no significant change in mature osteoclast viability between cells of uninfected, infected once, and infected twice (Fig. 3).
FIG. 3.
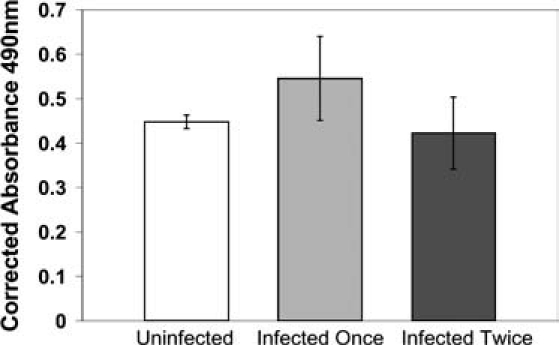
Viability of EGFP lentivirus-transduced human osteoclasts. Uninfected cells (control) were compared to the cells infected once (day 6) and infected twice (both days 6 and 7). EGFP-transduced cells were sorted at 10 days posttransduction. The cell viability assay was performed at 21 days post-transduction. The means and standard deviations were from three experiments.
Next, we assessed whether lentivirus gene transduction could affect human osteoclast differentiation by TRACP assay. Preosteoclasts were infected by EGFP lentiviruses on only day 6 or both days 6 and 7. Ten days posttransduction, 5000 EGFP-expressing preosteoclasts were sorted on a dentine slice in a single well of a 96-well plate, and continuously cultured in hRANKL and hM-CSF medium. As assessed by TRACP assay, mature osteoclasts of uninfected, infected once, or infected twice osteoclasts exhibited comparable numbers of TRACP-positive cells at 21 days posttransduction (Fig. 4).
FIG. 4.
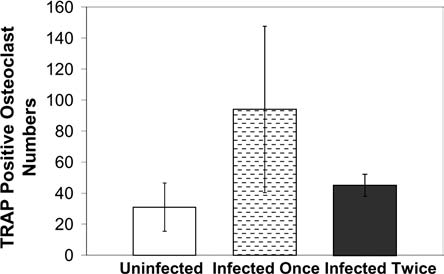
Differentiation of EGFP lentivirus-transduced human preosteoclasts. Uninfected cells (control) were compared to the cells infected once (day 6) and infected twice (both days 6 and 7). EGFP-transduced cells were sorted at 10 days post-transduction. The TRACP assay was performed at 21 days posttransduction. The means and standard deviations were from three experiments.
To further evaluate whether lentivirus transduction alters mature osteoclast function, pit formation assays were performed after the TRACP assays on the same dentine slices. Pit formation data were adjusted by the numbers of the TRACP-positive cells on the dentine slices. Although lentivirus-transduced osteoclasts resorbed bone at a slightly higher rate than untransduced cells, these differences were not statistically significant (Fig. 5). These data indicate that gene transduction by this HIV-1–based lentivirus delivery system did not affect human osteoclast differentiation or function.
FIG. 5.
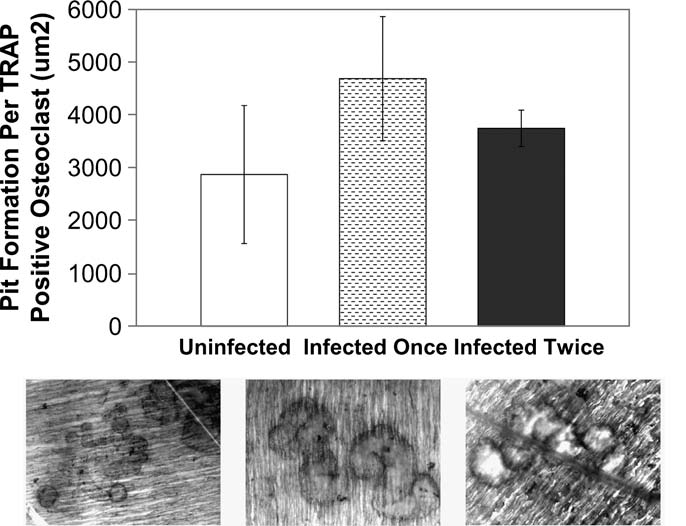
The pit formation of EGFP lentivirus-transduced human osteoclasts. Uninfected cells (control) were compared to the cells infected once (day 6) and infected twice (both days 6 and 7). EGFP-transduced cells were sorted at 10 days post-transduction. The pit formation assay was performed at 21 days posttransduction. The means and standard deviations were from three experiments.
Discussion
Osteoclasts play essential roles not only in normal skeletal development but also in pathological bone destruction such as that occurs in osteoporosis, rheumatoid arthritis, and bone metastasis. However, it has been difficult to transduce osteoclasts. This difficulty has hampered further understanding of the molecular events involved in osteoclast differentiation and activation, and also limited the development of potential gene therapy strategies. The aim of this study was to systematically investigate the ability of an HIV-1–based lentivirus gene delivery system to induce stable gene expression in human preosteoclasts. We recognized that differentiating human osteoclasts from peripheral monocytes created a mixture of monocytes, preosteoclasts, and mature osteoclasts with multinucleus. In our hands, the majority of this mixture is mature osteoclasts after 2 weeks differentiating under hM-CSF and hRANKL. Therefore, we describe here a reproducible and simple method for highly efficient and stable transduction of human preosteoclasts. This method results in a gene transduction rate of over 65% and expression that lasts at least 4 weeks, representing a major improvement over previously published methods on osteoclasts (Laitala-Leinonen, 2005).
Our results in osteoclasts are in good agreement with results obtained in other nondividing cells (Naldini et al., 1996b; Blomer et al., 1997; Lever, 2000; Amado and Chen, 2002; Watson et al., 2002). Although sustained knockdown of gene expression in the RAW246.7 osteoclast-like cell line has been reported using lentivirus-delivered RNA interference strategy, this is the first example of use of this technique in human osteoclasts (Humphrey et al., 2006). Additionally, the previous study did not provide information about long-term cell viability, formation, or function. Our data demonstrate that lentiviral-transduced human preosteoclasts had normal cell survival, osteoclast differentiation, and function. Importantly, these transduced preosteoclasts could differentiate into mature osteoclasts without a negative impact from lentiviruses. Collectively, these findings bode well for the future use of lentivirus-transduced preosteoclasts.
Lentivirus vectors derived from the HIV-1 are promising tools for gene therapy. They have several advantages over other vectors. First, they are able to infect nonproliferating cells such as hematopoietic stem cells, neurons, and muscle cells (Naldini et al., 1996b; Blomer et al., 1997; Lever, 2000; Amado and Chen, 2002; Watson et al., 2002). Therefore, osteoclasts (as nonproliferating cells) are ideal targets for the lentiviral vectors. Second, they can be generated to high titers without immunological complications, which could compromise transduced cell viability (Lever et al., 2004). Vector-related immunogenicity is a well-known problem with adenovirus vector and adeno-associated viral vector. Third, lentiviral vectors have an 8–10 kb transgene capacity. They have the ability to translocate across an intact nuclear membrane and integrate into the genome of nonproliferating cells and reside or progress through at least the G1b state of the cell cycle (Korin and Zack, 1998), contrasting to the other retroviral vectors based on murine retroviruses, which require passage of the cell through mitosis in order to integrate (Miller et al., 1990; Roe et al., 1993; Lewis and Emerman, 1994). In addition, they have evolved to efficiently replicate in human cells.
In conclusion, we have developed a simple method for highly efficient and stable gene expression into human preosteoclasts by using an HIV-1–based lentivirus gene delivery system. We have demonstrated that lentiviral infection did not alter osteoclast cell viability, differentiation, or function. These findings provide fundamental knowledge about lentiviral-gene-transduced human osteoclasts, which offers a new tool for the study of gene expression, signaling transduction, or gene knockdown in human osteoclast biology. Further work in this area may open a new avenue for gene therapy of disorders of osteoclast dysfunction.
Acknowledgments
We would like to thank the Indiana University Vector Production Facility, a designated NIH National Gene Vector Laboratory, for providing us the space and the technical assistant in lentiviral vector production and transduction. Technician Aparna Jasti helped to make lentivirus supernatant and with the RCL assay. We are grateful to Dr. Rachelle J.S. Galvin for discussion and review of the manuscript. This work was supported by National Institutes of Health grants R01 AR-43476 and M01 RR-00750.
References
- Amado R.G. Chen I.S.Y. Lentiviral vectors for gene therapy of HIV-induced disease. Curr Top Microbiol Immunol. 2002;261:229–243. doi: 10.1007/978-3-642-56114-6_12. [DOI] [PubMed] [Google Scholar]
- Blomer U. Naldini L. Kafri T. Trono D. Verma I.M. Gage F.H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K. Snyder R. Econs M.J. Disease status in autosomal dominant osteopetrosis type 2 is determined by osteoclastic properties. J Bone Miner Res. 2006;21:1089–1097. doi: 10.1359/jbmr.060409. [DOI] [PubMed] [Google Scholar]
- Dull T. Zufferey R. Kelly M. Mandel R.J. Nguyen M. Trono D. Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A. Hikita A. Wakeyama H. Akiyama T. Oda H. Nakamura K. Tanaka S. Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1. J Bone Miner Res. 2005;20:2245–2253. doi: 10.1359/JBMR.050816. [DOI] [PubMed] [Google Scholar]
- Humphrey M.B. Daws M.R. Spusta S.C. Niemi E.C. Torchia J.A. Lanier L.L. Seaman W.E. Nakamura M.C. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J Bone Miner Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- Kahl C.A. Marsh J. Fyffe J. Sanders D.A. Cornetta K. Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J Virol. 2004;78:1421–1430. doi: 10.1128/JVI.78.3.1421-1430.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. Take I. Yamashita T. Mizoguchi T. Ninomiya T. Hattori T. Kurihara S. Ozawa H. Udagawa N. Takahashi N. Prostaglandin E2 receptors EP2 and EP4 are down-regulated during differentiation of mouse osteoclasts from their precursors. J Biol Chem. 2005;280:24035–24042. doi: 10.1074/jbc.M500926200. [DOI] [PubMed] [Google Scholar]
- Korin Y.D. Zack J.A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitala-Leinonen T. Unsatisfactory gene transfer into bone-resorbing osteoclasts with liposomal transfection systems. J Negat Results Biomed. 2005;4:5. doi: 10.1186/1477-5751-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever A.M. Lentiviral vectors: progress and potential. Curr Opin Mol Ther. 2000;2:488–496. [PubMed] [Google Scholar]
- Lever A.M.L. Strappe P.M. Zhao J. Lentiviral vectors. J Biomed Sci. 2004;11:439–449. doi: 10.1007/BF02256092. [DOI] [PubMed] [Google Scholar]
- Lewis P.F. Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.G. Adam M.A. Miller A.D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. (Erratum appears in Mol Cell Biol 1992 Jan;12(1):433.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L. Blomer U. Gage F.H. Trono D. Verma I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996a;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L. Blomer U. Gallay P. Ory D. Mulligan R. Gage F.H. Verma I.M. Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector [see comment] Science. 1996b;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Roe T. Reynolds T.C. Yu G. Brown P.O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C.S. Her S.-J. Kim J.-A. Kim D.H. Kim S.W. Kim S.Y. Kim H.-S. Park K.H. Kim J.G. Kitazawa R. Cheng S.-L. Civitelli R. Dominant negative N-cadherin inhibits osteoclast differentiation by interfering with beta-catenin regulation of RANKL, independent of cell-cell adhesion. J Bone Miner Res. 2005;20:2200–2212. doi: 10.1359/JBMR.050809. [DOI] [PubMed] [Google Scholar]
- Tanaka S. Takahashi T. Takayanagi H. Miyazaki T. Oda H. Nakamura K. Hirai H. Kurokawa T. Modulation of osteoclast function by adenovirus vector-induced epidermal growth factor receptor. J Bone Miner Res. 1998;13:1714–1720. doi: 10.1359/jbmr.1998.13.11.1714. [DOI] [PubMed] [Google Scholar]
- Taylor A. Rogers M.J. Tosh D. Coxon F.P. A novel method for efficient generation of transfected human osteoclasts. Calcif Tissue Int. 2007;80:132–136. doi: 10.1007/s00223-006-0245-6. [DOI] [PubMed] [Google Scholar]
- Watson D.J. Kobinger G.P. Passini M.A. Wilson J.M. Wolfe J.H. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther. 2002;5:528–537. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]


