Abstract
This randomized, controlled, within-subjects (crossover design) study examined the effects of immersive virtual reality as an adjunctive analgesic technique for hospitalized pediatric burn inpatients undergoing painful physical therapy. Fifty-four subjects (6–19 years old) performed range-of-motion exercises under a therapist’s direction for one to five days. During each session, subjects spent equivalent time in both the virtual reality and the control conditions (treatment order randomized and counterbalanced). Graphic rating scale scores assessing the sensory, affective, and cognitive components of pain were obtained for each treatment condition. Secondary outcomes assessed subjects’ perception of the virtual reality experience and maximum range-of-motion. Results showed that on study day one, subjects reported significant decreases (27–44%) in pain ratings during virtual reality. They also reported improved affect (“fun”) during virtual reality. The analgesia and affect improvements were maintained with repeated virtual reality use over multiple therapy sessions. Maximum range-of-motion was not different between treatment conditions, but was significantly greater after the second treatment condition (regardless of treatment order). These results suggest that immersive virtual reality is an effective nonpharmacologic, adjunctive pain reduction technique in the pediatric burn population undergoing painful rehabilitation therapy. The magnitude of the analgesic effect is clinically meaningful and is maintained with repeated use.
Introduction
Physical therapy is a critical, though painful, component of burn rehabilitation therapy. Early and aggressive physical therapy can help counter the decreased range of motion and severe contractures that can develop secondary to burn injury or associated skin grafting [1,2]. As a result, burn rehabilitation therapy is often key to improving functional outcome and minimizing long-term disability. Unfortunately, patients may be reluctant to participate fully in physical therapy due to the significant procedural pain that can be triggered by the very activities that are intended to help them heal [3,4].
Traditionally, pre-procedure systemic opioid administration has been the treatment method of choice for management of rehabilitation-related pain [5]. However, opioid side effects (including nausea, vomiting, pruritis, urinary retention, constipation, respiratory depression, tolerance and risk of addiction are well known), and can limit their clinical use [6,7,8]. Despite the liberal use of opioids in the setting of burn injuries, burn pain -- particularly procedural pain -- continues to remain undertreated [9,10,11]. For example, recent studies demonstrate that burn patients report a mean procedural pain score as high as 7 on a scale of 1–10 despite the use of opioids [12], and frequently report severe pain during wound care [11].
Due to inadequate pain control with opioid analgesics alone, nonpharmacologic analgesic techniques have been promoted as clinically useful adjuncts [2,13]. Such techniques as hypnosis [2,14], imagery [15], and biofeedback [16] have been shown to significantly reduce pain when used to augment pharmacologic analgesics. More recently, immersive virtual reality has been suggested as an additional choice. This treatment may be particularly effective because it provides an interactive, computer-generated virtual environment that is highly distracting to the user/patient [17]. By drawing heavily upon conscious attention, virtual reality distraction theoretically leaves fewer cognitive resources available to evaluate nociceptive input, resulting in less pain during otherwise painful procedures [18,19,20]. Published investigations in settings of burn wound care [17,21,22] and post-burn physical therapy [23,24,25] suggest that immersive virtual reality may be particularly applicable to this challenging clinical pain population. Furthermore, a previous preliminary study in a small number of patients suggested that the analgesic effect of virtual reality does not appear to extinguish with repeated use [23]. However, previous assessments of virtual reality in the setting of post-burn physical therapy are limited in subject numbers and have not yet focused on children [17,23,24].
In the current study we utilized a within-subjects design to compare the effectiveness of adjunctive, immersive virtual reality with that of conventional pharmacologic treatment alone during one or more post-burn physical therapy sessions in the inpatient setting. We hypothesized that the addition of immersive virtual reality to each subject’s standard analgesic regimen for active-assisted range-of-motion physical therapy would result in better pain control, as well as increase the maximum range-of-motion that could be achieved by the therapist. We also hypothesized that with repeated use, the amount of pain reduction obtained with adjunctive virtual reality would be maintained.
Methods
Subjects
Subjects were recruited from the inpatient population at the University of Washington Burn Center at Harborview Medical Center, a Level 1 burn/trauma center in Seattle, WA. Eligible subjects were 19 years and younger who required post-burn, active-assisted range-of-motion physical therapy at least once during their stay in the hospital. Exclusion criteria included extreme susceptibility to motion sickness, burns on body regions that precluded use of the VR equipment (e.g., ear burns), or history of seizure activity. Participation was voluntary and participants were not reimbursed. With a protocol approved by the institutional human subjects review board, and with informed written consent (parent/guardian, and children 18–19 years old) and assent for children under 18 years old, 54 pediatric subjects with burn injuries requiring inpatient hospitalization participated. Ethnicity was classified by the subjects (or their parent/guardian) using standard NIH options provided by the investigator.
Procedures
An immersive, three-dimensional, interactive, computer-simulated environment was created using a personal computer workstation with dual 2GHz central processing units, 2GB of RAM, and a GeForce 6800 video card running the virtual reality software SnowWorld (www.vrpain.com) on a Windows 2000 operating system. This system was coupled with one of four head-mounted displays (helmets) that shared similar, highly immersive visual output characteristics: the nVisor SX (NVIS Inc., Reston VA), the VR-1280 (Virtual Research Systems, Aptos CA), the ProView XL 50 (Kaiser Electro-Optics, Carlsbad CA), and the ProView SR 80 (Kaiser Electro-Optics, Carlsbad CA). All helmets excluded the user’s view of the immediate care environment and provided a three-dimensional image with at least 1024 × 1280 pixel resolution (per eye), and at least 50 degree diagonal field-of-view. The helmet used for each subject was selected based upon availability at the time of study. A Polhemus Fastrak (Polhemus, Colchester VT) motion-sensing system (six degrees-of-freedom) measured the position of the user’s head and allowed independent user navigation of the virtual environment. The subjects in the study explored the SnowWorld virtual environment that was originally designed and created at the University of Washington using VEGA development software from MultiGen-Paradigm, Inc. (San Jose CA), and recently upgraded by Firsthand, Inc (Seattle WA). SnowWorld depicts a constantly changing, icy three-dimensional virtual canyon. Subjects “throw” virtual snowballs at snowmen, igloos, mammoths and penguins by aiming with their gaze and pressing a keyboard/mouse button. Stereophonic sound includes background music, as well as sound effects that are synchronized with the visual effects.
Patients were recruited by a research assistant alerted to potential study subjects by the burn care team and physical therapists. All enrolled subjects participated in the study on at least one study day, and for up to five study days, until they were discharged from the hospital, returned to the operating room for further surgery, or could no longer participate due to other clinical care decisions unrelated to the study. Each subject received standard pre-procedure pharmacologic analgesia/sedation, typically consisting of an oral opioid alone, or combined with an oral benzodiazepine. This regimen was administered at the discretion of the subject’s physicians and nurses, was independent of the study protocol, and consisted of non-intravenous opioids in nearly all cases and oral benzodiazepines in the minority of cases. The opioids typically used in our center include either transmucosal fentanyl lozenges (5–15 mcg/kg) or oral hydromorphone (0.05–0.1 mg/kg), while the most commonly used benzodiazepine is oral midazolam (0.5 mg/kg). Specific drugs/doses administered to each subject were not recorded due to the crossover study design (below).
A within-subject (crossover) study design that allowed both treatment conditions (i.e., virtual reality and no virtual reality) to be employed within a single physical therapy session was used to assure constant pharmacologic analgesia in both conditions for each subject. Thus, any difference in subjective pain reports identified between conditions on a given study day was the result of the virtual reality intervention and not related to changes in pharmacologic analgesia. During the physical therapy session, subjects participated in therapist-directed, slow stretching of the selected extremity to the end range of the joint in all planes of movement. If a second joint was also manipulated (at the discretion of the physical therapist), the proximal joint was manipulated first, followed by the distal joint. During both treatment conditions during the same therapy session, the subject’s injured limb(s) was moved through the identical predetermined sequence of exercises. Each physical therapy session lasted 6–20 minutes and was divided into two consecutive portions of identical duration (3–10 minutes each), consisting of identical active-assisted range-of-motion exercises guided by the same therapist. One portion of the session utilized standard pharmacologic therapy and no virtual reality (control condition) and the other portion utilized standard pharmacologic therapy plus immersive virtual reality (virtual reality condition). The order of conditions was randomized and counterbalanced (using a computer-generated randomization schedule created prior to subject enrollment), so that each experimental condition had an equal chance of occurring first or second for each subject during each therapy session.
The primary dependent variable (subjective pain) was measured by the research assistant using a standardized script during a brief pause immediately after each treatment condition. The research assistant was not part of the clinical care team, was previously not known to the subjects, and administered the same measures following both treatment conditions. Subjects were asked to report each of three subjective pain ratings using a 0–100 graphic rating scale (GRS), designed to assess three separable components of the pain experience: the cognitive component of pain (“time spent thinking about pain”, ranging from zero minutes to the entire time), the affective component of pain (“pain unpleasantness”, ranging from not unpleasant to most unpleasant), and the sensory component of pain (“worst pain”, ranging from no pain to worst pain) [19,26,27]. These pain rating scales have been validated through their strong associations with other measures of pain intensity and in their ability to detect treatment effects, including pediatric populations in this age range [28,29].
After completion of these pain ratings, subjects also provided the following subjective assessments using similar 0–100 GRS tools: (1) Rate the amount of “fun” you had during the therapy session (no fun to most fun); (2) To what extent did you feel nausea as a result of experiencing the virtual world (no nausea to severe nausea/vomiting [virtual reality condition only]); (3) How real did the objects in the virtual world seem to you (“completely fake” to “indistinguishable from a real object” [virtual reality condition only]); and (4) To what extent did you feel like you went into the virtual world while experiencing virtual reality (“I did not feel like I went into the virtual world at all” to “I went completely into the virtual world” [virtual reality condition only]). The experience of going “into” the virtual world is considered to be a measure of user “presence” in the virtual environment, a subjective illusion created in the user’s mind as a result of sensory input [30]. Hendrix and Barfield have described the reliability of similar subjective measures of presence [31]. In addition, the physical therapist measured the maximum range-of-motion of the first joint treated immediately prior to the physical therapy session and immediately after each treatment, using a goniometer to quantify the maximal joint range-of-motion achieved by the therapist under each treatment condition.
Statistical Analysis
As the result of the within-subjects design used in this study, each subject served as his/her own control. Regression models accounting for the longitudinal nature of these data were used to assess all outcomes. Valid standard errors of model parameter estimates were obtained using generalized estimating equations that accounted for correlations among repeated measures on the subjects. Separate regression models were utilized for each of the dependent variables (time spent thinking about pain, pain unpleasantness, worst pain, fun, nausea, virtual reality realness, virtual reality presence, and range-of-motion). Independent variables included the primary intervention (standard analgesia versus standard analgesia plus immersive virtual reality) and demographic variables (gender, age, and ethnicity), the latter in an attempt to identify potential modifiers of virtual reality analgesia. In the regression models, dummy variables were used to represent these independent variables. Linear regression models were utilized to analyze outcomes measured only in the virtual reality condition (nausea, virtual reality realness, and virtual reality presence). SAS v.9 (SAS Institute Inc., Cary NC) was used for all statistical analyses. Comparisons were considered significant if they differed at the level of p<0.05. Data variance is presented in tables as standard deviation, and in figures as standard error (for graphical clarity).
Results
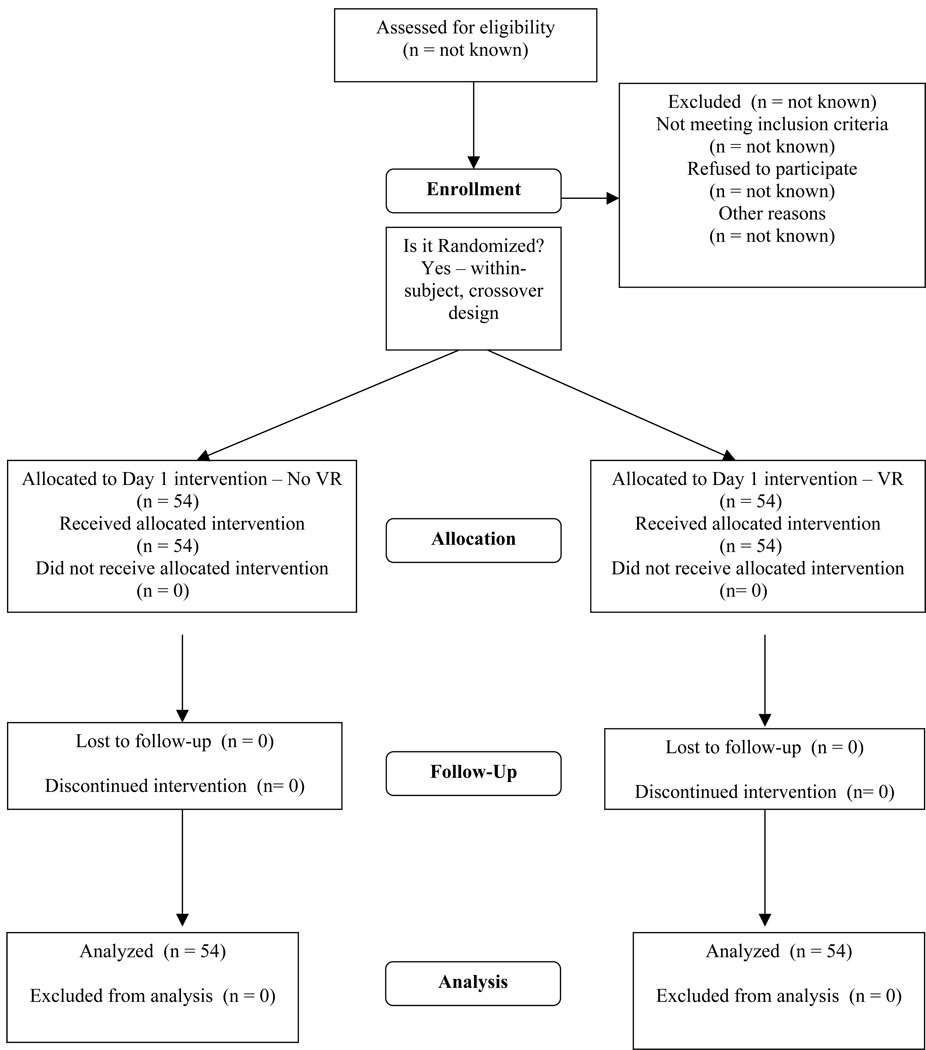
Fifty-four subjects aged 6–19 years old (mean age 12.0 ± 3.9 years) participated in the study on one or more (up to five) consecutive study days (Figure 1), with 31 participating for two days, 20 participating for three days, 9 participating for four days, and 5 participating for five days. Study withdrawals over this time were the result of standard clinical care decisions made by the primary care team resulting in changes in physical therapy, including achievement of full range-of-motion, discharge from the hospital, surgical intervention in the operating room, or pre-procedure discomfort (e.g., pain, pruritis) that prevented participation in the therapy session. Table 1 shows the demographics of the study population. Most of the subjects (81%) were male (reflecting the typical gender distribution of burn-injured children at our institution), and the majority of subjects (68.5%) were Caucasian. Burn injury size ranged from 1.5–50% of total body-surface area injured, but also included one child with 100% skin loss due to toxic epidermal necrolysis.
Figure 1.
CONSORT diagram for subject enrollment, allocation, and follow-up. The University of Washington IRB prohibits data collection on eligible research subjects who do not participate for any reason. Thus, information on potential subjects assessed, excluded, or who refused is not available.
Table 1.
Demographics of the Study Population (n=54 subjects who participated in the first day of study).
| Demographics | Subjects (n=54) |
|---|---|
| Males | 44 (81%) |
| Females | 10 (19%) |
| Caucasian | 37 (68%) |
| Hispanic | 7 (14%) |
| Native American | 5 (9%) |
| African American | 5 (9%) |
| TBSA burn 1.5–10% | 24 (47%) |
| TBSA burn 11–20% | 15 (29%) |
| TBSA burn 21–30% | 7 (14%) |
| TBSA burn 31–50% | 4 (8%) |
| TBSA burn >50% | 1 (2%) |
(TBSA, total body surface area. TBSA burn data missing for 3 subjects.)
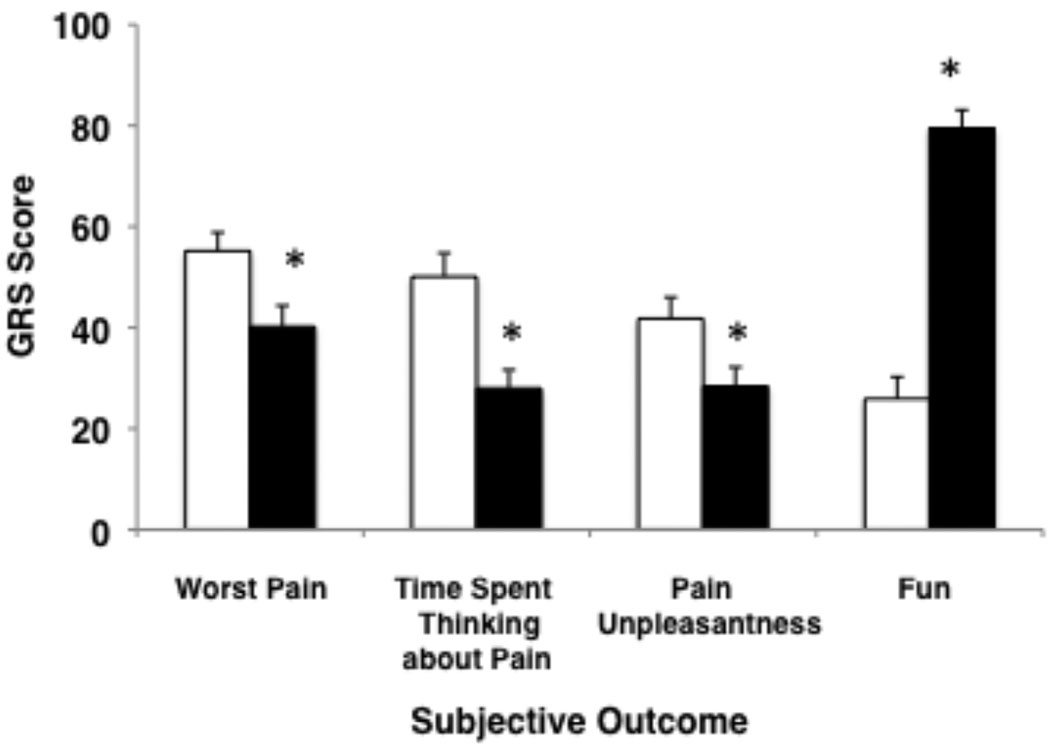
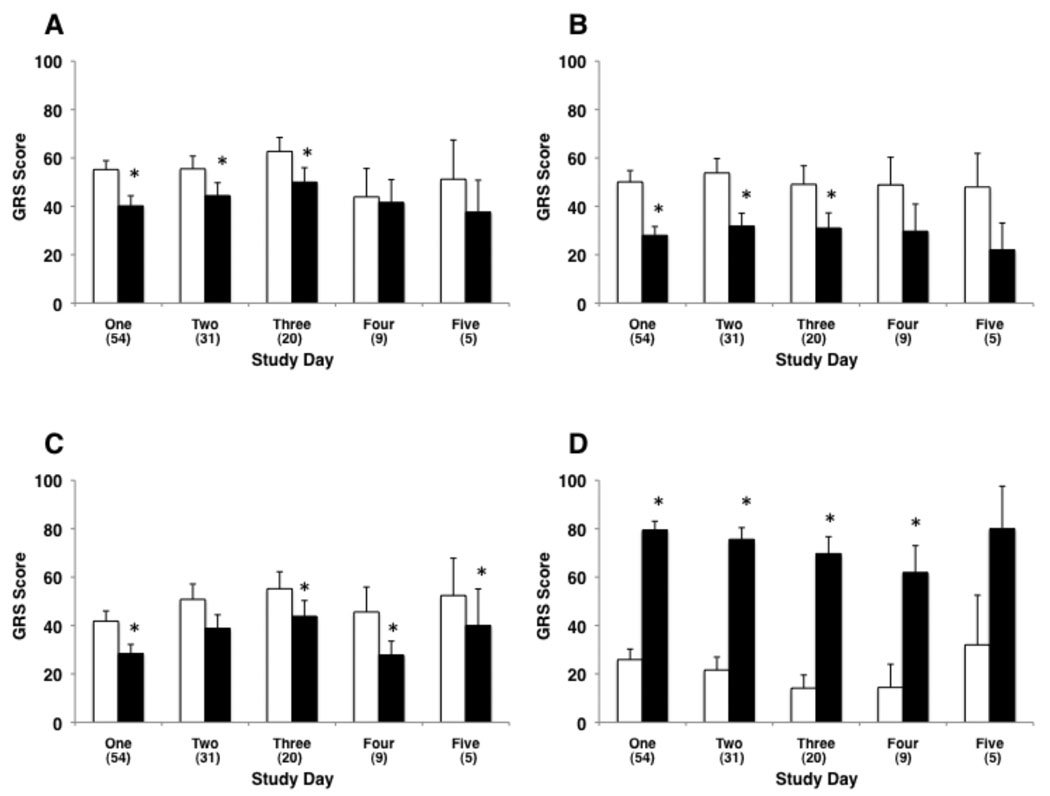
As shown in Figure 2, GRS assessments on study day one of cognitive pain (44% reduction), affective pain (32% reduction), and sensory pain (27% reduction) were significantly lower (p < 0.05 for each paired comparison) in the adjunctive virtual reality condition than in the control condition. Subjects reported a 3-fold increase in “fun” when in the virtual reality condition (p < 0.001). Age, ethnicity and gender had no effect on these pain outcome measures. The reductions in the three subjective pain reports and the increased report of “fun” during the adjunctive virtual reality portion of the physical therapy session did not diminish with repeated use of virtual reality over multiple days of therapy (Figure 3, including details of statistical comparisons).
Figure 2.
Subjective pain and fun ratings (0 to 100 GRS assessment) during the control condition (standard pharmacologic therapy without virtual reality – white bars) and the virtual reality condition (standard pharmacologic therapy plus immersive virtual reality – black bars) on the first study day (* p < 0.05).
Figure 3.
Subjective pain and fun ratings (0 to 100 GRS assessment) during the control condition (standard pharmacologic therapy without virtual reality – white bars) and the virtual reality condition (standard pharmacologic therapy plus immersive virtual reality – black bars) on multiple study days. Virtual reality results in a persistent decrease in “worst pain” (panel A), “time spent thinking about pain” (panel B), and “pain unpleasantness” (panel C) over several days of therapy (* p < 0.05). Virtual reality also results in a persistent increase in “fun” (panel D) over several days of therapy (* p < 0.05).
Immersive virtual reality did not result in a significant increase in maximal joint range-of-motion compared to the control condition (p = 0.21). However, treatment order did affect range-of-motion outcomes. There was a significant increase in the maximal range-of-motion (mean increase 6.8 degrees, p = 0.03) in the second treatment condition regardless of the order of the specific treatment conditions.
Table 2 summarizes the GRS scores for the three secondary outcomes assessed only in the immersive virtual reality condition (nausea, sense of “realness” of the virtual environment, and sense of “presence” in the virtual environment). Seventy-five percent of subjects reported a nausea rating of zero in the standard care plus virtual reality condition on the first study day, and more than 84% reported no nausea in the standard care plus virtual reality condition on subsequent study days. For the 16% of subjects who reported nausea, the symptom was mild (mean subjective magnitude of 9.2 on the first day and less than 4.0 on the subsequent days, on the 100-point GRS scale). Unfortunately, nausea ratings were not obtained from subjects in the control (no virtual reality) condition, for comparison purposes; thus, our ability to ascribe the nausea specifically to either the immersive virtual reality treatment or the systemic opioid treatment is not possible. Subjects’ reports of the virtual reality experience (realism and presence) were maintained throughout the 5-day study period.
Table 2.
Mean (SD) Graphic Rating Scale scores (0 to 100 rating) for secondary outcomes (defined in detail in the Methods section) reported on each of the five study days.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| Nausea | 9.3 (20.3) | 3.0 (8.1) | 1.0 (4.4) | 0 (0) | 4.0 (8.9) |
| Presence | 47.7 (37.0) | 55.0 (35.9) | 58.8 (29.7) | 53.3 (38.4) | 76.0 (28.8) |
| Realism | 35.7 (33.1) | 40.3 (31.4) | 46.4 (33.1) | 35.1 (33.0) | 46.0 (37.1) |
Discussion
Survivors of major burn injury are subjected to painful physical therapy and occupational therapy that are essential for successful treatment and rehabilitation of their burns. In particular, active-assisted range-of-motion physical therapy has many benefits for the burn patient, including the prevention of contractures and limited joint mobility that can result from hypertrophic scarring and decreased skin elasticity as the body tries to recover after burn injury [1,2]. In many cases these therapy sessions must occur on a daily basis in the recovery period, even though the pain associated with such therapy is not insignificant. Prior studies have documented the challenge of controlling such post-burn procedural pain with pharmacologic analgesia alone [10,11], a problem that can have significant impact on patient participation in rehabilitation therapy, particularly in children [3].
Standard care for procedural pain control in the inpatient burn population typically involves systemic opioids and/or benzodiazepines [4,32], yet burn patients frequently report severe pain during wound care [11]. In addition, a wide spectrum of side effects potentially limits the use of these agents [6,7,8]. Previous studies have suggested that immersive virtual reality has a significant analgesic effect in both the post-burn wound care and rehabilitation settings, as well as in non-burn settings such as dental pain [17,21,33,34,35,36]. However, the small sample sizes and short treatment durations of VR in these studies and their limited inclusion of pediatric patients make it difficult to generalize the conclusions of these studies to the inpatient pediatric burn population. Furthermore, few of these studies explored whether immersive virtual reality analgesia remains effective with repeated use [23].
The goal of the present study was to address these limitations by evaluating the effectiveness of immersive virtual reality analgesia over multiple days of treatment in a large, specifically pediatric, inpatient burn population. The results of this study provide further evidence that user interaction in a virtual environment can be a useful adjunct to pharmacologic analgesia in helping to control acute, procedural, rehabilitation-related pain in pediatric burn patients. Subjects reported significant and substantial decreases in three subjective pain experience components with virtual reality – sensory pain, cognitive pain, and affective pain. A reduction in pain severity of 30% or more is considered to be clinically meaningful by patients [37], and has also been deemed to represent a clinically meaningful decrease in pain by at least one consensus group of experts representing academic, industry, and government pain researchers [38]. The average effects of VR analgesia exceeded this benchmark for cognitive pain (44% reduction) and affective pain (32% reduction), and approached this benchmark for sensory pain (27% reduction) in this sample population. Moreover, mean pain ratings showed no indication that VR analgesia diminished in effectiveness when used repeatedly. Subjects also showed no indication of being less distracted by VR after using it several times, and VR continued to be fun day after day. These results are consistent with previous studies (primarily in adults) that showed a similar magnitude and effectiveness of adjunctive virtual reality distraction therapy in burn patients undergoing physical therapy [23,24].
We assessed the magnitude of “fun” experienced by subjects during the therapy session as a secondary outcome, in an exploratory assessment of subject mood or affect during this painful procedure. The concept of “fun” is related to mood/affect, but unlike more formal tools used to assess mood/affect in adults, is readily and easily understood in pediatric subjects spanning the wide age range in this study. We found that subjects reported a 3-fold increase in the subjective assessment of “fun” when performing physical therapy with immersive virtual reality, indirectly indicating an enhancement in mood or affect with the virtual reality experience. Such subjective improvement in mood, coupled with a decrease in pain perception during burn rehabilitation, may have important implications for patient compliance with burn rehabilitation activities. Since anticipatory anxiety can be associated with repeated painful procedures and may add to a patient’s subjective experience of pain [39], the increase in fun associated with immersive virtual reality therapy may prove valuable in increasing children’s cooperation with painful rehabilitation therapy and wound care.
Subjects’ reports of virtual reality “presence” (a subjective illusion created in the user’s mind based on sensory input from the virtual reality helmet and software) and virtual reality realism suggest that the virtual reality hardware and software components utilized in this study successfully captivated users’ attention. Previous studies have shown that improving virtual reality “immersiveness” through improvements to the virtual reality delivery system increases analgesic effect [40,41,42]. The role of virtual reality immersiveness is an important aspect of virtual reality distraction therapy that warrants further evaluation to examine the ideal balance between hardware cost (e.g., for improved helmet characteristics) and virtual reality analgesic effect.
Finally, virtual reality-assisted physical therapy was not associated with a statistically significant improvement in clinical joint range-of-motion in this short-term study, but is an important outcome to assess in future long-term assessments of virtual reality-assisted physical therapy. It is possible, although not tested in the current study, that ongoing reduced pain and increased “fun” with virtual reality treatment could translate into an increased willingness to participate in treatment, and therefore better long-term outcomes. Extended treatments (of longer durations and frequencies) could potentially reveal outcome benefits for VR analgesia, such as better range of motion post-discharge, shorter hospital stays, and reduced incidence of chronic pain. Ultimately, a long-term, randomized controlled, between-subjects trial with a large sample size and repeated virtual reality treatments is needed to determine whether there are any long term benefits to patients who receive systematic, repeated use of VR analgesia.
There were several limitations to this study. Although the treatment protocol was standardized, participating physical therapists were aware of the treatment condition and may have unintentionally treated patients differently in the two study conditions. Likewise, subjects were not blinded to the treatment condition and they may have been motivated to participate more vigorously in the physical therapy session with virtual reality or to underreport their pain scores following virtual reality exposure. Although very challenging, blinding of study researchers and subjects to the experimental condition would be useful. This limitation is inherent in the crossover study design, and could be overcome with a randomized, controlled, between-groups design. We chose the crossover design in order to standardize the pharmacologic analgesia under both treatment conditions, while still allowing randomization of treatment condition order. Future studies should ideally employ the randomized, between-groups design in order to minimize potential responder bias, but must also address the potential confound of non-standardized pharmacologic analgesia between treatment conditions. In addition, our use of several models of virtual reality helmet and more than one version of SnowWorld software may have increased treatment variance.
A second limitation is that missing data from subjects who did not continue beyond the initial study day (as a result of changes in their clinical care regimen) may have biased the results against virtual reality, since those subjects who underwent a greater number of physical therapy sessions and remained in the hospital longer may have been more sick, and did not represent a random subset of the original population. In addition, subject attrition (particularly by days 4 and 5) reduced the statistical power and may have affected our calculations of statistical significance for those days.
Third, nausea assessments were obtained only in the standard analgesia plus virtual reality condition and were not obtained in the standard analgesia without virtual reality condition, so it is difficult to assess whether the subjects’ nausea reports reflected potential virtual reality-induced “simulator sickness” or instead reflected a common opioid side effect. Although subjects were asked to rate their nausea resulting from virtual reality, it may well be that subjects are not able to discriminate between nausea caused by virtual reality and that caused by concurrent opioid use. Future studies should simply ask patients to rate their nausea after each condition.
In conclusion, the results of our study suggest that immersive virtual reality is a useful and powerful adjunct for enhancing pain control during rehabilitation therapy in the pediatric burn population. When added to standard pharmacologic analgesia, virtual reality distraction therapy produced a statistically significant and clinically meaningful reduction in subjective patient pain ratings, as well as a significant increase in perceived “fun.” The concern for potential negative side effects of opioids in the pediatric population, as well as the widespread need for effective nonpharmacologic adjuncts, supports continued research into the efficacy of virtual reality analgesia. Additional research is also warranted to investigate the mechanisms and ideal applications of virtual reality pain reduction techniques.
Acknowledgments
Funding for this study was provided by the National Institutes of Health (HD37683 and HD40954) and by the Paul G. Allen Family Foundation. The study sponsors had no involvement in the study design, data collection, analysis and interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication. The SnowWorld virtual environment was originally designed by the University of Washington Human Interface Technology Laboratory and created by Kristin Darken, Jeff Bellinghausen, and Chuck Walter (Multigen-Paradigm, Inc.), and later upgraded by Brian Stewart (SimWright Inc.) and Howard Abrams, with 3-dimensional modeling assistance from Duff Hendrickson (University of Washington Human Interface Technology Laboratory).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration (http://www.clinicaltrials.gov): #NCT00261690 ("Virtual Reality Pain Control During Burn Wound Care.")
Conflict of Interest Statement:
All authors deny any financial or personal relationships with other people or organizations that could inappropriately influence (bias) their work.
References
- 1.Ward RS. Physical rehabilitation. St. Louis, MO: Mosby; 1998. [Google Scholar]
- 2.Esselman PC, Thombs BD, Magyar-Russell G, Fauerbach JA. Burn rehabilitation: state of the science. Am J Phys Med Rehabil. 2006;85:383–413. doi: 10.1097/01.phm.0000202095.51037.a3. [DOI] [PubMed] [Google Scholar]
- 3.Ehde DM, Patterson DR, Fordyce WE. The quota system in burn rehabilitation. J Burn Care Rehabil. 1998;14:436–440. doi: 10.1097/00004630-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Patterson DR, Sharar SR. Burn pain. In: Loeser JD, Butler SH, Chapman CR, Turk DC, editors. Bonica's management of pain. Philadelphia, PA: Lippincott; 2001. [Google Scholar]
- 5.Cooper JO, Pavlin EG. Altered pharmacology in thermal injury. Crit Care Report. 1990;2:78–84. [Google Scholar]
- 6.Cherny N, Ripamonti C, Pereira J, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2942–2954. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 7.Brown C, Albrecht R, Pettit H, McFadden T, Schermer C. Opioid and benzodiazepine withdrawal syndrome in adult burn patients. Am Surg. 2000;66:367–370. [PubMed] [Google Scholar]
- 8.Ward RM. Opioid tolerance to sedation and analgesia. Pediatr Res. 2000;47:705–706. doi: 10.1203/00006450-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Melzack R. The tragedy of needless pain. Sci Am. 1990;262:27–33. doi: 10.1038/scientificamerican0290-27. [DOI] [PubMed] [Google Scholar]
- 10.Choiniere M, Melzack R, Rondeau J, Girard N, Paquin M. The pain of burns: characteristics and correlates. J Trauma. 1989;29:1531–1539. doi: 10.1097/00005373-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Perry S, Heidrich G, Ramos E. Assessment of pain by burn patients. J Burn Care Rehabil. 1981;2:322–327. [Google Scholar]
- 12.Carrougher GJ, Ptacek JT, Honari S, et al. Self-reports of anxiety in burn-injured hospitalized adults during routine wound care. J Burn Care Res. 2006;27(5):676–681. doi: 10.1097/01.BCR.0000238100.11905.AB. [DOI] [PubMed] [Google Scholar]
- 13.Adcock RJ, Boeve SA, Patterson DF. Psychological and emotional recovery. In: Carrougher G, editor. Burn Care and Therapy. St. Louis, MO: Mosby; 1998. [Google Scholar]
- 14.Patterson DR, Wiechman SA, Jensen M, Sharar SR. Hypnosis delivered through immersive virtual reality for burn pain: A clinical case series. Int J Clin Exp Hypn. 2006;54(2):130–142. doi: 10.1080/00207140500528182. [DOI] [PubMed] [Google Scholar]
- 15.Patterson DR, Everett JJ, Burns GL, Marvin JA. Hypnosis for the treatment of burn pain. J Consult Clin Psychol. 1992;60:713–717. doi: 10.1037//0022-006x.60.5.713. [DOI] [PubMed] [Google Scholar]
- 16.Knudson-Cooper MS. Relaxation and biofeedback training in the treatment of severely burned children. J Burn Care Rehabil. 1981;2:102–110. [Google Scholar]
- 17.Hoffman HG, Doctor JN, Patterson DR, Carrougher GJ, Furness TA., 3rd Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain. 2000;85(1–2):305–309. doi: 10.1016/s0304-3959(99)00275-4. [DOI] [PubMed] [Google Scholar]
- 18.McCaul KD, Malott JM. Distraction and coping with pain. Psychol Bull. 1984;95:516–533. [PubMed] [Google Scholar]
- 19.Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending, and a general theory. Psychol Rev. 1977;84:127–190. [Google Scholar]
- 20.Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–63. [Google Scholar]
- 21.Hoffman HG, Patterson DR, Magula J, et al. Water-friendly virtual reality pain control during wound care. J Clin Psychol. 2004;60(2):189–195. doi: 10.1002/jclp.10244. [DOI] [PubMed] [Google Scholar]
- 22.van Twillert B, Bremer M, Faber AW. Computer-generated virtual reality to control pain and anxiety in pediatric and adult burn patients during wound dressing changes. J Burn Care Res. 2007;28(5):694–702. doi: 10.1097/BCR.0B013E318148C96F. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman HG, Patterson DR, Carrougher GJ, Sharar SR. Effectiveness of virtual reality-based pain control with multiple treatments. Clin J Pain. 2001;17(3):229–235. doi: 10.1097/00002508-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clin J Pain. 2000;16(3):244–250. doi: 10.1097/00002508-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Sharar SR, Carrougher GJ, Nakamura D, Hoffman HG, Blough DK, Patterson DR. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: preliminary results from three ongoing studies. Arch Phys Med Rehabil. 2007;88(12 Suppl 2):S43–S49. doi: 10.1016/j.apmr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Melzack R. Psychological aspects of pain: implications for neural blockade. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 27.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 28.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of pain assessment. 2nd edition. New York, NY: Guilford Pr; 2001. [Google Scholar]
- 29.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 30.Slater M, Wilbur S. A framework for immersive virtual environments (FIVE): speculations on the role of presence in virtual environments. Presence Teleoper Virtual Environ. 1997;6:603–616. [Google Scholar]
- 31.Hendrix C, Barfield W. Presence in virtual environments as a function of visual and auditory cues. Los Alamitos, CA: Society Press; 1995. pp. 74–82. [Google Scholar]
- 32.Patterson DR. Non-opioid-based approaches to burn pain. J Burn Care Rehabil. 1995;16(3 Pt 2):372–376. doi: 10.1097/00004630-199505001-00007. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman HG, Garcia-Palacios A, Patterson DR, Jensen M, Furness T, 3rd, Ammons WF., Jr The effectiveness of virtual reality for dental pain control: a case study. Cyberpsychol Behav. 2001;4:527–535. doi: 10.1089/109493101750527088. [DOI] [PubMed] [Google Scholar]
- 34.Wright JL, Hoffman HG, Sweet RM. Virtual reality as an adjunctive pain control during transurethral microwave thermotherapy. Urology. 2005;66:1320. doi: 10.1016/j.urology.2005.06.123. [DOI] [PubMed] [Google Scholar]
- 35.Steele EB, Grimmer K, Thomas B, Mulley B, Fulton I, Hoffman H. Virtual reality as a pediatric pain modulation technique: a case study. Cyberpsychol Behav. 2003;6:633–638. doi: 10.1089/109493103322725405. [DOI] [PubMed] [Google Scholar]
- 36.Das DA, Grimmer KA, Sparnon AL, McRae SE, Thomas BH. The efficacy of playing a virtual reality game in modulating pain for children with acute burn injuries: a randomized controlled trial. BMC Pediatr. 2005;5:1. doi: 10.1186/1471-2431-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 38.Dworkin RH, Turk DC, Farrar JT, Jensen MP, Katz NP, Kerns RD, Stucki G, Allan RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Palermo TM, Drotar D. Prediction of children's postoperative pain: the role of presurgical expectations and anticipatory emotions. J Pediatr Psychol. 1996;21:683–698. doi: 10.1093/jpepsy/21.5.683. [DOI] [PubMed] [Google Scholar]
- 40.Prothero J, Hoffman H. Widening the field of view increases the sense of presence in immersive virtual environments. Technical Report TR-95-5. Seattle: Univ Washington, Human Interface Technology; 1995. [Google Scholar]
- 41.Gracely RH, McGrath PA, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman HG, Seibel EJ, Richards TL, Furness TA, Patterson DR, Sharar SR. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. J Pain. 2006;7(11):843–850. doi: 10.1016/j.jpain.2006.04.006. [DOI] [PubMed] [Google Scholar]