Abstract
The aim of this study was to determine whether peripheral N-methyl-D-aspartate (NMDA) receptors are involved in inflammation-induced mechanical hypersensitivity of the temporomandibular joint (TMJ) region. We developed a rat model of mechanical sensitivity to Complete Freund’s Adjuvant (CFA; 2μl containing 1μg Mycobacterium tuberculosis)-induced inflammation of the TMJ and examined changes in sensitivity following injection of NMDA receptor antagonists (DL-2-amino-5-phosphonovaleric acid (AP5) or Ifenprodil) with CFA. CFA injected into the TMJ resulted in an increase in mechanical sensitivity relative to pre-injection that peaked at day 1 and lasted for up to 3 days (n=8, P<0.05). There was no change in mechanical sensitivity in vehicle-injected rats at any time-point (n=9). At day 1, there was a significant increase in mechanical sensitivity in animals injected with CFA+vehicle (n=7) relative to those injected with vehicle alone (n=7; P<0.05), and co-injection of AP5 (n=6) or Ifenprodil (n=7) with CFA blocked this hypersensitivity. Subcutaneous injection of AP5 (n=7) and Ifenprodil (n=5) instead of into the TMJ had no significant effect on CFA-induced hypersensitivity of the TMJ region. Western blot analysis revealed constitutive expression of the NR1 and NR2B subunits in trigeminal ganglion lysates. Immunohistochemical studies showed that 99% and 28% of trigeminal ganglion neurons that innervated the TMJ contained the NR1 and NR2B subunits respectively. Our findings suggest a role for peripheral NMDA receptors in inflammation-induced pain of the TMJ region. Targeting peripheral NMDA receptors with peripheral application of NMDA receptor antagonists could provide therapeutic benefit and avoid side effects associated with blockade of NMDA receptors in the central nervous system.
Keywords: NMDA receptors, Temporomandibular joint, Inflammatory pain, Behavioural model, Peripheral sensitisation
INTRODUCTION
Temporomandibular joint (TMJ) disorders can cause significant pain and are often accompanied by inflammation (Gynther et al., 1998; Poveda-Roda et al., 2007; Sessle and Hu 1991). The pain is likely to result from an increased responsiveness and/or decreased activation thresholds of nociceptive neurons in the central nervous system (reflecting central sensitisation), and of primary afferent nociceptive neurons innervating the TMJ (reflecting peripheral sensitisation) (Denucci et al., 1996; Sessle 2000). Whilst it is clear that N-methyl-D-aspartate (NMDA) receptors on second-order nociceptive neurons in the spinal cord and medullary dorsal horn contribute to central sensitisation (Dickenson and Sullivan 1987; Hama et al., 2003; Herrero et al., 2000; Ren and Dubner 1993), the role of peripheral NMDA receptors is not well understood. NMDA receptors are composed of an obligatory NR1 and at least one of four NR2 (A, B, C and D) or an NR3 subunit, which are present in varying degrees in different populations of primary afferent neurons (Marvizon et al., 2002), implying different functional roles for some of the receptor subunits. NMDA receptors are located in the soma of primary afferent neurons in the dorsal root ganglia (Li et al., 2004; Ma and Hargreaves 2000; Marvizon et al., 2002; Sato et al., 1993; Wang et al., 1999), peripheral nerves and terminals of primary afferent fibres in skin and muscle (Alfredson et al., 2001; Carlton et al., 1995; Kinkelin et al., 2000). However, few studies have reported NMDA receptor expression in trigeminal ganglion neurons. Shigemoto et al. (1992) and Watanabe et al. (1994) reported mRNA expression for the NR1 subunit in trigeminal ganglion neurons, Lee and Ro (2007) reported NR1, NR2A and NR2B protein on immunoblots of trigeminal ganglion lysates, and Dong et al. (2007) reported NR2A and NR2B immunoreactivity in trigeminal ganglion neurons that innervate the masseter muscle.
Peripheral MK-801 (NMDA receptor antagonist) injection into the inflamed hind-paw returns heightened mechanical sensitivity to normal (pre-inflammation) levels (Du et al., 2003; Leem et al., 2001), as does application of AP5 (NMDA receptor antagonist) (Wang et al., 2000) and the more specific NR2B antagonist CP-101,606 (Taniguchi et al., 1997). These results imply that peripheral NMDA receptors have a role in inflammation-induced pain in the spinal system. In the trigeminal system, most studies of peripheral NMDA receptor involvement have focused on the role of glutamate in TMJ and masseter muscle pain (Lam et al., 2005a). Compared with injection of glutamate or NMDA alone, co-injection into the masseter muscle of either ketamine (NMDA antagonist) or Ifenprodil (specific NR2B antagonist) significantly attenuates glutamate or NMDA-evoked masseter nociceptive afferent fibre discharge (Cairns et al., 2003; Dong et al., 2007) and jaw reflex activity (Cairns et al., 1998). In addition, mustard oil (Yu et al., 1996) and capsaicin (Lam et al., 2005b) have been shown to induce similar reflex changes in jaw muscle activity (indicative of pain) when injected into the TMJ. Furthermore, ketamine injected with glutamate into the masseter muscle or TMJ reduces glutamate-induced pain in humans (Alstergren et al., 2010; Cairns et al., 2003). However, none of these studies examined behavioural sensitivity in response to inflammation of the TMJ, and so the present study was initiated to determine whether peripheral NMDA receptors are involved in inflammation-induced mechanical hypersensitivity of the TMJ.
METHODS
Adult male Sprague-Dawley rats, weighing between 100 and 300 g were used in this study. All rats were housed in a temperature-controlled room at 22°C under a 12 hour light/dark cycle with ad libitum access to food and water. Experiments were approved by the University of Melbourne Animal Experimentation Ethics Committee.
Behavioural Testing
All behavioural testing was carried out in a quiet environment, at the same time each day, and by the same experimenter (who was blind to the experimental procedure). Two days preceding surgery, each animal was habituated to having its body wrapped in a towel and being gently handled. The upper body of the animal was unrestrained, allowing the animal to move its head freely, and allowing us to observe head withdrawal in the subsequent behavioural testing. Habituation took no longer than 30 minutes per animal and resulted in animals that could be easily handled without causing stress. The animals were also acclimatised for 10 minutes, using the same manipulations, prior to testing on the behavioural testing days. On these experimental days, sensitivity to mechanical stimulation over the left TMJ was assessed by using an ascending series of calibrated Semmes-Weinstein monofilaments ranging in force from 0.4 g to 100 g. Each filament was applied five times, at five-second intervals, onto the skin directly over the TMJ, inferior to the posterior aspect of the zygomatic arch. Care was taken to ensure that the filament was applied to the same point on the joint each time it was applied. The animals were carefully observed for head withdrawal in response to each stimulus presentation. Head withdrawal was defined as a sudden retraction of the head immediately after filament application. Threshold of withdrawal was defined as the force of the first filament to elicit a head withdrawal in at least three out of the five presentations.
In the first series of behavioural experiments, we determined a time-line for sensitivity to mechanical stimulation in the region of the inflamed TMJ. Animals were anaesthetised with 4% Iso-flurane (Laser animal health). Two micro-litres of Complete Freund’s Adjuvant (CFA; 1μg Mycobacterium tuberculosis, Sigma) suspended in a 1:1 oil/saline mixture, were injected with a Hamilton syringe directly into the left TMJ (n=8). Isotonic saline was injected in a separate group of animals as a vehicle control (n=9). Fast Blue (Polysciences) was diluted in the injectate (to approximately 0.5%) so that we could confirm the site of injection after the behavioural testing protocol was completed. Rats were allowed to recover and were returned to their home cages. Behavioural testing was carried out at 5 hours, 1, 2, 3, 6, 9, 13, and 16 days post-CFA or vehicle injection, as well as 1 day pre-injection (baseline). During behavioural testing, there were no visible signs of CFA-induced inflammation in the skin over the joint or the surrounding tissue, and any inflammation that was present in the joint cavity could not be seen, ensuring the investigator was adequately blinded. At the conclusion of the testing period, rats were given an overdose of sodium pentobarbitone (80 mg/kg, i.p.; Lethobarb; Vibrac), perfused via the ascending aorta with 0.01M phosphate buffered saline (PBS), and the TMJ examined under ultra-violet illumination (for Fast Blue) to confirm injection localisation.
In the second series of behavioural experiments, we examined the effect of co-injecting NMDA receptor antagonists along with CFA or vehicle (saline) into either the TMJ or subcutaneously into the neck. Animals were anaesthetised as above and the left TMJ was injected with either 2 μl CFA + 2 μl vehicle (n=7), 4 μl vehicle alone (n=7), 2 μl CFA + 2 μl AP5 (40 μM; Sigma) (n=6), or 2 μl CFA + 2 μl Ifenprodil (10 μM; Sigma) (n=7). Two μl AP5 + 2 μl vehicle (n=7) and 2 μl Ifenprodil + 2 μl vehicle (n=5) controls were also performed by injection into the TMJ. In addition, we checked for systemic action of the antagonists by injecting 2 μl AP5 + 2 μl vehicle (n=7) or 2 μl Ifenprodil + 2 μl vehicle (n=5) subcutaneously into the neck instead of into the TMJ, in conjunction with injections of CFA + vehicle into the TMJ. The concentrations of AP5 (40μM) and Ifenprodil (10μM) were chosen as these concentrations block currents flowing through NMDA receptors in vitro (Kohno et al., 2008; Paoletti and Neyton 2007; Smith et al., 1998). As above, Fast Blue was included in the injectate to confirm injection localisation, and lack of visible inflammation of the joint cavity, surrounding tissues and overlying skin ensured the investigators performing the behavioural testing were adequately blinded. Behavioural testing was conducted one day pre-injection (baseline) and one day post-injection (at the time that we found the greatest change in mechanical sensitivity in the time-line experiments, see below). Rats were then given an overdose of sodium pentobarbitone (80 mg/kg, i.p.; Lethobarb), perfused via the ascending aorta with 0.01M PBS, and examined under ultra-violet illumination (for Fast Blue) to confirm injection localisation.
Animals were excluded from our analysis of behavioural testing if the injection was not confined entirely to the TMJ capsule (n=13), or the animals were clearly agitated and did not respond to acclimatisation on one of the days of testing (n=6). Prism (GraphPad Software Inc.) was used for statistical analysis. Repeated Measures ANOVA on Ranks followed by the Dunn’s post-hoc analysis was used to test for statistical differences between each post-injection time-point and the pre-injection (baseline) time-point in the time-line experiments. In the NMDA receptor antagonist studies, mechanical sensitivity (withdrawal threshold) for each animal at day 1 post-injection was expressed as a percentage of the baseline value for the same animal. As the force values generated by Semmes-Weinstein monofilaments are not linearly dispersed, we used log10 transformation to linearise data that were used to generate percentages. Thus, for animals that showed no change in mechanical sensitivity relative to baseline, for example animals of the vehicle control group, the value was close to 100% whereas for animals that were hypersensitive, for example animals injected with CFA + vehicle, the value was significantly less than 100%. A Student t-test was used to test for statistical differences in mechanical sensitivity between animals in specific pairs of experimental groups. P<0.05 was used to define significance for all tests performed.
Western Blot
Western blot analysis was performed in order to confirm that the NR1 and NR2B subunits of NMDA receptors are constitutively expressed in the rat trigeminal ganglia. Rats (n= 3) were decapitated and both trigeminal ganglia were removed, rinsed in cold sterile 0.01M PBS and sonicated immediately in ice-cold lysis buffer containing 0.3 μl Phenylmethylsulfonyl fluoride (1:1000, Sigma), 3 μl protease inhibitor cocktail (1:100, Sigma) and 300 μl of cell lysis buffer (CelLytic MT, Sigma). Lysates were centrifuged and supernatant protein concentration measured by using the Bio-Rad Protein assay (Regent’s Park). Fifty micrograms of protein were separated on an 8% sodium dodecyl sulphate-polyacrylamide gel, at 80V, and then transferred overnight (at 30V; 4°C) onto a polyvinylidene dufluoride membrane (Hybond-P, Amersham). Membranes were blocked in 5% skim milk powder in PBS + 0.1% tween-20 (PBS-T), incubated overnight (at 4°C) with the NR1 and NR2B primary antibodies (see Table 1), washed with PBS-T the next day, and incubated for one hour (at room temperature) in HRP-conjugated secondary antibodies (see Table 1). Protein immunoreactivity was visualized by enhanced chemiluminescence (Amersham) and exposed to Hyperfilm ECL (Amersham). Molecular weights were estimated by using Precision Plus Protein Standards (BioRad). Primary antibody omission was used to test antibody specificity.
Table 1.
| Primary antibody | Dilution | Specificity | Secondary antibody | Dilution |
|---|---|---|---|---|
| Goat anti-NR1 (Santa Cruz; #sc-1467) | 1:100 | Polyclonal antibody raised against carboxyl terminus, amino acids 918-938: RRAIEREEGQLQLCSRHREH [Marvizon et. al. (Marvizon et al., 2002)] | WB: HRP-conjugated donkey anti-sheep (Jackson Immunoresearch) IHC: Donkey anti-goat 488 (Molecular Probes)WB: |
1:3000 1:200 |
| Rabbit anti-NR2B (Millipore; #06-600) | 1:100 | Polyclonal antibody raised againstcarboxyl terminus, amino acids 1463-1482: FNGSSNGHVYEKLSSIESDV [Millipore] | WB: HRP-conjugated donkey anti-rabbit (Amersham) IHC: Donkey anti-rabbit 594 (Molecular Probes) |
1:2000 1:200 |
| Goat anti-GS (Millipore; MAB302) | 1:2000 | Monoclonal antibody raised against glutamine synthetase purified from sheep brain, clone GS-6 [Millipore] | IHC: Donkey anti-goat 488 (Molecular Probes) | 1:200 |
WB: Western blot experiments
IHC: Immunohistochemistry
Retrograde Tracing and Immunohistochemistry
Retrograde tracing was used to identify trigeminal primary afferent neurons that innervate the TMJ and immunohistochemistry was used to determine whether these neurons contained the NR1 and NR2B subunits of the NMDA receptor. Rats (n=3) were anaesthetized with ketamine (100 mg/kg i.p.; Ketamil; Troy Laboratories) and xylazine (5 mg/kg i.p.; Xylazil; Troy Laboratories). Two micro-litres of 2% Fast Blue (in distilled water) were injected with a Hamilton syringe directly into the left TMJ. After a seven day survival period, the rats were given an overdose of sodium pentobarbitone (80 mg/kg, i.p.; Lethobarb), and were perfused with 300 ml of 0.01M PBS followed by 300 ml of 4% paraformaldehyde in 0.01M phosphate buffer (pH 7.4). The TMJ was examined as above to confirm injection localisation. Trigeminal ganglia ipsilateral to the side injected with Fast Blue were dissected, washed in 0.01M PBS, and cryoprotected overnight in 30% sucrose in 0.01M PBS at 4°C. Twelve micron sections were cut at −17°C on a cryostat the next day, and mounted directly onto 1% gelatinised microscope slides. The sections were washed three times in 0.01M PBS and incubated for 1 hour in 10% normal horse serum with 1% Triton-X 100 and 0.1% sodium azide, then incubated for 48 hours (at 4°C) in the NR1 and NR2B primary antibodies (see Table 1). The following day, sections were washed three times in 0.01M PBS and then incubated for two hours in secondary antibodies (see Table 1). After three final washes in 0.01M PBS, sections were mounted on gelatinised slides, and cover-slipped with DAKO mounting media. NR1 and NR2B primary antibody omission and adsorption with synthetic peptides (1, 10 and 100 μg/ml) were also performed. In addition, double labelling (using the same wash and incubation protocol described above) with an antibody directed against glutamine synthetase (see Table 1) was used to identify whether NR2B immunoreactivity was in satellite glial cells.
Trigeminal ganglion sections were examined and photographed by using a Zeiss Axioskop fluorescence microscope (Zeiss, Oberkocken, Germany) fitted with a Zeiss 62 HE filter set and an AxioCam MRm camera. The size/frequency distribution of labelled neurons was determined by measuring cross-sectional area of retrogradely labelled neurons that were NR1 or NR2 immunoreactive with NIH Image J software. Neurons with cross-sectional area less than 800 μm2 were considered small, those between 800 and 1500 μm2 were considered medium, and those greater than 1500 μm2 were considered large. Counts of neurons immunoreactive for the NR1 and NR2B subunits were expressed as a percentage of the total number of retrogradely labelled neurons.
RESULTS
Time-course of mechanical hypersensitivity following CFA injection to the TMJ
CFA injected into the TMJ resulted in an increase in mechanical sensitivity to testing with the Semmes-Weinstein monofilaments (decrease in threshold of withdrawal to stimulation) (Figure 1A). The threshold of withdrawal following CFA injection was significantly lower than baseline at the 1 day and 2 day time-points (n=8, P<0.05). The effect of CFA injection was greatest at day 1, showing a reduction in withdrawal threshold from 26 g (25th percentile, 26 g; 75th percentile, 60 g) one day pre-CFA injection to 10 g (25th percentile, 8 g; 75th percentile, 22 g) one day post-CFA injection. The thresholds returned to pre-injection values by day 3. No significant changes in mechanical sensitivity to application of the Semmes-Weinstein monofilaments were observed in saline-injected rats (n=9, P>0.05) (Figure 1B). There was no significant difference in the withdrawal thresholds between CFA- and vehicle-injected animals at the baseline time-point (P>0.05)(Figure 1A). Whilst there appeared to be a decrease in withdrawal threshold in CFA-injected animals relative to vehicle-injected animals at days 1 and 2, this was not statistically significant (Figure 1A). However, when we pooled data taken from the day 1 time-point of this timeline study with raw data from the NMDA receptor antagonist studies outlined below, we found that CFA-injected animals (n=15) did indeed have significantly lower withdrawal thresholds than vehicle-injected animals (n=15) at day 1 (P<0.05; data not shown).
Figure 1.
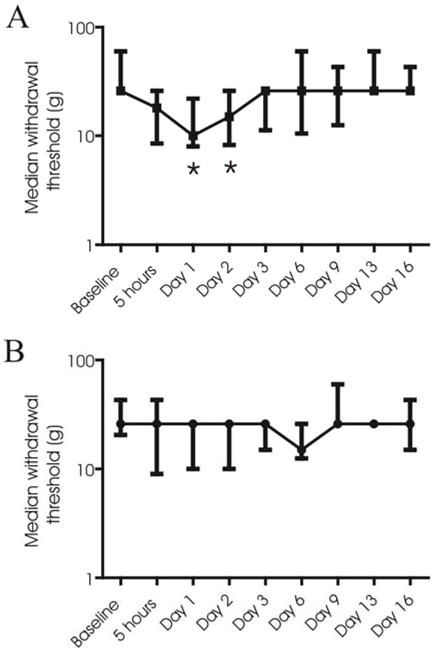
Time-course of mechanical sensitivity. Withdrawal thresholds (Median; 25th and 75th percentiles) to mechanical stimulation of the TMJ with Semmes-Weinstein monofilaments, pre-injection (baseline) and for 16 days after injection of CFA (n=8) (A) or vehicle (n=9) (B). Asterisks denote significant differences relative to baseline values (Repeated Measures ANOVA on Ranks followed by the Dunn’s post-hoc analysis; P<0.05).
NMDA receptors contribute to the mechanical hypersensitivity observed following CFA-induced inflammation of the TMJ
Behavioural testing was conducted to determine whether NMDA receptor antagonists could block the development of mechanical hypersensitivity following CFA injection into the TMJ. Testing was performed one day pre-injection (baseline) and one day post-injection (at the time when the greatest change in mechanical sensitivity occurred in the time-line experiments). The data presented in Figure 2 demonstrates that at day 1, there was a significant reduction in withdrawal threshold (decrease in percentage of baseline) in animals injected with CFA + vehicle (n=7) relative to those injected with vehicle alone (n=7; P<0.05). Co-injection of either AP5 (n=6) or Ifenprodil (n=7) with the CFA blocked the mechanical hypersensitivity generated by injection of CFA + vehicle (n=7). Co-injection of either AP5 (n=7) or Ifenprodil (n=5) with vehicle into the TMJ in the absence of CFA resulted in no change relative to the animals injected with vehicle alone. Simultaneous injection of CFA (into the TMJ) and subcutaneous administration of AP5 (n=7) or Ifenprodil (n=5) into the neck resulted in no change in sensitivity relative to animals injected with CFA (n=7) into the TMJ, but did produce statistically significant decreases in sensitivity relative to animals in the vehicle control group (P<0.05).
Figure 2.
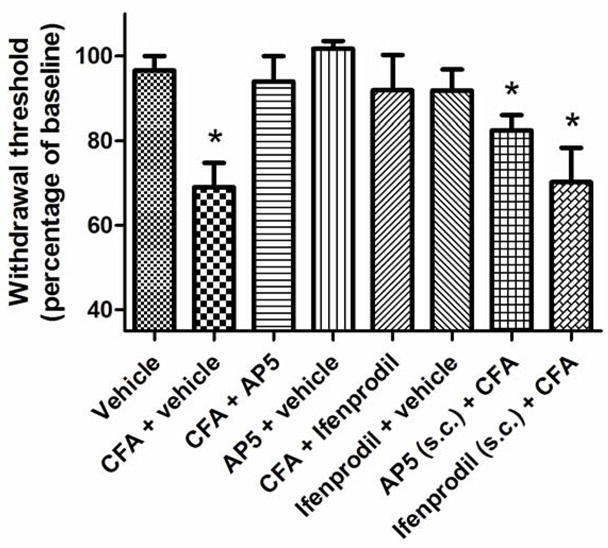
Effect of NMDA receptor antagonism on CFA-induced mechanical hypersensitivity. Each bar shows the mean withdrawal threshold (expressed as percentage of baseline) for each group of animals (mean±SEM). Asterisks denote significant differences relative to the vehicle control group (t-test; P<0.05).
Neurons that innervate the TMJ contain the NR1 and NR2B subunits
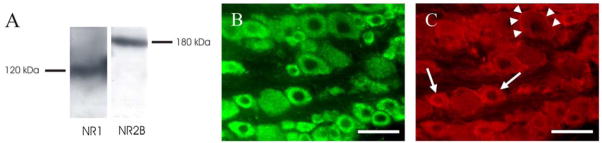
Western blot analysis was performed to determine the constitutive expression of the NR1 and NR2B subunits in the trigeminal ganglion. The NR1 and NR2B antibodies identified proteins in trigeminal ganglia lysates with molecular weights of 120 and 180 kDa (Figure 3A), and this is consistent with the reported molecular weights of the NR1 and NR2B subunits respectively (Marvizon et al., 2002). Primary antibody omission in the Western blot analysis resulted in no band (data not shown).
Figure 3.
Expression of NMDA receptor subunits in the trigeminal ganglion. (A) Western Blot analysis of the NR1 and NR2B subunits in a trigeminal ganglion lysate. (B) NR1 immunoreactivity of neurons in the trigeminal ganglion. (C) NR2B immunoreactivity of neurons (arrows) and satellite glial cells (arrowheads) in the trigeminal ganglion. Scale bars = 50 μm.
Immunohistochemistry was used to examine the localisation of the NR1 and NR2 subunits within the trigeminal ganglion. NR1 immunoreactivity revealed a clear and distinct staining of the cytoplasm in trigeminal ganglion neurons of all sizes (Figure 3B), whilst NR2B immunoreactivity revealed low level staining of the cytoplasm of small-sized trigeminal ganglion neurons and labelling of satellite glial cells around many of the larger neurons (Figure 3C). Double labelling with antibodies directed against glutamine synthetase confirmed that the labelling around the larger neurons was of satellite glial cells (data not shown). NR1 and NR2B primary antibody omission and adsorption with synthetic peptides (10 μg/ml and 100 μg/ml) completely abolished these staining patterns (data not shown).
Trigeminal afferent neurons that specifically innervate the TMJ were identified with retrograde labelling with Fast Blue. We examined a total of 119 retrogradely labelled neurons in the three animals studied. Ninety-eight percent of these showed NR1 immunoreactivity, and 28% showed NR2B immunoreactivity. Retrogradely labelled neurons were small or medium sized. NR1 immunoreactivity was found in the small and medium-sized retrogradely labelled neurons, and NR2B immunoreactivity was found only in small retrogradely labelled neurons (Figure 4).
Figure 4.
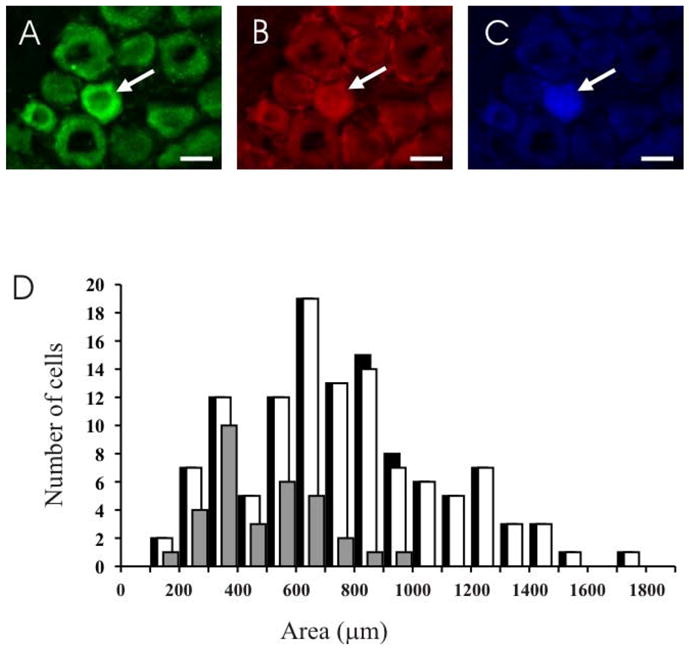
NMDA receptor expression in neurons that innervate the TMJ. The top panel consists of three images of the same field of a section through different filters to show a neuron (arrow) that is NR1 (A) and NR2B (B) immunoreactive, and contains Fast Blue (C). Scale bar = 20 μm. (D) Size distributions of neurons that innervate the TMJ (black), and TMJ afferents with NR1 (white) and NR2B (grey) subunit immunoreactivity.
DISCUSSION
This study has provided the first documentation that peripheral NMDA receptors are involved in mechanical hypersensitivity induced by injection of CFA into the rat TMJ, and that there is constitutive expression of NR1 and NR2B receptors in trigeminal ganglion, including primary afferent neurons that innervate the TMJ.
The time-to-peak of mechanical hypersensitivity observed in this rat behavioural model is consistent with that of other studies of CFA-induced inflammation. CFA-induced inflammation of the hind-paw (Inglis et al., 2005; Lin et al., 2007), parotid gland (Ogawa et al., 2003), masseter muscle (Ambalavanar et al., 2007), peri-oral skin (Morgan and Gebhart 2008; Ren 1999) and TMJ (Ren 1999; Wang et al., 2009) results in mechanical hypersensitivity that peaks between days 1 and 3 post-CFA injection. Some of the studies of TMJ sensitivity noted above used a larger volume of CFA (50 μl) and reported a longer-lasting hypersensitivity subsequent to TMJ inflammation (at least 7 days post-CFA injection) than that in the present study (Ren 1999; Wang et al., 2009). In preliminary experiments, we attempted to inject 50 μl of CFA or isotonic saline into the TMJ but found that rats that had this volume of isotonic saline injected showed some mechanical hypersensitivity (data not shown). It is difficult to compare our preliminary results directly to those of Ren (1999) and Wang and colleagues (2009) because Ren (1999) did not show data for vehicle controls and Wang and colleagues (2009) did not comment on the results of their vehicle control, although Figure 1 of Wang and colleagues (2009) suggests that there may have been some change in sensitivity due to the volume injected, albeit not statistically significant. Because of the small size of the TMJ and our strict criteria for injection localisation, we were concerned that 50 μl injections would produce a volume effect. We therefore used small injections in our study (2 μl for the time-line studies and 4 μl for the NMDA antagonist co-injection studies). These volumes injected into the TMJ produced no significant change in sensitivity relative to baseline at any time-point in our vehicle control group.
The finding that AP5 and Ifenprodil blocked the development of mechanical hypersensitivity at day 1 strongly implies that NMDA receptors, including those that contain the NR1 and NR2B subunits, are involved in inflammatory-induced pain associated with the TMJ. It is noteworthy that although Ifenprodil can also act on NR2A subunits, it is ~400 times more potent when acting on NR2B than NR2A subunits (Paoletti and Neyton 2007). Thus, the action of Ifenprodil at the concentration used in this study is likely to be specific to NR2B. Our finding that only 28% of neurons that innervate the TMJ express the NR2B receptor subunits highlights the importance of NR2B containing NMDA receptors in CFA-induced inflammatory TMJ pain. Furthermore, the effect of AP5 and Ifenprodil in this study can be attributed to actions on peripheral NMDA receptors in the TMJ and not to any systemic action on NMDA receptors outside of the TMJ, for several reasons: (1) the small volumes and concentrations of AP5 and Ifenprodil used; (2) AP5 penetrates the blood brain barrier poorly due to its highly charged nature and is thus unlikely to have an effect on central neurons (Kew and Kemp 2005); and (3) there was no difference in mechanical sensitivity, relative to injection of CFA + vehicle into the TMJ, when AP5 or Ifenprodil was administered systemically (subcutaneous injection into the neck) instead of directly into the TMJ. The mechanical testing used in the current study cannot differentiate between behavioural responses reflecting peripheral or central sensitisation, or both. Thus the attenuation of CFA-induced sensitisation by NMDA receptor antagonists could be explained by blockade of NMDA receptors associated with either peripheral sensitisation or afferent-related inputs into the brainstem that mediate central sensitisation.
Our studies of NMDA receptor localization in the trigeminal ganglion show that both NR1 and NR2B receptor subunits are present in the trigeminal ganglion and are expressed in the soma of small and medium sized primary afferent neurons that innervate the TMJ. Whilst the presence of receptor protein in the soma does not necessarily reflect the presence of functional receptors in the periphery, our finding that NMDA receptor antagonists applied to the TMJ block CFA-induced increases in mechanical sensitivity imply that NMDA receptors are localised in trigeminal primary afferent terminals in the TMJ, consistent with their presence in other deep tissues (Alfredson et al., 2001; Cairns et al., 1998; Cairns et al., 2003; Lam et al., 2005a). NR1 subunit immunohistochemistry in trigeminal ganglion neurons has not previously been reported. Our finding that 28% of the TMJ afferents contained the NR2B subunit is consistent with data of Dong et al (2007) who reported 31% of neurons innervating the masseter muscle of male rats express the NR2B subunit. Dong et al (2007) have further shown that injection of NMDA into the masseter muscle results in an increase in trigeminal primary afferent fibre discharge that could be blocked by ketamine and significantly reduced by Ifenprodil, implicating peripheral NMDA receptors, including those containing the NR2B subunit, in masseter peripheral sensitisation. Interestingly, they also highlighted significant sex differences by showing that female rats compared to male rats had a greater NMDA-evoked trigeminal primary afferent discharge and a greater number of masseter afferent neurons expressing NR2B. This suggests that NMDA receptor expression may contribute to sex differences in the perception of craniofacial musculoskeletal pain (Cairns et al., 2001; Cairns et al., 2006; Lam et al., 2005a). In the present study, we intentionally used male rats to avoid the possible effect of cyclic oestrogen levels on our results, but it is likely that sex differences may also play a role in our model and should be investigated in future studies. It would also be of interest if future studies tested whether NMDA receptor expression is altered in conditions of TMJ inflammation.
An examination of other NMDA receptor subunit types was not included in this study because of lack of appropriate and specific antibodies and antagonists to NR2A, C and D receptors, but we expect that they may also exist in TMJ afferents because they are present and form heteromeric complexes in the NMDA receptor in other peripheral nervous tissues (Marvizon et al., 2002). In addition, NMDA receptors have been reported on circulating immune (and other) cells (Gill and Pulido 2001; Piani et al., 1991) and thus it is possible that activation or blockade of NMDA receptors on non-neuronal cells in the TMJ could contribute to changes in mechanical sensitivity in the present study by affecting the inflammatory process. However, direct injection of glutamate into the TMJ or masseter muscle does not cause plasma extravasation or oedema but does result in a nociceptive jaw muscle reflex activity in animals and pain in humans (Cairns et al., 2001; Cairns et al., 2003; Lam et al., 2005a). This implies that NMDA receptors on immune (or other) cells are unlikely to mediate inflammation in the TMJ in rats and thus are not responsible for the changes in mechanical sensitivity observed in the present study.
Our findings suggest a role for peripheral NMDA receptor antagonism in the treatment of inflammation-induced pain of the TMJ. Importantly, peripheral application of NMDA receptor antagonists may help to avoid the serious side effects that occur following exposure of the central nervous system to NMDA antagonists in the treatment of trigeminal pain (Eide et al., 1994; Eide et al., 1995; Klepstad et al., 1990; Mathisen et al., 1995). Pre-emptive targeting of peripheral NMDA receptors with the application of NMDA receptor antagonists to the masseter muscle and TMJ has been successful in reducing glutamate-induced pain in humans and nociceptive responses in animals (Alstergren et al., 2010; Cairns et al., 2003). However, when the NMDA receptor antagonist, ketamine, was applied relatively late into the onset of pathology, it was not successful in reducing pain associated with temporomandibular disorders when injected into the masseter (Castrillon et al., 2008) or temporomandibular joint arthralgia when injected into the TMJ (Ayesh et al., 2008) in humans. These differences may reflect the time of application of the NMDA antagonists. Indeed there are a number of studies that support a role for early local administration of NMDA antagonists in the reduction of human experimental pain (including hyperalgesia) in non-craniofacial tissues (Pedersen et al., 1998; Poyhia and Vainio 2006; Tan et al., 2007; Warncke et al., 1997) but none of these have examined the effect of local administration of NMDA antagonists at later time-points relative to pain onset. The results of the current study are consistent with the idea that NMDA receptor antagonists may be most effective at treating induction of inflammatory pain conditions (Weyerbacher et al., 2010). Whilst these studies suggest that early application or pre-treatment with local NMDA antagonist application may be critical to therapeutic benefit, a clearer definition of effects at later time-points in human experimental pain models is required.
Acknowledgments
This work was supported by funding attained from the Australian National Health and Medical Research Council (#454606) and NIH grant (#DE04786).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res. 2001;19(5):881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- Alstergren P, Ernberg M, Nilsson M, Hajati A, Sessle B, Kopp S. Glutamate-induced temporomandibular joint pain in healthy individuals is partially mediated by the peripheral NMDA receptor. J Orofac Pain. 2010;24(2):172–180. [PubMed] [Google Scholar]
- Ambalavanar R, Yallampalli C, Yallampalli U, Dessem D. Injection of adjuvant but not acidic saline into craniofacial muscle evokes nociceptive behaviors and neuropeptide expression. Neuroscience. 2007;149(3):650–659. doi: 10.1016/j.neuroscience.2007.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayesh EE, Jensen TS, Svensson P. Effects of intra-articular ketamine on pain and somatosensory function in temporomandibular joint arthralgia patients. Pain. 2008;137(2):286–294. doi: 10.1016/j.pain.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001;86(2):782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Evidence That Excitatory Amino Acid Receptors within the Temporomandibular Joint Region Are Involved in the Reflex Activation of the Jaw Muscles. J Neurosci. 1998;18(19):8056–8064. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res. 2006;169(4):467–472. doi: 10.1007/s00221-005-0158-z. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang KL, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003;90(4):2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197(1):25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P. Effect of peripheral NMDA receptor blockade with ketamine on chronic myofascial pain in temporomandibular disorder patients: a randomized, double-blinded, placebo-controlled trial. J Orofac Pain. 2008;22(2):122–130. [PubMed] [Google Scholar]
- Denucci DJ, Dionne RA, Dubner R. Identifying a neurobiologic basis for drug therapy in TMDs. J Am Dent Assoc. 1996;127(5):581–593. doi: 10.14219/jada.archive.1996.0270. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience. 2007;146(2):822–832. doi: 10.1016/j.neuroscience.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl--aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118(2):547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Eide PK, Jorum E, Stubhaug A, Bremnes J, Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain. 1994;58(3):347–354. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Eide PK, Stubhaug A, Stenehjem AE. Central dysesthesia pain after traumatic spinal cord injury is dependent on N-methyl-D-aspartate receptor activation. Neurosurgery. 1995;37(6):1080–1087. doi: 10.1227/00006123-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Gill SS, Pulido OM. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol Pathol. 2001;29(2):208–223. doi: 10.1080/019262301317052486. [DOI] [PubMed] [Google Scholar]
- Gynther GW, Dijkgraaf LC, Reinholt FP, Holmlund AB, Liem RS, de Bont LG. Synovial inflammation in arthroscopically obtained biopsy specimens from the temporomandibular joint: a review of the literature and a proposed histologic grading system. J Oral Maxillofac Surg. 1998;56(11):1281–1286. doi: 10.1016/s0278-2391(98)90609-7. discussion 1287. [DOI] [PubMed] [Google Scholar]
- Hama A, Woon Lee J, Sagen J. Differential efficacy of intrathecal NMDA receptor antagonists on inflammatory mechanical and thermal hyperalgesia in rats. Eur J Pharmacol. 2003;459(1):49–58. doi: 10.1016/s0014-2999(02)02828-5. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther. 2005;7(4):R807–816. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179(1):4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kinkelin I, Brocker EB, Koltzenburg M, Carlton SM. Localization of ionotropic glutamate receptors in peripheral axons of human skin. Neurosci Lett. 2000;283(2):149–152. doi: 10.1016/s0304-3940(00)00944-7. [DOI] [PubMed] [Google Scholar]
- Klepstad P, Maurset A, Moberg ER, Oye I. Evidence of a role for NMDA receptors in pain perception. Eur J Pharmacol. 1990;187(3):513–518. doi: 10.1016/0014-2999(90)90379-k. [DOI] [PubMed] [Google Scholar]
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28(17):4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Cairns BE, Hu JW. Neural mechanisms of temporomandibular joint and masticatory muscle pain: a possible role for peripheral glutamate receptor mechanisms. Pain Res Manag. 2005a;10(3):145–152. doi: 10.1155/2005/860354. [DOI] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Cairns BE, Hu JW. Peripheral NMDA receptor modulation of jaw muscle electromyographic activity induced by capsaicin injection into the temporomandibular joint of rats. Brain Res. 2005b;1046(1–2):68–76. doi: 10.1016/j.brainres.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Lee J, Ro JY. Differential regulation of glutamate receptors in trigeminal ganglia following masseter inflammation. Neurosci Lett. 2007;421(2):91–95. doi: 10.1016/j.neulet.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Hwang JH, Hwang SJ, Park H, Kim MK, Choi Y. The role of peripheral N-methyl-D-aspartate receptors in Freund’s complete adjuvant induced mechanical hyperalgesia in rats. Neurosci Lett. 2001;297(3):155–158. doi: 10.1016/s0304-3940(00)01662-1. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109(3):443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Lin T, Li K, Zhang FY, Zhang ZK, Light AR, Fu KY. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J Neuroimmunol. 2007;192(1–2):40–48. doi: 10.1016/j.jneuroim.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Hargreaves RJ. Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience. 2000;101(3):699–707. doi: 10.1016/s0306-4522(00)00419-x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446(4):325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Mathisen LC, Skjelbred P, Skoglund LA, Oye I. Effect of ketamine, an NMDA receptor inhibitor, in acute and chronic orofacial pain. Pain. 1995;61(2):215–220. doi: 10.1016/0304-3959(94)00170-J. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Gebhart GF. Characterization of a model of chronic orofacial hyperalgesia in the rat: contribution of NA(V) 1.8. J Pain. 2008;9(6):522–531. doi: 10.1016/j.jpain.2008.01.326. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Ren K, Tsuboi Y, Morimoto T, Sato T, Iwata K. A new model of experimental parotitis in rats and its implication for trigeminal nociception. Exp Brain Res. 2003;152(3):307–316. doi: 10.1007/s00221-003-1538-x. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharm. 2007;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Pedersen JL, Galle TS, Kehlet H. Peripheral analgesic effects of ketamine in acute inflammatory pain. Anesthesiology. 1998;89(1):58–66. doi: 10.1097/00000542-199807000-00011. [DOI] [PubMed] [Google Scholar]
- Piani D, Frei K, Do KQ, Cuenod M, Fontana A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991;133(2):159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- Poveda-Roda R, Bagan JV, Diaz-Fernandez JM, Hernandez-Bazan S, Jimenez-Soriano Y. Review of temporomandibular joint pathology. Part I: classification, epidemiology and risk factors. Med Oral Patol Oral Cir Bucal. 2007;12(4):E292–298. [PubMed] [Google Scholar]
- Poyhia R, Vainio A. Topically administered ketamine reduces capsaicin-evoked mechanical hyperalgesia. Clin J Pain. 2006;22(1):32–36. doi: 10.1097/01.ajp.0000149800.39240.95. [DOI] [PubMed] [Google Scholar]
- Ren K. An Improved Method for Assessing Mechanical Allodynia in the Rat. Physiol Behav. 1999;67(5):711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. NMDA receptor antagonists attenuate mechanical hyperalgesia in rats with unilateral inflammation of the hindpaw. Neurosci Lett. 1993;163(1):22–26. doi: 10.1016/0304-3940(93)90220-f. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4(11):1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11(1):57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Hu JW. Mechanisms of pain arising from articular tissues. Can J Physiol Pharmacol. 1991;69(5):617–626. doi: 10.1139/y91-092. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Ohishi H, Nakanishi S, Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci Lett. 1992;144(1–2):229–232. doi: 10.1016/0304-3940(92)90756-w. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512 ( Pt 1):149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PH, Cheng JT, Kuo CH, Tseng FJ, Chung HC, Wu JI, Hsiao HT, Yang LC. Preincisional subcutaneous infiltration of ketamine suppresses postoperative pain after circumcision surgery. Clin J Pain. 2007;23(3):214–218. doi: 10.1097/AJP.0b013e31802e3377. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Shinjo K, Mizutani M, Shimada K, Ishikawa T, Menniti FS, Nagahisa A. Antinociceptive activity of CP-101,606, an NMDA receptor NR2B subunit antagonist. Br J Pharmacol. 1997;122(5):809–812. doi: 10.1038/sj.bjp.0701445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Wang Y-W, Zhao Z-Q. Peripheral NMDA and non-NMDA receptors contribute to nociception: an electrophysiological study. Brain Res Bull. 2000;52(1):31–34. doi: 10.1016/s0361-9230(99)00277-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang R-x, Wang R, Qiao J-T. Decreased expression of N-methyl--aspartate (NMDA) receptors in rat dorsal root ganglion following complete Freund’s adjuvant-induced inflammation: an immunocytochemical study for NMDA NR1 subunit. Neurosci Lett. 1999;265(3):195–198. doi: 10.1016/s0304-3940(99)00246-3. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Mao J, Sung B, Mao J. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain. 2009;141(1–2):97–103. doi: 10.1016/j.pain.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke T, Jorum E, Stubhaug A. Local treatment with the N-methyl-D-aspartate receptor antagonist ketamine, inhibit development of secondary hyperalgesia in man by a peripheral action. Neurosci Lett. 1997;227(1):1–4. doi: 10.1016/s0304-3940(97)00263-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct gene expression of the N-methyl-D-aspartate receptor channel subunit in peripheral neurons of the mouse sensory ganglia and adrenal gland. Neurosci Lett. 1994;165(1–2):183–186. doi: 10.1016/0304-3940(94)90740-4. [DOI] [PubMed] [Google Scholar]
- Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-Methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain. 2010;148(2):237–246. doi: 10.1016/j.pain.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Sessle BJ, Haas DA, Izzo A, Vernon H, Hu JW. Involvement of NMDA receptor mechanisms in jaw electromyographic activity and plasma extravasation induced by inflammatory irritant application to temporomandibular joint region of rats. Pain. 1996;68(1):169–178. doi: 10.1016/S0304-3959(96)03181-8. [DOI] [PubMed] [Google Scholar]