Abstract
The quality of the social environment can have profound influences on the development and activity of neural systems with implications for numerous behavioral and physiological responses, including the expression of emotionality. Though social experiences occurring early in development may be particularly influential on the developing brain, there is continued plasticity within these neural circuits amongst juveniles and into early adulthood. In this review, we explore the evidence derived from studies in rodents which illustrates the social modulation during development of neural systems, with a particular emphasis on those systems in which a long-term effect is observed. One possible explanation for the persistence of dynamic changes in these systems in response to the environment is the involvement of epigenetic mechanisms, and here we discuss recent studies which support the role of these mechanisms in mediating the link between social experiences, gene expression, neurobiological changes, and behavioral variation. This literature raises critical questions about the interaction between neural systems, the concordance between neural and behavioral changes, sexual dimorphism in effects, the importance of considering individual differences in response to the social environment, and the potential of an epigenetic perspective in advancing our understanding of the pathways leading to variations in mental health.
Keywords: social, anxiety, epigenetic, maternal, enrichment, isolation, stress
Introduction
Though our understanding of the neurobiology of mood disorders has advanced through the use of modern imaging and pharmacological techniques, there are still significant gaps in our knowledge regarding the origins of increased susceptibility to psychopathology. Epidemiological studies of the impact of early life abuse and neglect suggest that the quality of early social experiences are associated with a an altered risk of depression and anxiety in adulthood (Batten et al., 2004; Bradley et al., 2008; Neigh et al., 2009; Stirling and Amaya-Jackson, 2008). These studies suggest that the quality of the early life social environment may lead to a cascade of neurobiological changes with implications for numerous behavioral outcomes, including enhanced emotionality. Further support for the role of the social environment in shaping the brain comes from experimental studies in rodents, where targeted manipulation of postnatal mother-infant interactions has been demonstrated to induce long-term changes in behavior associated with effects on a wide range of neural systems. These results suggest that plasticity in brain development in response to the early social environment may account for the vulnerability that exists in individuals when they are exposed to disruptions in the quality of that environment. Interestingly, the social influence on risk and resilience may not be limited to these early developmental time-points. Here we will highlight literature from recent experimental studies in rodents illustrating how social experiences occurring during postnatal and juvenile periods, and in early adulthood, can shape neural systems that in some cases may lead to altered emotionality (i.e. behavioral and physiological responses to stress, novelty etc.). Though questions still remain regarding the time-course, specificity, and behavioral consequences of these changes, there is emerging evidence that epigenetic regulation of gene expression may be a critical feature of this neural plasticity. We will explore the recent evidence supporting an epigenetic perspective on the origins of individual differences in behavior and identify critical issues raised by these studies that can guide future studies on the role of the social environment in shaping brain development.
Social Influence on the Developing Brain
Studies of the impact of social experiences on development have examined a wide range of physiological, metabolic, immune, neurobiological, and behavioral outcomes. Within the framework of neurobiological consequences, there has been exploration of structural neuroanatomical changes, cell death/survival, synaptic plasticity, and region-specific variation in neurotransmitter and receptor levels/gene expression, all of which may lead to behavioral phenotypes associated with increased or decreased emotionality. Here we will highlight evidence from rodent studies (a discussion of studies in humans is reviewed elsewhere in this issue) for the influence of social experiences occurring during postnatal, juvenile, and in some cases, early adulthood on several neural systems, including monoamine (with a focus of serotonin and dopamine), GABAergic, glutamatergic, vasopressin, oxytocin, estrogen sensitivity and estrogen receptors, and receptors for corticotrophin releasing hormone (CRH) and glucocorticoids. Though the neurobiological substrates of emotionality spans beyond these systems as do the effects of the social environment, discussion of these select targets illustrates the wide range of social experiences that can shape the brain and explores neurotransmitter/receptor systems that may be regulated via epigenetic modifications (as will be discussed in subsequent sections).
A. Dopamine & Serotonin
In rodents, social experiences during postnatal development are dominated by mother-infant interactions, with dams spending the majority of the first week postpartum in contact with litters (Champagne et al., 2003). Long-term developmental consequences of variations in this early social environment have been most commonly addressed through utilization during the first few weeks of life of the maternal separation (pups are removed from the dam daily for at least one hour per day) and ‘handling’ (pups are removed from the dam for 15 minutes per day – also known as brief maternal separations or maternal augmentation) paradigms (see Table 1 for a summary of paradigms). Prolonged periods of maternal separation during the postpartum period are typically associated with increased anxiety-like and depression-like behavior, while brief separations stimulate interactions between mother and pups (Boccia and Pedersen, 2001; Liu et al., 1997) leading to an attenuation of the stress response and decreases in anxiety-like and depression-like behavior, especially in male offspring. Several studies have demonstrated that these behavioral changes are due in part to long-term modifications of the dopaminergic system. For instance, male and female rat pups who are isolated from both mother and sibling contact daily for one hour from postnatal (PN) days 2–9 were found to have cocaine-induced elevations in dopamine (DA) within the ventral striatum at PN10, with both males and females showing 100–300% DA increases compared to controls (Kosten et al., 2003). Elevated levels of DA in the nucleus accumbens (NAc) also persist into adulthood (Hall et al., 1999; McCormick et al., 2002). Similarly, increased DA and decreased DOPAC (a dopamine metabolite) in adult mice have been documented in the striatum in offspring separated from their dams for five hours/day from PN days 2–6 compared to handled mice (Ognibene et al., 2008). Moreover, compared to individuals who were handled (15 minutes/day) during the first two weeks of life, adults who are separated from their mother daily for three hours per day are more hyperactive in a novel environment and exhibit a dose-dependent higher sensitivity to cocaine-induced locomotor activity (Brake et al., 2004). These behavioral changes are associated with increased dopamine D1 receptor binding levels in the NAc core and caudate putamen (CP), increased D3 receptor mRNA in the NAc shell, and a trend for increased D2 receptor levels in the NAc core. Additionally, dopamine transporter (DAT – which uptakes DA from the synapse) levels are significantly decreased in maternally separated animals in the NAc core and CP but not the ventral tegmental area (VTA) or prefrontal cortex (PFC) (Brake et al., 2004). Similar consequences to DA activity have been observed as a function of low levels of maternal care experienced in infancy, such that offspring of low licking/grooming (LG) dams have elevated stress-induced dopamine release within the medial prefrontal cortex (mPFC) (Zhang et al., 2005). Hence, these studies indicate that prolonged maternal separation and reduced maternal care is associated with long-term sensitization of dopaminergic activity via alterations of site-specific dopamine, dopamine receptor and dopamine transporter gene expression that may contribute to the observed increases in anxiety-like and depression-like behavior in these offspring.
Table 1.
Summary of rodent paradigms for the study of social modulation of the brain
| Developmental Period | Paradigm | Description* |
|---|---|---|
| early postnatal | maternal separation | daily removal of pups from the nest for 1 hour or more during pre-weaning development |
| postnatal abuse | disruption of cage environment to induce increased stepping-on and dragging of pups | |
| handling, brief maternal separation, maternal augmentation | daily removal of pups from the nest for 15–20 minutes during pre-weaning development | |
| natural variations in licking/grooming (LG) | comparison of offspring reared by high LG compared to low LG dams (LG status determined via home-cage observations) | |
| communal nursing | offspring reared in nests by 2–3 lactating females with multiple litters | |
| single vs. biparental rearing | removal of father from rearing environment amongst biparental species | |
| late postnatal | early weaning | e.g. in mice, weaning pups at PN14 compared to PN21 |
| juvenile | late weaning | e.g. in mice, weaning pups at PN28 compared to PN21 |
| social isolation | post-weaning housing of pups in social isolation | |
| social enrichment | post-weaning housing of pups in large, complex cages, with numerous conspecifics | |
| mixed-sex housing vs. same-sex housing | comparisons of males and females reared with or without exposure to the opposite sex | |
| adulthood | dominant vs. subordinate status | comparison of individuals based on social status when housed in same-sex pairs or when housed in same-sex or mixed-sex groups |
| social isolation | prolonged housing in isolation | |
| social enrichment | prolonged housing in enrichment | |
| social defeat | repeated exposure to an aggressive conspecific involving increased agonistic encounters |
wide variations in methodology are used in some paradigms
Early life maternal separation also significantly alters serotonin (5-HT) signaling and metabolism in adulthood. Maternally separated rat pups (3 hours of separation twice daily from PN days 1–13) have decreased 5-HIAA and HVA (5-HT metabolites) levels in the amygdala and increased stress-induced 5-HT and 5-HIAA levels in the dorsal raphe nucleus (DRn), cingulate cortex, and NAc at 4 months of age (Arborelius and Eklund, 2007). Similar increases in 5-HT levels are found in the PFC, hippocampus, and striatum of mice that are maternally separated for five hours daily during the first week postpartum (Ognibene et al., 2008). Significantly, postnatal maternal separation not only affects 5-HT levels in the brain, but also modulates long-term changes in the functioning of 5-HT receptors and the serotonin transporter (5-HTT - which removes 5-HT from synapses). For instance, the maternal separation of rats (with separation carried out for 6 hour sessions on 10 days distributed from PN days 5–20) induces a reduced sensitivity of α1-adrenoceptors on 5-HT neurons in the DRn, an increased desensitization of frontal cortex 5-HT1A autoreceptors (Gartside et al., 2003). Amongst rat offspring exposed to 3 hours of daily maternal separation from PN days 2–14, an enhancement of the inhibitory effect of the serotonin reuptake inhibitor (SRI) citalopram on serotonergic cell firing in the DRn (Arborelius et al., 2004) and an increase in 5-HTT mRNA expression in the DRn following exposure to a novel stressor is observed (Gardner et al., 2009). Finally, another aspect of 5-HT functioning that is modulated by maternal separation is the site-specific pre-mRNA editing of 5-HT2C receptor transcripts in the adult forebrain (Bhansali et al., 2007). The pre-mRNA for the 5-HT2C receptor possesses five editing sites, which can undergo adenosine to inosine nucleotide conversions leading to altered encoding of triplet codons and hence different isoforms and functional activity of the encoded G-protein-coupled receptor. Maternally separated mice (3 hours of daily separation from PN days 2–15) have significantly increased pre-mRNA editing at four of these five sites which is associated with reduced sensitivity to 5-HT and increased depression-like behavior. Interestingly, both post-weaning social enrichment and treating mice with fluoxetine from PN32-61 are able to reverse the behavioral effects of maternal separation, but only the fluoxetine treatment reverses the pre-mRNA editing phenotype (Bhansali et al., 2007).
The frequency and diversity of social contact with siblings and conspecifics during juvenile development can also induce shifts in adult behavioral phenotypes associated with multiple changes within monoamine pathways. Deprivation of both maternal and littermate contact through early weaning is associated with reduced social play, increased stress responses, and elevated anxiety-like and depression-like behavior (Janus, 1987; Kikusui et al., 2006; Shimozuru et al., 2007). Mice weaned at an early age (PN14) have significantly reduced levels of the 5-HT1B receptor in the hippocampus compared to standard weaned controls (weaned at PN21), although they have equivalent levels of 5-HT1A receptors (Nakamura et al., 2008). Post-weaning social isolation, typically starting at PN 21 and lasting from 6–8 weeks, is generally associated with an increase in anxiety-like and depression-like behaviors and in mice is associated with decreased expression of several 5-HT receptor subtypes in the PFC, hypothalamus, and midbrain (Bibancos et al., 2007). In rats, social isolation during the late juvenile period (PN38-51) is associated with latent elevations in DA levels in the NAc (Shao et al., 2009). Comparisons of rats reared in combinations of enriched or isolated environments suggest that exposure to social isolation during adolescence (from PN 21 for 6 weeks), regardless of exposure to subsequent enrichment (for an additional 6 weeks), leads to consistently reduced sucrose preference, suggesting permanently altered DA signaling (Hellemans et al., 2004). Like social isolation, social defeat stress during adolescence (PN35) leads to disordered emotional behavior and is associated with reduced DA in the mPFC, increased norepinephrine (NE) and 5-HT in the hippocampus, and increased NE in the DRn. Defeated rats also have significantly higher DOPAC levels in the substantia nigra pars compacta (SNc) but no change in DA, NE, or 5-HT in this region (Watt et al., 2009). In contrast, social enrichment during the post-weaning period (PN 23 to PN 43) is associated with increased levels of the DAT within the NAc and a trend for increased phosphorylated-tyrosine hydroxylase (TH – an enzyme in the dopamine production pathway) and DARPP-32 (an inhibitor of protein phosphatase 1 which integrates the activity between dopaminergic and glutamatergic signaling pathways) (Zakharova et al., 2009).
This plasticity of monoamine pathways in response to the social environment continues throughout adulthood, though effects are typically more transient compared to those emerging in response to earlier experiences. Though long-term social isolation (3 months) of adult rats leads to a significant decrease in TH mRNA, there are increased protein levels of TH and two other catecholamine biosynthesis enzymes, dopamine-beta-hydroxylase (DBH) and phenylethanolamine N-methyltransferase (PNMT) within the adrenal medulla in isolated rats compared to controls (Gavrilovic et al., 2008). A 4-week period of adult social isolation in rats (amongst 11 week old males) is associated with increases in DOPAC and 5-HIAA and an increase in the DOPAC/DA ratio (Miura et al., 2005). Adult isolation housing (7 weeks in duration) also reduces hippocampal neuronal proliferation and expression of genes associated with plasticity, including mRNA levels of the 5-HT2A receptor, brain derived neurotrophic factor (BDNF), and neuropeptide-Y (NPY) in the hippocampus compared to socially housed animals (Bjornebekk et al., 2007). Social enrichment of adult rats (30 days of enrichment at 3 months of age) has been found to increase NE in the dorsal hippocampus and decrease 5-HT and 5-HIAA in the ventral hippocampus (Galani et al., 2007). A 40-day period of enrichment in adulthood is associated with increased NE content within the parieto-temporo-occipital cortex, the cerebellum, and the pons/medulla, without any change in 5-HT or DA levels in these regions (Naka et al., 2002). Daily exposure to social defeat for 10 consecutive days results in an increase in anxiety-like and depression-like behaviors as well as a reduction in social investigation in adult mice and involves long-term changes in monoamine functioning as evidenced by the efficacy of chronic treatment with the SRIs fluoxetine or imiprimine in ameliorating the behavioral phenotypes associated with social defeat (Berton et al., 2006; Tsankova et al., 2006). These studies clearly demonstrate that the modulation of multiple monaminergic systems as a result of the social environment, with implications for emotional behaviors, though the precise mechanisms through which these effects occur and their interrelationship to one another remain to be elucidated.
B. GABA
Gamma-aminobutyric acide (GABA) is the principal inhibitory neurotransmitter in the CNS. GABA is synthesized from glutamate via the rate limiting enzyme glutamic acid decarboxylase (GAD) which is found in two forms, GAD65 and GAD67, in the nervous system (Erlander et al., 1991). There are two main types of receptors through which GABA exerts its actions – GABAA and GABAB. The inhibitory actions of GABA arise primarily through the GABAA receptor, a chloride ionophore, which allows negatively charged Cl- ions into the cell resulting in hyperpolarization. The receptor complex itself is a heteropentameric assembly that is comprised of 2α, 2β and one other subunit (either γ, δ or ε). The presence or absence of particular subunits has important implications for the synaptic localization and functional properties of the receptor (Barnard et al., 1998). Since the α and γ subunits form the benzodiazepine binding site, different subunit isoforms that make up the receptor may result in different degrees of Cl- influx and resulting neuronal potential (reviewed in(D'Hulst et al., 2009)). The GABAB receptor, on the other hand, is a G-protein coupled metabotropic receptor and is present at much lower levels in the brain. In contrast to adulthood, during development, activation of GABAA receptors results in membrane depolarization (via a chloride efflux), activating a host of second messenger molecules and transcription factors (e.g. CaMKI-IV, pCREB) (Ben-Ari et al., 2007; Obrietan et al., 2002; Owens et al., 1996). The resulting gene products (e.g. BDNF) exert widespread trophic actions, promoting neurogenesis, cell survival, cell differentiation, synapse formation and the morphological maturation of neural circuits (Borodinsky et al., 2003; Cancedda et al., 2007; Represa and Ben-Ari, 2005; Tozuka et al., 2005).
Early social experiences that engage the GABAergic system during early postnatal development are likely to have multiple effects. First, there are those that engage the trophic actions of excitatory GABA during its discrete excitatory period, which refine neuronal circuits and promote plasticity, and second, there are those effects that shape the inhibitory system for the remainder of life. Together, these effects have widespread implications for the physiology and behavioral functioning of offspring, some of which may be independent of GABA-specific changes (i.e. in receptor number or composition). Indeed, it has been shown that increases in trophic factors (e.g. neurotrophin-3 (NT-3) and BDNF) following a single episode of early handling at PN1 can be attenuated by antagonizing either NMDA, GABAA, 5-HT receptors or L-type voltage gated calcium channels (VGCC), emphasizing the role of excitatory activity and highlighting a potential role for excitatory GABA in mediating the effects of early handling in the neonate brain (Garoflos et al., 2005; Garoflos et al., 2007). Long-term changes in GABAA receptor number and subunit composition have also been reported following the early handling procedure in both the brain and periphery. Postnatal (PN1 to PN14) handling-induced increases in GABAA/central benzodiazepine (CBZ) receptors have been detected in a number of brain regions including the mPFC, locus ceruleus (LC), hippocampus and lateral (LA) and central amygdaloid (CeA) nuclei as well as peripheral tissues such as the adrenals and kidney (Bodnoff et al., 1987; Caldji et al., 2000; Weizman et al., 1999). These molecular changes have been correlated with increased exploration in a novel open-field, reduced startle responsivity, and decreased novelty-induced suppression of feeding; all of which suggest a reduction in anxiety-like behavior (Bodnoff et al., 1987; Caldji et al., 1998; Caldji et al., 2000). Further, variations in maternal behavior in the rat and between different strains of mice have been shown to regulate GABAA receptor subunit composition with implications for its receptor pharmacology (Caldji et al., 2000; Caldji et al., 2003; Caldji et al., 2004). It should be noted that cross-fostering studies confirm that shifts in the characteristics of the GABAergic system are associated with the quality of postnatal maternal care received.
The expression of specific GABAA receptor subunit genes and the resulting subunit composition of the receptor are also influenced by social isolation experienced during juvenile development. Social isolation (3 months in duration) reduces the extent of radioligand binding to brain GABAA/CBZ receptors (Insel, 1989; Miachon et al., 1990) and impairs the anxiolytic effect of diazepam in rats subjected to a social interaction test (Wongwitdecha and Marsden, 1996). In particular, increased immunoreactivity of the α4 and δ subunits (involved in seizure susceptibility and extrasynaptic localization) has been detected throughout the hippocampus of rats socially isolated for 30 days post-weaning compared to group-housed rats (Serra et al., 2008). Together, these changes likely act in concert to affect the overall functioning of the GABAA receptor. Further, the social isolation appears to effect the functional coupling between the recognition site of GABAA and its allosteric modulators (such as benzodiazepines and neurosteroids) as evidenced by the marked decrease in GABA-evoked Cl− currents in response to diazepam in isolated synaptosomes and within the cerebral cortex and hippocampus of brain slice preparations from isolated rats (Serra et al., 2000). In contrast, fewer studies have examined the consequences of social environmental enrichment on the GABAergic system, although there is evidence for increased GAD enzyme activity and extracellular GABA concentrations within the hippocampus following 30 days of enrichment (Frick et al., 2003; Segovia et al., 2006). In adulthood, an episode of social defeat results in transient changes in GABA receptor (those with benzodiazepine binding sites) levels in cortex, cerebellum and hypothalamus (Miller et al., 1987). Adult social defeat (a single prolonged episode) also increased α1 and and γ subunit mRNA expression in the cortex (but not cerebellum and hippocampus); an effect that persisted for 72h but returned to baseline after seven days (Kang et al., 1991). Thus, though the adult social environment can induce transient changes in GABA, any long-term effects are likely maintained by alterations within other neurotransmitter systems.
C. Glutamate
Glutamate is the main excitatory neurotransmitter in the brain, which acts on metabotropic and iontropic receptors. Of the iontropic receptors, NMDA, which permits entry of Ca2+ ions into the cell when activated, is considered to be critically important in plasticity, and underlies the construction and storage of memories throughout life. Other receptors of glutamate include the fast conducting Na+ ionophore, AMPA, and the metabotropic glutamate receptors (mGluR) which are G-protein coupled and activate intracellular signaling molecules to regulate excitation and transmitter release (reviewed in (Cartmell and Schoepp, 2000; Dingledine et al., 1999)). The subunits that comprise these receptors have important implications for the kinetic and functional properties of glutamate transmission. For example, a shift in expression from the NR2A to the NR2B subunit (one that keeps NMDA channels open for longer) results in a heightened response to glutamate (Monyer et al., 1994). On the other hand, a higher ratio of the NR2A:NR2B subunit might constrain plasticity (reviewed in (Cull-Candy et al., 2001)). Like GABA, changes in glutamate transmission following various social experiences can have a modulatory role in shaping long-term changes in other neurotransmitter or peptide systems (Korosi and Baram, 2008). Alternatively, social experiences can induce long-term changes in glutamergic components leading to permanent changes in the regulation of neural circuits (Barkus et al.; Bird and Lawrence, 2009).
Extended periods of maternal separation at, or prior to PN9 has been reported to cause reductions in the expression of the hippocampal NMDA receptor subunits, NR1 and NR2A and NR2B, as well as the AMPA receptor subunits, GluR1 and GluR2, in adult rat offspring (Bellinger et al., 2006; Pickering et al., 2006; Roceri et al., 2002). Moreover, these effects are mirrored by findings in offspring of low LG rat mothers who exhibit a deficit in NR1, NR2A and NR2B hippocampal subunit mRNA expression as adults (Bredy et al., 2003; Bredy et al., 2004; Liu et al., 2000). Similarly, in biparental species such as the California deer mouse, reduced paternal contact results in increased NR2A and decreased NR2B mRNA expression in the hippocampus (Bredy et al., 2007). Housing rats in isolation from the time of weaning for 4–13 weeks induces distinct cognitive impairments that include deficits in spatial and recognition memory, visuospatial attention and impulsivity, reversal learning and sensorimotor gating/pre-pulse inhibition (Bianchi et al., 2006; Dalley et al., 2002; Lu et al., 2003; Weiss et al., 2004). These effects are correlated with deficits in neural plasticity (e.g. decreased rates of hippocampal neurogenesis and decreased forebrain spine density), which are thought to stem from glutamatergic hypofunction (Abdelrahman et al., 2005; Lu et al., 2003; Silva-Gomez et al., 2003; Stranahan et al., 2006). Not surprisingly, isolation-rearing of rats, in some cases combining postnatal maternal separation and post-weaning isolation of varying durations, has been shown to induce increases in mGluRs, AMPA, and NR2A NMDA receptor subunit expression in the mPFC (Levine et al., 2007; Turnock-Jones et al., 2009; Zhao et al., 2009). In the hippocampus, similar increases in NR2A subunit expression are accompanied by decreases in NR1 mRNA in rats isolated for 9 weeks from the time of weaning (Hall et al., 2002). Social enrichment (50 days in duration) during the post-weaning period has been found to reverse the deficits in neural plasticity and cognition associated with low levels of maternal care in infancy by partially reversing the accompanying reductions in NMDA receptor subunit expression and initiating new changes (e.g. increases in AMPA receptor expression) in the hippocampus (Bredy et al., 2003; Bredy et al., 2004). It would appear that the recruitment of glutamatergic signaling mechanisms is a general mechanism whereby social enrichment is able to facilitate and compensate for deficits in neural plasticity. Indeed, post-weaning enrichment has been used to reverse cognitive deficits that result from prenatal stress (Koo et al., 2003), postnatal lead exposure (Guilarte et al., 2003), social isolation (Lu et al., 2003), and gene-knockout models of cognitive disease (e.g. Fragile X, (Rampon et al., 2000; Restivo et al., 2005). In some cases, these effects have been shown to occur through changes in glutamate receptor expression or signaling cascades associated with glutamate transmission (Arai et al., 2009; Rampon et al., 2000; Restivo et al., 2005). Furthermore, the experience of agonistic behaviors in adulthood during social defeat has been demonstrated to increase NMDA receptor binding in the CA3 of the hippocampus and decrease AMPA receptor binding in several other hippocampal regions (Krugers et al., 1993) which may indicate a sensitivity to glutamate transmission following social defeat. Moreover, social defeat stress has been shown to increase the expression of the GluR1 subunit of the AMPA receptor in the VTA (Covington et al., 2008) and blockade of NMDA receptors in the basolateral amygdala (BLA) prevents the acquisition of conditioned defeat confirming that long-term changes to glutamergic activity are required for the acquisition of the changes in emotional behaviors associated with social defeat (Jasnow et al., 2004a).
D. Vasopressin
The function of the peptide hormone arginine vasopressin (AVP) has been explored in a broad range of contexts, including physiology/metabolism, social behavior, aggression, reproduction, and stress responsivity. In rodents, postnatal maternal separation has generally been associated with elevated AVP mRNA expression and immunoreactivity in the paraventricular nucleus of the hypothalamus (PVN) and a subsequent enhanced release of adrenocorticotropic hormone (ACTH) and corticosteroids, through activation of vasopressin 1B receptors (V1Br), of adult and juvenile rats and mice (Vazquez et al., 2006; Veenema et al., 2006; Veenema et al., 2007), though some studies only find this increase in subjects undergoing a subsequent exposure to stress (Veenema et al., 2006). In adulthood, chronic social defeat leads to the increased expression of AVP mRNA in the parvocellular neurons of the PVN and related increases in mouse anxiety-like and depression-like behavior (Erhardt et al., 2009; Keeney et al., 2006; Veenema et al., 2006), and 6 weeks of post-weaning social isolation in male prairie voles has also been found to elevate both anxiety-like behavior and AVP mRNA expression in the PVN (Pan et al., 2009). However, paradigms using other forms of social stress, such as long-term social isolation (early weaning combined with 2 months of post-weaning isolation) in male rats (Sanchez et al., 1998) and social hierarchy formation in large groups of mixed-sex adult rats (Choi et al., 2006) have found no effect on AVP mRNA expression in the PVN. Moreover, reduced AVP mRNA expression in the PVN has been found in response to chronic subordinate colony housing (Veenema et al., 2008), 21 days of post-weaning social isolation in female prairie voles (Ruscio et al., 2007) and 2 weeks post-weaning social isolation of male rats (Tanaka et al., 2010), though these experiences still lead to increased anxiety-like behavior. Similarly, one study has found an increase in the number of AVP immunopositive cells in the magnocellular neurons of the PVN in male rats that were briefly maternally separated (1 minute/day from PN1 to PN10) during the early postnatal period (Todeschin et al., 2009). These studies highlight the importance of considering plasticity in other neuroendocrine systems as well as the type and timing of social experiences, gender, species and genetic background when attempting to establish associations between variations in the social environment and changes in the AVP system and emotional behavior.
Social experiences may also induce variation in the expression and distribution of vasopressin receptors, particularly vasopressin receptor 1A (V1Ar). During early development, high LG by rat dams has been found to lead to elevated levels of V1Ar in the CeA of male offspring (Francis et al., 2002b). Maternal separation leads to region-specific shifts in V1Ar binding during different developmental periods of male rats and notably V1Ar binding in the lateral septum (LS) is enhanced in maternally juveniles exposed to maternal separation for 3 hours daily from PN1 to PN14 (Lukas et al., 2010). Adult male rats who become subordinate following being housed with a dominant male have elevated V1Ar in the LS (Askew et al., 2006), whereas a later age of weaning (PN28 vs. PN21) in mice is associated with elevated levels of V1Ar in several hypothalamic regions of female mice (Curley et al., 2009b). Female mice who are communally reared (an early form of social enrichment) throughout the postnatal/pre-weaning period display reduced anxiety-like behaviors and have reduced V1Ar binding in the LS (Curley et al., 2009a). Significantly, V1Ar agonists and antagonists administered into the LS lead to enhanced and reduced anxiety-like behavior respectively and over-expression of the V1Ar gene in the LS also increases anxiety-like behavior, suggesting that the expression of V1Ar in this region may indeed be fundamental to modulating the central effects of vasopressin on emotional phenotypes (Bielsky et al., 2005; Caldwell et al., 2008; Landgraf et al., 1995; Liebsch et al., 1996).
E. Oxytocin
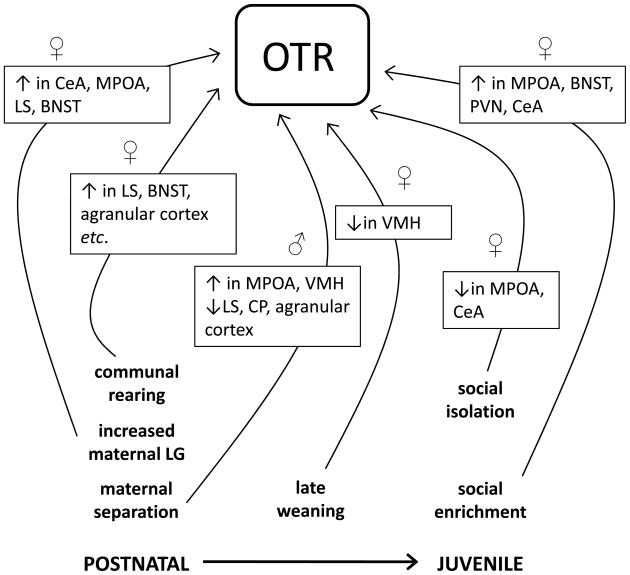
The nonapeptide oxytocin (OT) is synthesized primarily in the PVN and supraoptic nucleus (SON) and plays a key role in social, sexual and parental behaviors. It acts upon the oxytocin receptor (OTR), which is located in several brain regions, including the cortex, limbic system, hypothalamus, and brainstem. OTR expression and binding have been shown to be particularly sensitive to long-term change in response to differential social experiences (see Figure 1). For instance, OTR binding in several areas of the female brain is altered in offspring reared by high LG rat dams compared to low LG dams, with OTR binding elevated in the CeA, medial preoptic area (MPOA), LS and bed nucleus of the stria terminalis (BNST) of female offspring of high LG dams (Champagne et al., 2001; Francis et al., 2000; Francis et al., 2002b). Male rats exposed to maternal separation 3 hours daily from PN1 to PN14 have lower OTR binding in the agranular cortex (in young adulthood) and the LS and caudate putamen (as adults), but higher OTR binding in the MPOA (as juveniles) and ventromedial hypothalamus (VMH) (as adults) compared to control rats (Lukas et al., 2010). Female mice that are communally reared throughout postnatal development have elevated OTR binding in several brain regions (LS, BNST, agranular cortex, endopiriform cortex) as adults compared to female mice reared by just one dam (Curley et al., 2009a). Moreover, adult female mice that are weaned at day PN21 had higher OTR binding in the VMH compared to mice that had extended mother-infant and sibling contact through being weaned at day PN28 (Curley et al., 2009b). Juvenile social enrichment in female rats from weaning (PN21) for 50 days has also been found to elevate OTR binding in a number of forebrain and hypothalamic areas including the CeA (Champagne and Meaney, 2007). Thus, there is a strong link between developmental social experiences, occurring in infancy and later amongst juveniles, and alterations within the OT system.
Figure 1. Illustration of the social modulation of oxytocin receptor levels in the rodent brain during postnatal and juvenile development.
Social experiences occurring during the postnatal period, in the form of variation in mother-infant interactions, maternal separation, and communal nursing, and extending into juvenile development (late weaning, post-weaning variation in social housing), have been demonstrated to alter oxytocin receptor (OTR) levels in the brain. Indicated in the figure are findings highlighting these influences, which illustrate region-specific effects on OTR in response to the social environment. It is important to note that the impact of a particular social experience will vary dependent on the species, sex, and timing of the experience. The complexity of effects on OTR levels highlights the diverse pathways through which experiences occurring during development can alter functioning within neural systems which shape behavior. (BNST – bed nucleus of the stria terminalis; CeA – central amygdala; CP – caudate putamen; LS – lateral septum; MPOA – medial preoptic area; PVN –paraventricular nucleus; VMH – ventromedial hypothalamus).
The link between the social environment, modulation of OT pathways, and behavioral outcomes is not always straightforward. For example, maternal separation during the first two weeks of life (3 hours daily) has been found to increase anxiety-like behavior in both male and female mice but separation-induced decreases in levels of OT are only found in the PVN of lactating female mice (Veenema et al., 2007). Conversely, brief maternal separations induce consistent decreases in anxiety-like behavior in males and inconsistent behavioral effects in females. These variable behavioral findings are mirrored by variable neurobiological changes. For instance, in one study, brief maternal separations (1-2minutes daily from PN 1 to PN 10) are associated with a decrease in the number of OT positive neurons in the parvocellular region of the PVN in adult female but not male rats (Winkelmann-Duarte et al., 2007), while in another study both male and female offspring exhibit decreases in the number of OT positive neurons (Todeschin et al., 2009). Furthermore, prairie voles reared with one mother exhibit reduced anxiety-like behavior and have higher OT mRNA expression in the PVN compared to biparentally reared prairie voles (Ahern and Young, 2009). Post-weaning, social isolation for 3 weeks did not alter OT immunoreactivity in female prairie voles (Ruscio et al., 2007) whereas in another set of studies, 4 weeks of post-weaning social isolation induced increased depressive behaviors and elevated stress reactivity in female prairie voles accompanied by a higher number of OT positive neurons in the PVN, with limited isolation effects reported in males (Grippo et al., 2007a; Grippo et al., 2007b). In a third study, a similar up-regulation of OT mRNA in the PVN was found after six weeks of social isolation in males who also showed enhanced anxiety-like behavior (Pan et al., 2009). Finally, in female rats, two weeks of post-weaning social isolation induced decreases in the number of OT positive cells in the parvocellular neurons of the PVN, though this manipulation did not alter anxiety-like behavior (Tanaka et al., 2010). In contrast, male rats isolated for the same period of time did develop an anxiogenic profile but had no change in OT immunoreactivity in the PVN. These variable findings suggest that the effects of the social environment in modulating behavior may not necessarily be as a direct result of long-term modifications to the OT system but occur through indirect interactions between OT and other neuroendocrine systems.
F. Estrogen & Estrogen Receptors
The influence of gonadal hormones can be observed on cellular morphology throughout the body, on physiological responses, and on neurobiological and behavioral outcomes. Within the brain, estrogen and can act either directly to modify neural activity or indirectly through cascades of changes in gene expression. Genomic effects of estrogen are achieved through activation of nuclear estrogen receptors (ERα and ERβ). ERα and ERβ have distinct expression patterns within the CNS such that ERα mRNA is highly expressed within the MPOA and VMH and ERβ is expressed within the hippocampus, DRn, mPFC, and PVN (Shughrue et al., 1997; Shughrue et al., 1998). Disruptions to the early life environment have been demonstrated to alter the cyclic variations in behavior of females related to estrus state, possibly through effects on responsiveness to estrogen signaling. Neonatal maternal isolation (1 hour daily from PN2 to PN9) in female rats has been found to eliminate estrus dependent changes in cocaine-induced locomotor activity (Kosten et al., 2005). Female offspring reared under conditions of complete maternal deprivation, through use of an artificial rearing paradigm, display reduced levels of hormonally-primed maternal behavior (Novakov and Fleming, 2005). Reduced levels of mother-infant interactions induced through maternal exposure to predator odor on the day of parturition are associated with elevated levels of ERα and ERβ mRNA in the MPOA of female offspring compared to control-reared females (McLeod et al., 2007). Natural variations in maternal care have been associated with altered gene expression and receptor levels within the MPOA and females reared by dams that exhibit low levels of LG have a reduced sensitivity to estrogen-mediated increases in neuronal activation within the MPOA (Champagne et al., 2001, Champagne et al., 2003b). Analysis of levels of ERα in the offspring of High and Low LG dams suggest that differences in estrogen sensitivity are mediated by variations in ERα levels such that expression of ERα in the MPOA of both lactating and non-lactating female offspring of Low LG dams is significantly reduced compared to that of the offspring of High LG dams (Champagne et al., 2003b). Conversely, ERα levels in the anterioventral paraventricular nucleus is elevated amongst the female offspring of Low LG dams and these females are more sensitive to estrogen induced ERα activation within this region (Cameron et al., 2008). Thus, site-specificity is a critical feature of the impact of social experiences on estrogen receptor levels and this specificity will have implications for the behavioral consequences of early life exposures.
The experience of extended periods of social isolation during juvenile development has also been found to alter estrogen levels. E2 biosynthesis from testosterone in gonadal tissue was found to increase in female rats housed in isolation from weaning and slightly decrease in isolated males when compared to group housed males and females (a housing condition consisting of mixed sex groups; (Fulgheri et al., 1975). Amongst 60 day-old rats, the effects of juvenile isolation vary dependent on the nature of the isolation, with plasma estradiol being altered in unisex vs. mixed-sex housing and isolated vs. isolated with contact (a wire mesh separation between individual rats). Amongst both males and females, estradiol has been found elevated in mixed-sex socially housed rats compared to isolates (with and without contact) and amongst males, post-weaning isolation rearing results in decreased estradiol compared to both unisex and mixed-sex reared offspring (Lupo di Prisco et al., 1978). These data highlight the dynamic influence of reproductively relevant stimuli on the levels of gonadal hormones that has been demonstrated to shift patterns of affiliative, aggressive, and sexual behaviors in adult males and females. Within the brain, levels of ERα can be altered in response to variations in juvenile housing conditions experienced by prairie voles. Comparison of isolated males and females to those housed with either a sibling or an unfamiliar same-sex conspecific for 3 weeks post-weaning indicated decreased levels of ERα in the MPOA and BNST of isolated females (particularly compared to those housed with an unfamiliar female) though levels of ERα were not significantly decreased in the MPOA and BNST of isolated males (Ruscio et al., 2009). Consequently, sex differences in ERα (males having elevated levels compared to females) were apparent in isolation-reared but not socially reared offspring. Thus, the quality of the social environment experienced at the time of puberty onset and into adulthood can, much like the postnatal environment, shift responsiveness to gonadal hormones with implications for sexual dimorphism in behavior.
E. Corticotropin Releasing Hormone (CRH) and Glucocorticoid Receptors
The hypothalamic-pituitary-adrenal (HPA) response to stress, resulting in elevated circulating levels of glucocorticoids, can have both acute and long-term consequences for behavior. CRH is also released from neurons with the amygdala, hippocampus and LC and can have local neuromodulatory effects on target neurons possessing CRH1 or CRH2 receptors thereby regulating behaviors centrally independently of the HPA-axis (Korosi and Baram, 2008). Within the brain, glucocorticoids act on glucocorticoid (GR) or mineralocorticoid receptors (MR) located in several brain regions (MRs –hippocampus, LS, amygdala, PVN, LC; GRs – expressed throughout the brain particularly in the hippocampus, LS and PVN) and the pituitary. MRs have a strong affinity for glucocorticoids and so are generally occupied even at low levels such as basal conditions, however, GRs have a lower affinity for glucocorticoids and as such tend to only be occupied following stress. Levels of GR within the hippocampus have been demonstrated to enhance negative-feedback of the HPA response to stress, thus serving as a critical neural system in the regulation of emotionality (Meaney et al., 1991b).
The effects of social experiences on modulating the actions of CRH and HPA reactivity have been explored extensively (for reviews see (Korosi and Baram, 2008; Korosi and Baram, 2009; Lupien et al., 2009)). Here, we will focus on studies in which region-specific variations in CRH receptors, MR, or GR in the brain have been explored and highlight broad findings that have been most consistently reported, though it is important to acknowledge that there is variation in these results depending upon contextual factors such as the type of paradigm used and species, strain, sex and age of the animals. In early development, social experiences that promote increased levels of tactile stimulation generally lead to long-term reduction in CRH-mediated HPA stress reactivity. Studies in mice illustrate that offspring reared in communal nests have a blunted neuroendocrine response social stress (Branchi et al., 2010). Adult rats that are reared by high LG dams have attenuated stress responsivity and higher expression of GRs in the hippocampus, compared to rats reared by dams that provide low levels of LG during the first week (Francis et al., 1999; Plotsky and Meaney, 1993). Likewise, mice and rats (particularly males) that undergo daily brief maternal separations (15 minutes daily) during the first weeks of life have an attenuated response to stress (Meaney et al., 1991a; Plotsky and Meaney, 1993; Viau et al., 1993), associated with increased GR mRNA expression in the hippocampus, that are induced in part through increased sensory stimulation derived from the mother on reunion with her pups (Avishai-Eliner et al., 2001; Fenoglio et al., 2005; Liu et al., 1997). Mechanistically, it appears that a reduction in CRH mRNA expression facilitates this effect, as this maternally induced difference can be seen as early as PN9 which precedes both the increase in hippocampal GR expression at PN23 and the altered stress phenotype (Avishai-Eliner et al., 2001). Moreover, this same effect can be achieved developmentally through reducing the accessibility of CRH to its CRH1 receptor (Fenoglio et al., 2005). In contrast, postnatal maternal separation (3 hours daily throughout the postnatal period) induces increased stress-reactivity (Caldji et al., 1998; Plotsky and Meaney, 1993; Wigger and Neumann, 1999) associated with reduced GR and MR expression in the hypothalamus and hippocampus, and regional changes in CRH1r expression (Ladd et al., 2005; Plotsky and Meaney, 1993). Another form of maternal deprivation, the early permanent removal of mouse pups from their mother on PN14 (early weaning), elevates HPA activity in adult males under basal and stressful conditions and induces a reduction in GR mRNA expression in the hippocampus (Kikusui et al., 2006). Overall, these results would suggest significant plasticity in the levels of GR in response to early social stimuli that may induce long-term changes in HPA functioning.
Juvenile social isolation and enrichment effects on HPA responses or CRH/glucocorticoid receptors have also been explored extensively, however, the majority of studies report few or inconsistent long-term effects on this system (for a review see (Lukkes et al., 2009c)). For instance, in several studies in rats, no effect of post-weaning social isolation was found upon GR and MR levels in the hippocampus (Lukkes et al., 2009b; Schrijver et al., 2002; Weiss et al., 2004). In studies which do report a significant influence of these juvenile experiences, effects are dependent upon sex, the type of stressor utilized and the degree of social isolation and re-socialization (Lukkes et al., 2009c). For instance, male rats that were socially isolated for three weeks and then re-socialized for two weeks, exhibited an increase in NAc 5-HT release that is mediated by CRH2 receptor activation in the DRn (Lukkes et al., 2009a; Lukkes et al., 2009c). Indeed, using this paradigm, socially isolated male rats were found to have elevated levels of CRH2 receptors in the DRn which project directly to the NAc, and the behavioral phenotype exhibited by these males, characterized by elevated social anxiety, can be reversed through CRF2r blockade. In the case of juvenile social enrichment, though in general there is an attenuation of the HPA response to stress (Belz et al., 2003) , some studies report no change in basal levels (Morley-Fletcher et al., 2003) and others even find an increase (Marashi et al., 2003). Indeed, in cases where social enrichment is able to attenuate elevated stress responses, it appears that this may not be achieved by reversing altered GR expression. For instance, while 7 weeks of post-weaning social enrichment was found to reverse the elevated HPA-axis stress response induced by maternal separation in male rats, no differences in GR levels in the hippocampus were found (Francis et al., 2002a). However, adult male rats that are unmanipulated prior to weaning and then subjected to 30 days of social and physical enrichment have been shown to have elevated GR levels in the hippocampus accompanied by higher GR mRNA expression, induced by elevated levels of nerve growth factor inducible protein A (NGFIA) (Olsson et al., 1994). Alternatively, the anxiolytic effect of post-weaning environmental enrichment may occur through alterations to CRH receptors as this experience in female mice leads to a reduced expression of CRH1r mRNA in the BLA (Sztainberg et al., 2010).
In adulthood, a single social defeat is associated with prolonged alterations to the HPA stress response and emotional behavior, and changes to the expression of central CRH receptors may underlie some of these long-term changes (Buwalda et al., 1999; Cooper and Huhman, 2007). Studies using selective and nonselective CRH receptor antagonists injected into the DRn of male Syrian hamsters suggest that CRH1r is utilized for the acquisition of conditioned defeat (i.e. a persistent subordinate/inhibited phenotype) following social defeat, and that CRH2r regulates the expression of these stress-related behaviors after defeat (Cooper and Huhman, 2007). Studies have also implicated the activity of CRH receptors in other brain regions as being significant. Acute social defeat followed by 75 minutes of housing with the aggressor male leads to an increase in CRH2r mRNA expression in activated c-fos expressing neurons of the medial amygdala following defeat (Fekete et al., 2009). In hamsters, administration of a selective CRH2r antagonist into the BNST (but not amygdala) before social defeat prevented defeated males from developing conditioned defeat (Cooper and Huhman, 2007; Jasnow et al., 2004b). However, selective blockade of CRH1r infused into the BLA following social defeat in male mice leads to defeated males failing to show long-term changes in generalized fear responses (Robison et al., 2004). Therefore, there is strong evidence for the role of CRH and its receptors in the long-term acquisition and expression of behavioral changes following social defeat, though the precise role of these neural systems and its interaction with other systems remains to be delineated.
The Role of Epigenetic Mechanisms in Shaping the Brain
In considering the behavioral variation that occurs in response to variations in the social environment, the mechanisms underlying persistent increases or decreases in neurotransmitter release, receptor levels and hormonal activation described in the previous sections must be determined. Advances in molecular biology have identified processes through which dynamic yet stable alterations in the activity of genes can be induced. The epigenetic regulation of transcription is a critical feature of the link between genotype and phenotype and refers to those factors which control accessibility of DNA to transcription and which can alter the levels of gene expression (either silencing genes or increasing transcriptional activity) without altering the sequence of DNA. The molecular mechanisms through which these epigenetic effects are achieved are diverse, including histone protein modifications and DNA methylation (Feng et al., 2007; Razin, 1998). Within the cell nucleus, DNA is wrapped around a core of histone proteins which can undergo multiple post-translational modifications including methylation, acetylation and ubiquination (Peterson and Laniel, 2004; Zhang and Reinberg, 2001). These modifications alter the dynamic interactions between the histones and DNA which either reduce or enhance the accessibility of DNA. In particular, histone acetylation is associated with increased transcriptional activity whereas histone deacetylation or methylation is typically associated with transcriptional repression. Acetylation of histones is mediated by the enzyme histone acetyltransferase (HAT) whereas histone deacetylase (HDAC) promotes removal of the acetyl group from the histone tails. Thus, through alterations in the conformation of histones, the accessibility of DNA can be rapidly and reversibly altered. In contrast, DNA methylation represents what is generally considered a more stable and enduring modification to the activity of genes. DNA methylation occurs when cytosine nucleotides, usually located in CpG islands, within DNA become converted to 5-methylcytosine. This process is mediated by methyltransferases which either promote maintenance (i.e. DNMT1) or de novo DNA methylation (i.e. DNMT3) (Feng et al., 2007; Razin, 1998; Turner, 2001). The conversion to 5-methylcytosine does not alter the DNA sequence but does reduce the likelihood that that sequence of DNA will be transcribed. Methylated DNA attracts methyl-binding proteins, such as MeCP2, which further reduce the accessibility of the gene and is associated with transcriptional repression (Fan and Hutnick, 2005). The stability of DNA methylation patterns within the genome permits the stable regulation of gene expression associated with cellular differentiation. Importantly, during cell division, both DNA and DNA methylation patterns are inherited by daughter cells, thus allowing differentiated cells to transmit their phenotype to the next generation of cells (Fukuda and Taga, 2005).
There is emerging evidence for the role of epigenetics in understanding the persistent effects of environmental exposures on the activity of genes with implications for brain and behavior. Moreover, these epigenetic effects are not limited to nutritional and toxicological exposures, as there is support for the hypothesis that the sustained effects of variations in social experience can be linked to epigenetic variation, particularly in DNA methylation patterns. The link between variations in postnatal maternal care, altered DNA methylation, and long-term changes in behavior in rodents, supports the role of this molecular mechanism in shaping individual differences in neurobehavioral outcomes. As described in the previous sections, natural variations in postnatal maternal LG in the rat are associated with changes in numerous receptor pathways, with effects on hippocampal GR being implicated in the high levels of HPA reactivity observed amongst offspring who receive low levels of LG (Liu et al., 1997). Analysis of the GR 17 promoter region suggests that low levels of LG are associated with increased GR 17 methylation, decreased GR expression and an increased HPA response to stress. Time course analysis has indicated that these maternally induced epigenetic profiles emerge during the postnatal period and are sustained into adulthood (Weaver et al., 2004). The pathways through which these effects are achieved are currently being elucidated and it appears likely that maternal LG mediated up-regulation of NGFI-A in infancy may be critical to activating GR transcription and maintaining low levels of DNA methylation within the GR 17 promoter amongst the offspring of High LG dams (Weaver et al., 2007) (see Figure 2). Similarly, increased levels of ERα observed in the MPOA of the offspring of High LG dams may be induced through LG-mediated activation of the promoter region of this gene. Analysis of levels of DNA methylation within the 1B promoter region of the ERα gene in MPOA tissue indicates that High LG is associated with decreased promoter methylation whereas Low LG is associated with increased promoter methylation, leading to reduced gene expression and an attenuated response to hormonally-primed behaviors (Champagne et al., 2006). Chromatin immunoprecipitation assays demonstrate that this differential DNA methylation has consequences for the binding of transcription factors such as STAT5a to the 1B promoter. Maternal LG is associated with increased levels of STAT5a during the postnatal period and the increased levels of this factor may lead to sustained activation of transcription and reduced DNA methylation.
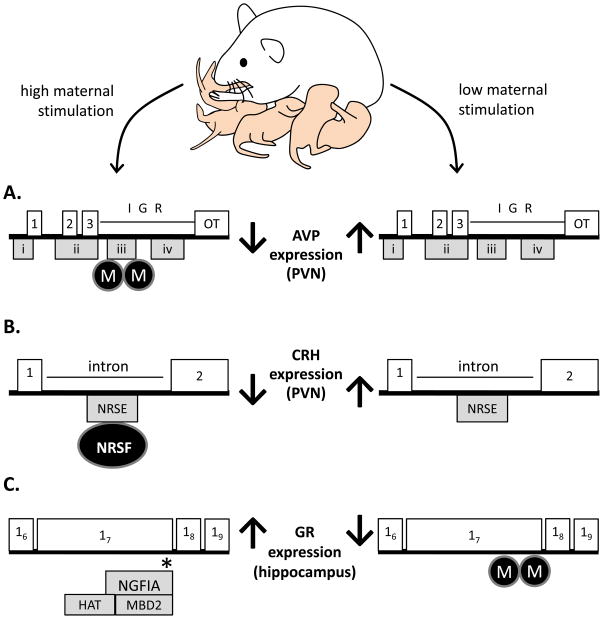
Figure 2. Variations in early life maternal stimulation can alter offspring gene expression via epigenetic mechanisms.
A) AVP exons 1, 2, and 3 (numbered white boxes) are separated from oxytocin exons (white box labeled OT) by a highly conserved intergenic region (IGR). CpG DNA methylation sites cluster in four islands (CGI 1–4; grey boxes marked i, ii, iii, iv), with DNA methylation at specific CpG sites within CGI3 (such as CpG10 marked by * which acts as a binding site for MeCP2) being the most significant for AVP mRNA expression. By PN10, maternal separation induces the phosphorylation of MeCP2 (via activation of the protein kinase CaMKII) preventing its functioning, reducing DNA methylation (M) and increasing AVP mRNA expression. These changes in methylation and gene expression persist into adulthood. B) In the intronic region between exons 1 and 2 (white boxes) of the CRH gene lies its regulatory region that contains a 21 bp sequence (NRSE) that is specifically bound to by the transcriptional repressor NRSF, which then recruits cofactors and induces epigenetic modification of gene expression. By day PN9, handled rat offspring show increased binding of NRSF to the NRSE that reduces CRH gene expression in the PVN permanently into adulthood. C) Exon 1 of the rat hippocampal GR gene contains multiple regulatory regions that are able to activate gene transcription including the brain-specific exon 17 promoter which contains an NGFI-A binding sequence (marked by *). High levels of tactile stimulation in the form of maternal LG increases the levels of intracellular 5-HT in the hippocampus which activates protein kinase and CREB signaling pathways leading to elevated levels of NGFI-A. When NGFI-A is bound to its binding site on the exon 17 promoter it recruits the HAT CREB-binding protein (CBP) and the DNA demethylase MBD2 to increase histone acetylation and decrease DNA methylation (M). This demethylation takes place by day PN6 and persists to adulthood.
An epigenetic response to variations in the quality and frequency of mother-infant interactions has also been demonstrating using postnatal maternal separation and handling. Daily and prolonged maternal separation has effects on a broad range of neurotransmitter and neuropeptide systems, as described in the previous sections, and in a recent study, significant increase in AVP mRNA in the parvocellular neurons of the PVN which persist for a year following this early social experience were confirmed in mice (Murgatroyd et al., 2009). Demonstrating the functional importance of this increased expression, the HPA hyperactivity and behavioral indices of anxiety-like and depressive responses observed amongst maternally separated mice could be attenuated if the mice were treated with a V1Br antagonist. Within the AVP gene, there are four regions rich in CpG islands that could potentially regulate gene expression through DNA methylation. Analysis of PVN tissue reveals that at one of these four regions (CGI3) maternally separated males have significantly reduced DNA methylation compared to control males at 6 weeks, 3 months and 1 year of age. Furthermore, this hypomethylation is significantly correlated with increased mRNA expression, and these effects are brain region specific as no changes in AVP mRNA or DNA methylation exist between maternally separated and control males in the SON. Analysis of the time-course of the molecular changes involved in this differential methylation suggests that short-term activation of the methyl binding protein, MeCP2, may be a critical factor within the pathways leading to AVP hypomethylation and increased AVP mRNA levels within the PVN (see Figure 2). Conversely, in response to brief postnatal maternal separation (handling), reductions in CRH mRNA in the parvocellular neurons of the PVN can be observed as earlier as PN9 and at this developmental time-point, PVN levels of the vesicular transporter of glutamate (vGlut2) (a marker for the activity of excitatory glutametergic inputs into CRH neurons) is also significantly lower in handled pups (Korosi et al., 2010). However, these alterations in vGlut2 levels are transient and are not observed at later stages of development and, while they may be responsible for short-term changes in CRH mRNA, they cannot explain the long-term changes. Within the regulatory region of the CRH gene resides a binding element (NRSE) for the repressor neuron-restrictive silencer factor (NRSF) (Seth and Majzoub, 2001). When bound, this factor recruits co-factors and other enzymes/proteins involved in epigenetic regulation leading to the repression of gene expression (Zheng et al., 2009). Amongst handled offspring, protein levels of NRSF are dramatically higher in PVN tissue at PN9 and throughout adulthood, suggesting a possible mechanism for the initiation and maintenance of reduced CRH gene expression in response to handling-induced stimulation of mother-infant interactions (see Figure 2). Overall, these studies suggest that both up- and down-regulation of gene expression relevant to emotional phenotypes can be achieved through the recruitment of epigenetic mechanisms in response to early life social experiences.
In considering the neurobiological targets that are modulated by the social environment, one of the common downstream mediators of many of the neural systems described in this review is BDNF. For example, BDNF plays a critical role in the synaptic maturation triggered by GABA signaling (Represa and Ben-Ari, 2005), and increases in BDNF are thought to mediate changes in structural plasticity in the brain associated with elevated levels of estradiol (Sato et al., 2007) and glutamate (Marini et al., 1998). Activation of the HPA response to stress is associated with increases in BDNF (Tapia-Arancibia et al., 2004) and CRH mediated increases in BNDF can be inhibited through antagonism of CRH1r (Bayatti et al., 2005). Interactions between BDNF and the mesolimbic dopamine system may modulate the response to reward, effects of psychostimulants, and depression (Berton et al., 2006; Nestler and Carlezon, 2006; Thomas et al., 2008), and BDNF acts during development to shape dopaminergic, serotonergic, and GABAergic neuronal populations (Eaton et al., 1995; Lu et al., 2009; Zhou et al., 1996). Epigenetic effects on BDNF of both postnatal and adult social experiences have been explored, and suggest that this activity of this gene may be highly susceptible to environmental regulation. In a rodent model of postnatal abuse, in which females engage in an increased frequency of rough handling, dragging, dropping, and stepping on pups (Roth and Sullivan, 2005), offspring exposed to these abusive social interactions are found to have reduced expression of BDNF in the prefrontal cortex in adulthood associated with increased DNA methylation within the BDNF IV promoter region (Roth et al., 2009). In adulthood, social defeat has likewise been demonstrated to alter BDNF levels. BNDF gene expression is significantly decreased in the hippocampus of socially defeated male mice and this effect appears to be mediated by specific decreases in the BDNF III and IV transcripts (Tsankova et al., 2006). These effects are observed a month following exposure to the social stress, indicating a persistent effect on gene expression. Chromatin immunoprecipitation assay analysis indicates increased histone H3-K27 dimethylation at the BDNF III and IV promoters amongst socially defeated males which may account for the reduced BDNF expression. Histone deacetylase (HDAC5) mRNA levels are also found to be decreased in socially defeated males (Tsankova et al., 2006) and HDAC5 appears to be important in mediating the effects of anti-depressant treatment in males exposed to chronic social stress (Renthal et al., 2007). The differential levels histone H3-K27 dimethylation are also found across the genome within the NAc, both in response to chronic social defeat and prolonged adult social isolation (Wilkinson et al., 2009). Analysis of histone acetylation in the NAc indicates that H3-K14 acetylation is initially decreased and then increased following chronic social defeat associated with decreases in HDAC2 levels. Interestingly, post-mortem analysis of brain tissue from depressed patients indicates increases in H3-K14 acetylation and decreased HDAC2 levels similar to those observed in socially defeated mice (Covington et al., 2009), suggesting that there may be an environmentally induced-epigenetic substrate associated with mood disorders in humans.
It is important to note that studies exploring the relationship between social experiences, epigenetic variation, gene expression, and behavioral outcomes have relied primarily on a target gene approach, rather than exploring the global effects of these manipulations. Given the very broad influences of social experiences occurring at different developmental time-points that are illustrated even amongst the subset of targets we have explored in this review, there are likely multiple systems that are regulated through DNA methylation and histone modifications. Investigation of epigenetic regulation within these systems may yield important new insights into the processes through which long-term variations in phenotype are achieved.
Challenges and Future Directions
Though there is significant empirical support for the role of epigenetic mechanisms in mediating the effects of social experiences on neurobiological development and behavioral, it is clear that the relationship between the social environment and specific neurobehavioral outcomes, particularly when considering indices of emotionality, is complex. Thus addressing this complexity, and identifying issues that need to be considered in the study of the social modulation of brain and behavior, is a critical step in advancing our understanding of the origins of depression and anxiety in humans. Here we will highlight several of these issues.
i) Establishing a consistent link between environmental experiences, neuronal changes, and behavior throughout development
Though we typically design studies to explore the role of a particular neurotransmitter, hormone, or brain region in mediating anxiety or depression, this approach fails to capture the complexity of the interactions between neural systems. Importantly, the impact of activity of one system can only be considered within the context of the activity in other brain regions and neurotransmitters/receptors, and it is clear from the literature highlighted in this review that there are complex and interdependent effects of the social environment on multiple neural systems. A closely related issue is that though there may be some degree of consistency in the effect of social environment on the direction of changes within specific neural systems, however, this consistency does not always extend to the behavioral phenotypes that emerge in response to particular social experiences. For example, though reductions in AVP are typically associated with reduced anxiety-like behavior, the decreases in AVP mRNA expression in the PVN that has been demonstrated in response to various manipulations of the social environment, including subordinate colony housing (Veenema et al., 2008) and post-weaning social isolation (Ruscio et al., 2007; Tanaka et al., 2010) are associated with increased indices of anxiety-like behavior. One possible explanation for the discordance between neurobiological vs. behavioral changes may be related to the differential time-course through which environmental effects are achieved on these systems. To address this issue, a greater focus on biobehavioral measurements across development rather that at a single time-point in adulthood needs to be incorporated into the study of social modulation of behavior and a consideration of the particular sensitive periods for different neural systems and subsequent behavioral outcomes would help inform the experimental design of these studies. Though challenging to implement, this strategy may also advance our understanding of the pathways through which environmentally-induced short-term effects may lead to specific long-term outcomes, as was illustrated by the recent studies of the epigenetic effects on AVP and CRH (Korosi et al., 2010; Murgatroyd et al., 2009) in response to early life adversity.
ii) Interactive and interdependent influences of early and later life social experiences on the brain
The influence of the social environment extends beyond the perinatal period, as illustrated by the impact of juvenile and adult social experiences on shaping neurobiological pathways. This plasticity across the lifespan suggests that 1) amelioration of deficits induced by exposures occurring early in development may be achieved through later environmental intervention, and 2) the nature of the effects of later life experiences may vary dependent on the quality of the social experiences occurring early in development. The effectiveness of post-weaning social enrichment to reverse the effects of maternal separation or low levels of maternal LG illustrates this plasticity. In some cases, these studies suggest that although behavioral deficits can be ameliorated by these later experiences, the underlying neurobiological and molecular vulnerabilities induced by early-life adversity are not altered. For example, though post-weaning social enrichment reversed the heightened stress reactivity of maternally separated male rats, it did not alter levels of CRH in the PVN or GR in the hippocampus (Francis et al., 2002a). Similarly, post-weaning social enrichment reverses the depression-like behavior of maternally separated mice, however, it does not reverse the 5-HT2C pre-mRNA editing phenotype associated with maternal separation (Bhansali et al., 2007). Moreover, the impact of the post-weaning environment may only be observed in individuals with specific early-life experiences. For example, juvenile enrichment induces increases in hypothalamic and CeA OTR binding exclusively in the offspring of low LG dams, whereas OTR levels are not altered in enrichment-housed offspring of high LG dams (Champagne and Meaney, 2007). These findings highlight the need to take a developmental perspective in which consistency and change in environmental conditions is used as a tool to better understand the influences of social experiences on long-term biobehavioral changes.
iii) Sex-specific effects of the social environment
Many of the long-term behavioral and neuroendocrine changes induced by variations in the social environment during development are sex-specific. For example, maternal separation increases the anxiety-like behavior of both male and female mice but decreases in the levels of OT in the PVN are female-specific (Veenema et al., 2007). Likewise, elevated levels of OTR binding in the BNST and CeA of offspring of high LG dams compared to the offspring of low LG are found in females and not males, while increases in V1Ar binding in the CeA are found in male but not female offspring of high LG dams (Francis et al., 2002b) . In most cases, comparisons of the effects of the social environment on males versus females is omitted entirely due to the practical constraints of including a sufficient sample size of both sexes and the difficulty in controlling for hormonally-induced behavioral and neuroendocrine changes occurring in cycling females. However, given the observed sex differences in the prevalence of anxiety and depression, the current male-bias in experimental work on the neural mechanisms mediating increased emotionality may overlook key variables relevant to these sex-specific effects. This is particularly important given the role of variations in gonadal hormones on mood and evidence for the influence of social experiences on hormone and steroid receptor levels. In addition, recent studies have demonstrated how variations in the social environment induce sex-specific structural and functional changes in brain regions such as the hippocampus (Oomen et al., 2009), cingulate cortex (Park et al., 2003) and the mPFC (Stack et al., 2010) that are known to mediate emotional behaviors. Interestingly, there is emerging evidence for the role of DNA methylation patterns, DNMT activity, methyl binding protein levels and co-repressor proteins in shaping sex differences in brain development and behavior (reviewed in (McCarthy et al., 2009). These findings may suggest an epigenetic route through which sex differences in vulnerability to early life experiences and later life emotional disorder are achieved.
iv) Individual differences in responses to social experiences
One key emerging finding from research into the ontogeny of emotionality is that not all individuals are equally responsive to variations in the social environment, with some being more resilient or vulnerable to particular experiences. These individual differences can emerge from environmental factors such as variations in LG experienced by rat offspring during the first week postpartum or from differences in genetic background. A challenge for future research in this field is to identify those genetic or epigenetic variations that mediate this vulnerability (Landgraf et al., 2007). An example of the value of such an approach comes from the studies of social defeat, in which a subgroup of mice have been identified that do not develop the depression-like behavioral changes following agonistic social experiences (Krishnan et al., 2007). Resilience to social defeat may be achieved through both genetic and epigenetic pathways. Mice with a naturally occurring single nucleotide polymorphism in the BDNF gene, which results in a 50% reduction in BDNF protein levels in the NAc, are not susceptible to the long-term effects of chronic defeat (Krishnan et al., 2007). At the epigenetic level, resilient mice show a strikingly different profile of dimethylK9/K27-H3 methylation amongst genes expressed in the NAc compared to vulnerable and undefeated mice demonstrating that becoming resilient is an active process (Wilkinson et al., 2009). These studies highlight the diverse origins of individual differences in vulnerability and resilience and demonstrate how insights into the molecular and cellular substrates of emotionality can be achieved through incorporating the study of individual variation into research designs.
v) Translational Studies on Epigenetic Effects in Humans
The translation to humans of the findings regarding epigenetic changes within the brain which are dynamically altered by social experiences is limited by the inaccessibility of brain tissue and the lack of in vivo strategies for studying these effects during development. One approach has been to study postmortem brain tissue in cases where there are either well characterized developmental experiences or diagnoses of particular psychiatric outcomes. In a recent series of studies, the long-term effects of childhood abuse on hippocampal DNA methylation patterns was examined, with findings suggesting that a history of childhood abuse is associated with decreased GR expression and elevated GR 1F promoter methylation (McGowan et al., 2008; McGowan et al., 2009). These studies also confirm the potential role of NGFI-A as a mediator of differential GR promoter methylation. Amongst suicide victims with a diagnosis of major depression, levels of DNMT are elevated and within the frontopolar cortex, and there are elevated levels of DNA methylation within the promoter region of the GABAA receptor α1 subunit, which has reduced expression within suicide/major depressive disorder brains (Poulter et al., 2008). Within the NAc of depressed patients, there are increases in H3-K14 acetylation and decreased HDAC2 levels, which mirror the effects found in response to social defeat in mice. Epigenetic dysregulation has been identified in the brains of bipolar and schizophrenia patients (Veldic et al., 2005) and, in particular, on methylation levels within the reelin gene promoter (Abdolmaleky et al., 2005; Chen et al., 2003; Grayson et al., 2005). Though these findings suggest an epigenetic pathway associated with psychiatric illness, it is important to note that in using this approach, it is not possible to determine whether these DNA methylation changes are causes or consequences of mental illness.
A second approach to studying epigenetic variation in humans in response to early life experiences has been to examine DNA methylation within peripheral blood mononuclear cells (PBMC). Using this approach, monozygotic twins discordant for schizophrenia have been found to have differential methylation within the 5’-regulatory region of the dopamine D2 receptor gene (DRD2) (Petronis et al., 2003). As noted in the previous sections, analysis of cord blood samples of neonates born to depressed mothers indicates increased methylation within the GR promoter which is correlated with stress responses measured in infancy (Oberlander et al., 2008). Early life effects on GR signaling pathways in humans are further illustrated by a recent genome wide analysis of gene expression of PBMCs from healthy adults who had experienced conditions of low vs. high socioeconomic (SES) status during childhood, with low childhood SES associated with a down-regulation of genes containing GR response elements (Miller et al., 2009). Though epigenetic mechanisms were not explored in this study, these findings provide support for the hypothesis that social regulation of gene expression may predispose individuals to heightened stress and immune reactivity with implications for subsequent risk of mood disorders. The validation of the use of peripheral gene expression and DNA methylation levels to predict changes in the brain relevant to the pathophysiology of mood disorders will be a critical methodological step in developing novel strategies for studying the molecular targets linking social experiences and brain development in humans.
vi) Implications for Designing Novel Therapeutic Strategies
The role of DNA methylation and histone modifications in sustaining the effects of early-life experiences suggests a possible epigenetic target for therapeutic intervention. In the rodent studies discussed in previous sections, this approach has been successfully implemented to reverse the effects of postnatal experiences on DNA methylation, gene expression, physiology and behavior. Infusion of trichostatin A (TSA), a drug that promotes histone acetylation and reduced methylation, in adult offspring who had received low levels of maternal LG in infancy, induces decreased hippocampal GR methylation, increased GR mRNA and decreased response to stress (Weaver et al., 2004). In contrast, adult offspring who had received high levels of maternal LG in infancy can be given a two week treatment with methionine, which results in increased availability of methyl groups, and observed to have increased hippocampal GR methylation, decreased GR expression and a heightened behavioral response to stress (Weaver et al., 2005). Central administration of zebularine, a compound that reduces DNA methylation, leads to increased BDNF expression in maltreated rats such that BDNF levels are equivalent amongst abused and non-abused offspring (Roth et al., 2009). Moreover, the depressive-like phenotype induced by social defeat, can be reversed with an HDAC inhibitor infused into the NAc (Covington et al., 2009). The effect on gene expression of this pharmacological targeting of histones is very similar to that achieved using fluoxetine. Thus, there is strong support for the validity of epigenetic therapy in the amelioration of the effects of early- and later-life social experiences which may provide novel therapeutic strategies. However, the side-effects of such a strategy, when applied to human interventions must be carefully considered, particularly when peripheral routes of drug administration are being used.
Conclusions
Advancements in the study of the biological basis of mood disorders have been achieved through developmental studies in which detailed characterization of the social environment is combined with neurobiological, behavioral and, more recently, with epigenetic analysis. Continued refinement of this approach, incorporating assessment of multiple neural targets, particularly those which have been demonstrated to be modulated by social experiences, within research designs where individual differences, sex-specific effects, and differentiation of short- and long-term mechanisms in mediating neuroendocrine and behavioral outcomes is essential to moving this field forward. Experimental approaches in which consistency in the link between social experiences and biobehavioral outcomes is established will be essential for creating a framework for examining the epigenetic pathways through which dynamic yet stable variations in gene activity serve as a biological marker of increased risk or resilience to psychiatric illness.
Acknowledgments
This research was supported by Grant Number DP2OD001674 from the Office of the Director, National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelrahman AM, Lin Lim S, Pang CC. Influence of urocortin and corticotropin releasing factor on venous tone in conscious rats. Eur J Pharmacol. 2005;510:107–11. doi: 10.1016/j.ejphar.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–6. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai JA, Li S, Hartley DM, Feig LA. Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J Neurosci. 2009;29:1496–502. doi: 10.1523/JNEUROSCI.5057-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB. Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology (Berl) 2004;176:248–55. doi: 10.1007/s00213-004-1883-x. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Eklund MB. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience. 2007;145:738–50. doi: 10.1016/j.neuroscience.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Askew A, Gonzalez FA, Stahl JM, Karom MC. Food competition and social experience effects on V1a receptor binding in the forebrain of male Long-Evans hooded rats. Horm Behav. 2006;49:328–36. doi: 10.1016/j.yhbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–54. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Hermann H, Lutz B, Behl C. Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology. 2005;146:1205–13. doi: 10.1210/en.2004-1154. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Davidson MS, Bedi KS, Wilce PA. Ethanol prevents NMDA receptor reduction by maternal separation in neonatal rat hippocampus. Brain Res. 2006;1067:154–7. doi: 10.1016/j.brainres.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–6. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–84. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J Neurosci. 2007;27:1467–73. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Fone KF, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci. 2006;24:2894–902. doi: 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- Bibancos T, Jardim DL, Aneas I, Chiavegatto S. Social isolation and expression of serotonergic neurotransmission-related genes in several brain areas of male mice. Genes Brain Behav. 2007;6:529–39. doi: 10.1111/j.1601-183X.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–13. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Gruber SH, Brene S. Social isolation increases number of newly proliferated cells in hippocampus in female flinders sensitive line rats. Hippocampus. 2007;17:1193–200. doi: 10.1002/hipo.20352. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–72. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. Postnatal handling reduces novelty-induced fear and increases [3H]flunitrazepam binding in rat brain. Eur J Pharmacol. 1987;144:105–7. doi: 10.1016/0014-2999(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, O'Leary D, Neale JH, Vicini S, Coso OA, Fiszman ML. GABA-induced neurite outgrowth of cerebellar granule cells is mediated by GABA(A) receptor activation, calcium influx and CaMKII and erk1/2 pathways. J Neurochem. 2003;84:1411–20. doi: 10.1046/j.1471-4159.2003.01638.x. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–74. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Branchi I, D'Andrea I, Cirulli F, Lipp HP, Alleva E. Shaping brain development: mouse communal nesting blunts adult neuroendocrine and behavioral response to social stress and modifies chronic antidepressant treatment outcome. Psychoneuroendocrinology. 2010;35:743–51. doi: 10.1016/j.psyneuen.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–6. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur J Neurosci. 2004;20:1355–62. doi: 10.1111/j.1460-9568.2004.03599.x. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Brown RE, Meaney MJ. Effect of resource availability on biparental care, and offspring neural and behavioral development in the California mouse (Peromyscus californicus) Eur J Neurosci. 2007;25:567–75. doi: 10.1111/j.1460-9568.2006.05266.x. [DOI] [PubMed] [Google Scholar]
- Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, Tilders FJ, Bohus B, Koolhaas JM. Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol. 1999;11:513–20. doi: 10.1046/j.1365-2826.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–9. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29:1344–52. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–35. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor–alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–15. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353–63. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–9. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006;89:301–10. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology (Berl) 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–16. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–60. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–35. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009a;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev Neurosci. 2009b;31:318–31. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hulst C, Atack JR, Kooy RF. The complexity of the GABAA receptor shapes unique pharmacological profiles. Drug Discov Today. 2009;14:866–75. doi: 10.1016/j.drudis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Pereira EA, Li PM, Robbins TW. Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berl) 2002;164:329–40. doi: 10.1007/s00213-002-1215-y. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Eaton MJ, Staley JK, Globus MY, Whittemore SR. Developmental regulation of early serotonergic neuronal differentiation: the role of brain-derived neurotrophic factor and membrane depolarization. Dev Biol. 1995;170:169–82. doi: 10.1006/dbio.1995.1205. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Muller MB, Rodel A, Welt T, Ohl F, Holsboer F, Keck ME. Consequences of chronic social stress on behaviour and vasopressin gene expression in the PVN of DBA/2OlaHsd mice--influence of treatment with the CRHR1-antagonist R121919/NBI 30775. J Psychopharmacol. 2009;23:31–9. doi: 10.1177/0269881108089813. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Fan G, Hutnick L. Methyl-CpG binding proteins in the nervous system. Cell Res. 2005;15:255–61. doi: 10.1038/sj.cr.7290294. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–6. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002a;22:7840–3. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002b;14:349–53. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–98. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005;80:12–8. doi: 10.1111/j.1447-073x.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- Fulgheri F, Di Prisco CL, Verdarelli P. Influence of long-term isolation on the production and metabolism of gonadal sex steroids in male and female rats. Physiol Behav. 1975;14:495–9. doi: 10.1016/0031-9384(75)90017-7. [DOI] [PubMed] [Google Scholar]
- Galani R, Berthel MC, Lazarus C, Majchrzak M, Barbelivien A, Kelche C, Cassel JC. The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol Learn Mem. 2007;88:1–10. doi: 10.1016/j.nlm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 2009;1305:47–63. doi: 10.1016/j.brainres.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoflos E, Stamatakis A, Mantelas A, Philippidis H, Stylianopoulou F. Cellular mechanisms underlying an effect of “early handling” on pCREB and BDNF in the neonatal rat hippocampus. Brain Res. 2005;1052:187–95. doi: 10.1016/j.brainres.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Garoflos E, Stamatakis A, Pondiki S, Apostolou A, Philippidis H, Stylianopoulou F. Cellular mechanisms underlying the effect of a single exposure to neonatal handling on neurotrophin-3 in the brain of 1-day-old rats. Neuroscience. 2007;148:349–58. doi: 10.1016/j.neuroscience.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Johnson DA, Leitch MM, Troakes C, Ingram CD. Early life adversity programs changes in central 5-HT neuronal function in adulthood. Eur J Neurosci. 2003;17:2401–8. doi: 10.1046/j.1460-9568.2003.02668.x. [DOI] [PubMed] [Google Scholar]
- Gavrilovic L, Spasojevic N, Tanic N, Dronjak S. Chronic isolation of adult rats decreases gene expression of catecholamine biosynthetic enzymes in adrenal medulla. Neuro Endocrinol Lett. 2008;29:1015–20. [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–6. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007a;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol. 2003;53:50–6. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hall FS, Ghaed S, Pert A, Xing G. The effects of isolation rearing on glutamate receptor NMDAR1A mRNA expression determined by in situ hybridization in Fawn hooded and Wistar rats. Pharmacol Biochem Behav. 2002;73:185–91. doi: 10.1016/s0091-3057(02)00796-7. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–15. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Insel TR. Decreased in vivo binding to brain benzodiazepine receptors during social isolation. Psychopharmacology (Berl) 1989;97:142–4. doi: 10.1007/BF00442234. [DOI] [PubMed] [Google Scholar]
- Janus K. Early separation of young rats from the mother and the development of play fighting. Physiol Behav. 1987;39:471–6. doi: 10.1016/0031-9384(87)90375-1. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Cooper MA, Huhman KL. N-methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004a;123:625–34. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004b;118:1052–61. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Kang I, Thompson ML, Heller J, Miller LG. Persistent elevation in GABAA receptor subunit mRNAs following social stress. Brain Res Bull. 1991;26:809–12. doi: 10.1016/0361-9230(91)90179-n. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary–adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–8. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Nakamura K, Kakuma Y, Mori Y. Early weaning augments neuroendocrine stress responses in mice. Behav Brain Res. 2006;175:96–103. doi: 10.1016/j.bbr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Koo JW, Park CH, Choi SH, Kim NJ, Kim HS, Choe JC, Suh YH. The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. FASEB J. 2003;17:1556–8. doi: 10.1096/fj.02-1032fje. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The central corticotropin releasing factor system during development and adulthood. Eur J Pharmacol. 2008;583:204–14. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother's love to baby's future. Front Behav Neurosci. 2009;3:27. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, Horvath TL, Baram TZ. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci. 2010;30:703–13. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. Chronic neonatal isolation stress enhances cocaine-induced increases in ventral striatal dopamine levels in rat pups. Brain Res Dev Brain Res. 2003;141:109–16. doi: 10.1016/s0165-3806(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Koolhaas JM, Bohus B, Korf J. A single social stress-experience alters glutamate receptor-binding in rat hippocampal CA3 area. Neurosci Lett. 1993;154:73–7. doi: 10.1016/0304-3940(93)90174-j. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–33. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–8. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, Nussbaumer M, Czibere L, Turck CW, Singewald N, Rujescu D, Frank E. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase–I. Neurosci Biobehav Rev. 2007;31:89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Levine JB, Youngs RM, MacDonald ML, Chu M, Leeder AD, Berthiaume F, Konradi C. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145:42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Wotjak CT, Landgraf R, Engelmann M. Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci Lett. 1996;217:101–4. [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Lu B, Wang KH, Nose A. Molecular mechanisms underlying neural circuit formation. Curr Opin Neurobiol. 2009;19:162–7. doi: 10.1016/j.conb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–9. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Neumann ID, Veenema AH. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58:78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009a;55:248–56. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009b;158:845–55. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009c;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lupo di Prisco C, Lucarini N, Dessi-Fulgheri F. Testosterone aromatization in rat brain is modulated by social environment. Physiol Behav. 1978;20:345–8. doi: 10.1016/0031-9384(78)90230-5. [DOI] [PubMed] [Google Scholar]
- Marashi V, Barnekow A, Ossendorf E, Sachser N. Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm Behav. 2003;43:281–92. doi: 10.1016/s0018-506x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Marini AM, Rabin SJ, Lipsky RH, Mocchetti I. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-D-aspartate. J Biol Chem. 1998;273:29394–9. doi: 10.1074/jbc.273.45.29394. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–23. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol Biochem Behav. 2002;73:77–85. doi: 10.1016/s0091-3057(02)00758-x. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, Huang TC, Unterberger A, Suderman M, Ernst C, Meaney MJ, Turecki G, Szyf M. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PLoS One. 2008;3:e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bhatnagar S, Sapolsky RM. Postnatal handling attenuates certain neuroendocrine, anatomical, and cognitive dysfunctions associated with aging in female rats. Neurobiol Aging. 1991a;12:31–8. doi: 10.1016/0197-4580(91)90036-j. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Bhatnagar S, Betito K, Iny LJ, O'Donnell D, Mitchell JB. Cellular mechanisms underlying the development and expression of individual differences in the hypothalamic-pituitary-adrenal stress response. J Steroid Biochem Mol Biol. 1991b;39:265–74. doi: 10.1016/0960-0760(91)90072-d. [DOI] [PubMed] [Google Scholar]
- Miachon S, Manchon M, Fromentin JR, Buda M. Isolation-induced changes in radioligand binding to benzodiazepine binding sites. Neurosci Lett. 1990;111:246–51. doi: 10.1016/0304-3940(90)90269-f. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LG, Thompson ML, Greenblatt DJ, Deutsch SI, Shader RI, Paul SM. Rapid increase in brain benzodiazepine receptor binding following defeat stress in mice. Brain Res. 1987;414:395–400. doi: 10.1016/0006-8993(87)90023-0. [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Kitagami T, Ohta T, Ozaki N. Effects of fluvoxamine on levels of dopamine, serotonin, and their metabolites in the hippocampus elicited by isolation housing and novelty stress in adult rats. Int J Neurosci. 2005;115:367–78. doi: 10.1080/00207450590520984. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci. 2003;18:3367–74. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Naka F, Shiga T, Yaguchi M, Okado N. An enriched environment increases noradrenaline concentration in the mouse brain. Brain Res. 2002;924:124–6. doi: 10.1016/s0006-8993(01)03257-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kikusui T, Takeuchi Y, Mori Y. Changes in social instigation- and food restriction-induced aggressive behaviors and hippocampal 5HT1B mRNA receptor expression in male mice from early weaning. Behav Brain Res. 2008;187:442–8. doi: 10.1016/j.bbr.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse. 2009;10:389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88:1005–15. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Caprioli A, Ghirardi O, Ali SF, Aloe L, Laviola G. The effect of early maternal separation on brain derived neurotrophic factor and monoamine levels in adult heterozygous reeler mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1269–76. doi: 10.1016/j.pnpbp.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Olsson T, Mohammed AH, Donaldson LF, Henriksson BG, Seckl JR. Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Brain Res Mol Brain Res. 1994;23:349–53. doi: 10.1016/0169-328x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Girardi CE, Cahyadi R, Verbeek EC, Krugers H, Joels M, Lucassen PJ. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One. 2009;4:e3675. doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–23. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Hoang TA, Belluzzi JD, Leslie FM. Gender specific effect of neonatal handling on stress reactivity of adolescent rats. J Neuroendocrinol. 2003;15:289–95. doi: 10.1046/j.1365-2826.2003.01010.x. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–51. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Petronis A, Gottesman II, Kan P, Kennedy JL, Basile VS, Paterson AD, Popendikyte V. Monozygotic twins exhibit numerous epigenetic differences: clues to twin discordance? Schizophr Bull. 2003;29:169–78. doi: 10.1093/oxfordjournals.schbul.a006988. [DOI] [PubMed] [Google Scholar]
- Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res. 2006;1099:101–8. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress–induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, Szyf M, Anisman H. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64:645–52. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–44. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. Embo J. 1998;17:4905–8. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–83. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci U S A. 2005;102:11557–62. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison CL, Meyerhoff JL, Saviolakis GA, Chen WK, Rice KC, Lumley LA. A CRH1 antagonist into the amygdala of mice prevents defeat-induced defensive behavior. Ann N Y Acad Sci. 2004;1032:324–7. doi: 10.1196/annals.1314.052. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–16. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–31. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Gomez A, Parker K, Carter CS. Social environment alters central distribution of estrogen receptor alpha in juvenile prairie voles. Physiol Behav. 2009;98:296–301. doi: 10.1016/j.physbeh.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Aguado F, Sanchez-Toscano F, Saphier D. Neuroendocrine and immunocytochemical demonstrations of decreased hypothalamo-pituitary-adrenal axis responsiveness to restraint stress after long-term social isolation. Endocrinology. 1998;139:579–87. doi: 10.1210/endo.139.2.5720. [DOI] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–20. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73:209–24. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- Segovia G, Yague AG, Garcia-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res Bull. 2006;70:8–14. doi: 10.1016/j.brainresbull.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–40. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Mostallino MC, Sanna E, Biggio G. Changes in neuroactive steroid content during social isolation stress modulate GABAA receptor plasticity and function. Brain Res Rev. 2008;57:520–30. doi: 10.1016/j.brainresrev.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Seth KA, Majzoub JA. Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin–releasing hormone gene transcription. J Biol Chem. 2001;276:13917–23. doi: 10.1074/jbc.M007745200. [DOI] [PubMed] [Google Scholar]
- Shao F, Jin J, Meng Q, Liu M, Xie X, Lin W, Wang W. Pubertal isolation alters latent inhibition and DA in nucleus accumbens of adult rats. Physiol Behav. 2009;98:251–7. doi: 10.1016/j.physbeh.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Shimozuru M, Kodama Y, Iwasa T, Kikusui T, Takeuchi Y, Mori Y. Early weaning decreases play-fighting behavior during the postweaning developmental period of Wistar rats. Dev Psychobiol. 2007;49:343–50. doi: 10.1002/dev.20202. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–36. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35:570–80. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling J, Jr, Amaya-Jackson L. Understanding the behavioral and emotional consequences of child abuse. Pediatrics. 2008;122:667–73. doi: 10.1542/peds.2008-1885. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–33. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztainberg Y, Kuperman Y, Tsoory M, Lebow M, Chen A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.151. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Osako Y, Yuri K. Juvenile social experience regulates central neuropeptides relevant to emotional and social behaviors. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todeschin AS, Winkelmann-Duarte EC, Jacob MH, Aranda BC, Jacobs S, Fernandes MC, Ribeiro MF, Sanvitto GL, Lucion AB. Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm Behav. 2009;56:93–100. doi: 10.1016/j.yhbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–15. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Turner B. Chromatin and Gene Regulation. Blackwell Science Ltd; Oxford: 2001. [Google Scholar]
- Turnock-Jones JJ, Jennings CA, Robbins MJ, Cluderay JE, Cilia J, Reid JL, Taylor A, Jones DN, Emson PC, Southam E. Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. Synapse. 2009;63:836–46. doi: 10.1002/syn.20665. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121:83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–20. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–50. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–36. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–7. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. J Neurosci. 1993;13:1097–105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–76. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, D'Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Weizman R, Lehmann J, Leschiner S, Allmann I, Stoehr T, Heidbreder C, Domeney A, Feldon J, Gavish M. Long-lasting effect of early handling on the peripheral benzodiazepine receptor. Pharmacol Biochem Behav. 1999;64:725–9. doi: 10.1016/s0091-3057(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–32. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann-Duarte EC, Todeschin AS, Fernandes MC, Bittencourt LC, Pereira GA, Samios VN, Schuh AF, Achaval ME, Xavier LL, Sanvitto GL, Mandarim-de-Lacerda CA, Lucion AB. Plastic changes induced by neonatal handling in the hypothalamus of female rats. Brain Res. 2007;1170:20–30. doi: 10.1016/j.brainres.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–7. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci. 2005;25:1493–502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–60. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, He S. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment–related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1173–7. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Zheng D, Zhao K, Mehler MF. Profiling RE1/REST–mediated histone modifications in the human genome. Genome Biol. 2009;10:R9. doi: 10.1186/gb-2009-10-1-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Bradford HF, Stern GM. Induction of dopaminergic neurotransmitter phenotype in rat embryonic cerebrocortex by the synergistic action of neurotrophins and dopamine. Eur J Neurosci. 1996;8:2328–39. doi: 10.1111/j.1460-9568.1996.tb01196.x. [DOI] [PubMed] [Google Scholar]