Abstract
Aims
Circulating microRNAs (miRNAs) may represent a novel class of biomarkers; therefore, we examined whether acute myocardial infarction (MI) modulates miRNAs plasma levels in humans and mice.
Methods and results
Healthy donors (n = 17) and patients (n = 33) with acute ST-segment elevation MI (STEMI) were evaluated. In one cohort (n = 25), the first plasma sample was obtained 517 ± 309 min after the onset of MI symptoms and after coronary reperfusion with percutaneous coronary intervention (PCI); miR-1, -133a, -133b, and -499-5p were ∼15- to 140-fold control, whereas miR-122 and -375 were ∼87–90% lower than control; 5 days later, miR-1, -133a, -133b, -499-5p, and -375 were back to baseline, whereas miR-122 remained lower than control through Day 30. In additional patients (n = 8; four treated with thrombolysis and four with PCI), miRNAs and troponin I (TnI) were quantified simultaneously starting 156 ± 72 min after the onset of symptoms and at different times thereafter. Peak miR-1, -133a, and -133b expression and TnI level occurred at a similar time, whereas miR-499-5p exhibited a slower time course. In mice, miRNAs plasma levels and TnI were measured 15 min after coronary ligation and at different times thereafter. The behaviour of miR-1, -133a, -133b, and -499-5p was similar to STEMI patients; further, reciprocal changes in the expression levels of these miRNAs were found in cardiac tissue 3–6 h after coronary ligation. In contrast, miR-122 and -375 exhibited minor changes and no significant modulation. In mice with acute hind-limb ischaemia, there was no increase in the plasma level of the above miRNAs.
Conclusion
Acute MI up-regulated miR-1, -133a, -133b, and -499-5p plasma levels, both in humans and mice, whereas miR-122 and -375 were lower than control only in STEMI patients. These miRNAs represent novel biomarkers of cardiac damage.
Keywords: Circulating miRNA, Myocardial infarction, miR-1, miR-133a, miR-133b, miR-499
Introduction
See page 2705 for the editorial comment on this article (doi:10.1093/eurheartj/ehq221)
MicroRNAs (miRNAs) are ∼22 nucleotides long non-coding RNAs which inhibit mRNA translation or induce its degradation; each miRNA can target several mRNAs and each mRNA can be the target of different miRNAs, therefore their effects can be very complex.1 It is estimated that the human genome encodes ∼1000 miRNAs, and to date, 721 human miRNAs have been identified.2 Many miRNAs exhibit a tissue-specific distribution and they appear to play a key role in cell function both under physiological and pathological conditions; indeed, marked changes in the tissue level of some miRNAs have been found in myocardial infarction (MI),3–5 cardiac hypertrophy,6 heart failure,7 acute hind-limb ischaemia,8 as well as in isolated cells exposed to hypoxia.9
Recent studies have shown that some miRNAs are present in the systemic circulation, both in humans and animals, and that they can be associated with exosomes10 and microparticles.11 The level of some circulating miRNAs has been reported to vary significantly during pregnancy,12 in the presence of a variety of cancers,13,14 and in acetaminophen-induced liver damage in the mouse.15 Studies on circulating miRNAs and heart disease have shown a modulation in a rat model of isoproterenol-induced cardiac injury16 and in patients with heart failure.17 Finally, two very recent studies have examined circulating miRNAs in patients with acute MI.18,19 In light of these findings, it has been hypothesized that at least in some pathological conditions, miRNAs in the systemic circulation may reflect tissue damage and, for this reason, they may be regarded as biomarkers of the disease.
The objective of the present work was to examine miRNAs plasma levels in humans with acute MI. It was found that miR-1, -133a, -133b, and -499-5p increased within few hours after the onset of MI symptoms and after 5 days had returned towards control values. In contrast, miR-122 and -375 were lower than control. The same miRNAs which increased in humans with ST-segment elevation MI (STEMI) were also elevated in a mouse model of MI, whereas their level did not rise in a mouse model of skeletal muscle damage due to acute hind-limb ischaemia.
Methods
Patients' characteristics and blood sample collection
Blood samples were obtained from 17 healthy donors and 33 STEMI patients (see Supplementary material online, Tables S1 and S3). There were no statistically significant differences between healthy donors and STEMI patients for any of the considered variables except for age and for hypertension which was more prevalent in STEMI patients. A comparison of STEMI-miRNAs expression between controls, with or without hypertension, showed no statistically significant difference, thus suggesting that hypertension is not a confounding variable in this context (data not shown).
The study was approved by the Ethics Committee of participating hospitals and written informed consent was obtained from each patient.
For details, see Supplementary material online.
Mouse models of myocardial infarction and hind-limb ischaemia
C57BL/6 female mice, 2–3 months of age, were used for all experiments. Myocardial infarction and hind-limb ischaemia were induced as described previously.20,21 For details, see Supplementary material online.
RNA isolation
Total RNA was isolated from human plasma and serum, from mouse plasma, and from mouse cardiac and skeletal muscle. For details, see Supplementary material online.
MicroRNAs profiling and validation
TaqMan Human MicroRNA A and B Arrays, version 2.0 (Applied Biosystems, Foster City, CA, USA), were used for miRNA expression screening of 667 miRNAs. Only miRNAs that differed from controls more than eight-fold were considered for the subsequent validation step by real-time RT–PCR [quantitative RT–PCR (qRT–PCR)]. For details, see Supplementary material online.
Troponin I determination
Plasma troponin I (TnI) blood levels were determined with a commercially available ELISA assay. For details, see Supplementary material online.
Statistical analysis
Values are expressed as mean ± standard deviation unless otherwise indicated. It was established whether the continuous data followed the normal distribution by the Shapiro–Wilk test. Statistical testing on patients' baseline characteristics was conducted using Student's t-test and Fisher's exact test. Results were evaluated by ANOVA adjusted for age as covariate and miRNAs expression levels in MI patients were compared with those in healthy controls. Similarly, miRNAs expression levels in mice with acute MI and hind-limb ischaemia were compared with those in sham-operated animals. miRNAs and TnI time courses were analysed by repeated-measures ANOVA adjusted for age as covariate. All P-values are two-sided and P < 0.05 was considered the threshold for statistically significant differences.
Statistical analyses were performed using the GraphPad Prism 5 statistical package.
Results
Circulating microRNAs in humans
MicroRNAs plasma and serum levels in healthy human subjects
In preliminary experiments, it was established that which miRNAs were detectable in the systemic circulation of healthy human subjects; in six individuals, 667 miRNAs were screened and 259 were present (see Supplementary material online, Table S4). Further, miRNAs were profiled both in plasma and serum and no differences were found (data not shown). Therefore, all subsequent miRNAs determinations were performed on plasma samples.
MicroRNAs plasma levels in ST-segment elevation myocardial infarction patients
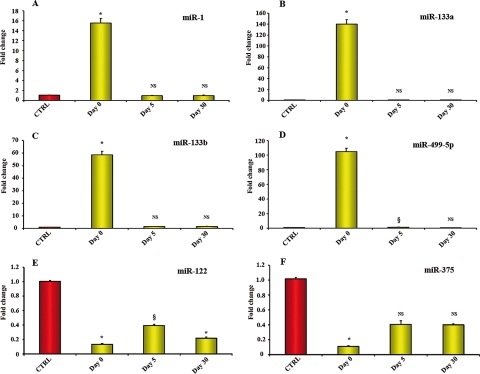
Patients with acute anterior STEMI (Group 1) were enrolled in the study and their first plasma sample was collected 517 ± 309 min after the onset of symptoms (Day 0). The screening procedure described above was repeated on the first blood sample collected from six of these patients (see Supplementary material online, Table S5). Only miRNAs which were detectable in the plasma of healthy subjects exhibited a significant change in the presence of acute MI; out of the 259 miRNAs previously identified in the plasma 34 differed more than eight-fold from healthy controls, 20 were up-regulated and 14 were down-regulated (see Supplementary material online, Table S5e). The levels of these 34 miRNAs were validated by qRT–PCR and this approach led to the identification and selection of only six miRNAs of interest which were quantified in all healthy subjects (n = 17) and STEMI patients in this cohort (n = 25). It was found that miR-1, -133a, -133b, and -499-5p were up-regulated, whereas miR-122 and -375 were already lower than in healthy subjects. Additional determinations of the six selected miRNAs were performed at later time points; 5 days after MI, miR-1, -133a, -133b, -499-5p, and -375 were back to control levels. In contrast, at Day 5, miR-122 was ∼70% lower than in healthy control subjects and these low levels persisted throughout the 30-day time course of the experiment (Figure 1).
Figure 1.
MicroRNAs plasma levels in patients with ST-segment elevation myocardial infarction (Group 1). (A–D) miR-1, -133a, -133b, and -499-5p exhibited a 15- to 140-fold increase in plasma samples collected 517 ± 309 min after the onset of myocardial infarction symptoms, i.e. Day 0. At Day 5 after myocardial infarction, these microRNAs were back to levels comparable to those in healthy subjects. (E and F) At Day 0, miR-122 (E) and miR-375(F) were lower than in control subjects and miR-122 level remained below control both at Day 5 and at Day 30. (Control healthy subjects = 17; ST-segment elevation myocardial infarction patients = 25 at Day 0; 7 at Day 5 and 7 at Day 30. Values indicate fold changes of each microRNA vs. its level in control healthy subjects, arbitrarily set at 1 as indicated by the red bar; yellow bars indicate microRNA values after myocardial infarction; results are reported as mean ± SEM; *P < 0.01; §P < 0.05 vs. control; NS, not significant.)
In light of a recent study which has shown an increase in circulating miR-208a in patients with acute MI,19 we have examined the level of this miRNA also in our control subjects and STEMI patients. Human plasma samples from healthy controls did not show any expression of miR-208a. In STEMI patients, miR-208a exhibited very low levels of expression in three of nine patients and it was undetectable in the others (data not shown).
Simultaneous microRNAs and troponin I plasma levels determination in ST-segment elevation myocardial infarction patients
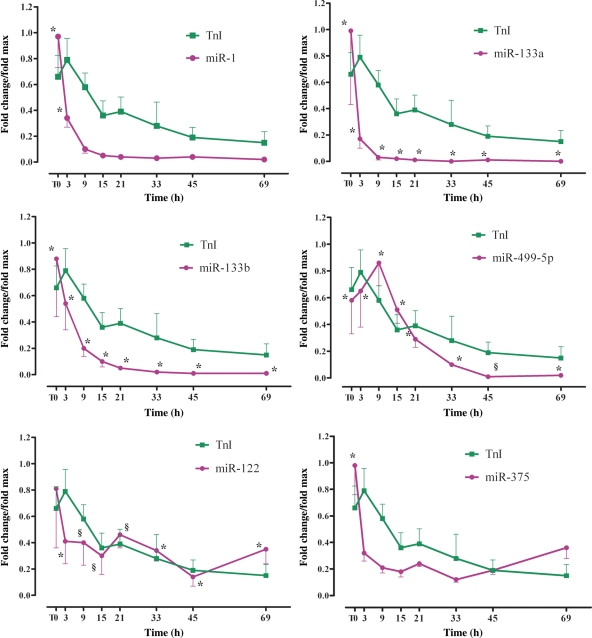
In eight STEMI patients (Group 2), miRNAs and TnI were measured in the same plasma samples. The first sample was obtained 156 ± 72 min after the onset of MI symptoms (T0) and additional samples were obtained 3, 9, 15, 21, 33, 45, and 69 h after T0. Interestingly, in these patients, miR-1, -133a, and -133b plasma levels were already at their peak at T0, i.e. at a time point very close to the peak increase in TnI. In contrast, miR-499-5p exhibited a slower time course and peaked after TnI (Figure 2). At the end of the 3-day time course, miR-1, -133a, -133b, and -499-5p had returned close to their control levels. It is noteworthy that miRNAs peak-fold increase was enhanced in these patients in comparison to the patients in Group 1 (Figure 1A–D), possibly because in Group 1, the time of the first blood collection after the onset of MI symptoms was 517 ± 309 vs. 156 ± 72 min in Group 2. Finally, miR-122 and -375 were close to baseline or below control throughout the duration of the experiment.
Figure 2.
Time course of microRNAs plasma levels and TnI in patients with ST-segment elevation myocardial infarction (Group 2). In these patients, the first plasma sample was obtained 156 ± 72 min after the onset of symptoms (T0); other samples were obtained at different times thereafter, as indicated. miR-1, -133a, and -133b achieved their peak before troponin I, whereas miR-499-5p exhibited a slower time course. The data have been normalized to the peak level that each microRNA and troponin I achieved in each patient and the time of the peak-fold increase vs. healthy control was not identical among patients. On the average, miR-1 achieved a 48.3 ± 17.4-fold peak at T0; miR-133a achieved a 5426 ± 3047-fold peak at T0; miR-133b achieved a 312.2 ± 182.6-fold peak at T0; miR 499-5p achieved a 299.1 ± 106.4-fold peak at 9 h. miR-122 and -375 were never above control; their lowest level was 0.26 ± 0.12 and 0.53 ± 0.10 control and occurred at the 45 and 33 h time points, respectively. Troponin I peak increase of 1066 ± 200-fold was achieved at the 3 h time point. (Control healthy subjects = 17; ST-segment elevation myocardial infarction patients = 5–8 at each time point; results for each microRNA and troponin I are reported as mean ± SEM; *P < 0.01; §P < 0.05 vs. control.)
MicroRNAs plasma and tissue levels in mice
MicroRNAs plasma and heart levels in mice with acute myocardial infarction
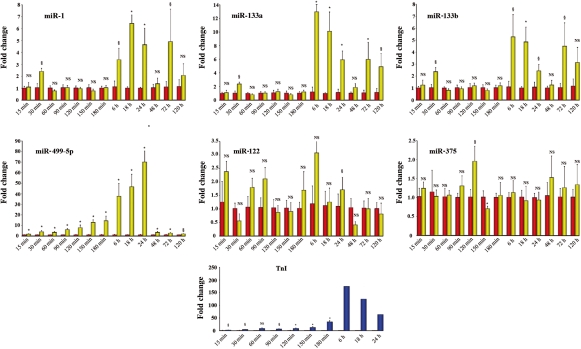
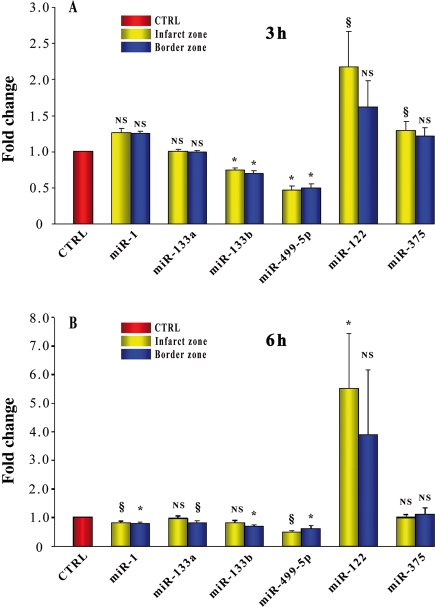
These experiments were aimed at establishing whether the effects of acute MI on miRNAs plasma levels observed in humans were reproduced in a murine model of MI. Circulating miRNAs were monitored from 15 min to 5 days and TnI from 15 min to 24 h, after permanent coronary ligation (Figure 3). In this model, miR-1, -133a, -133b, and -499-5p plasma levels increased; however, their peak-fold increase was not as marked as in humans. Interestingly, miR-499-5p was an extremely sensitive indicator of cardiac damage; it closely paralleled the increase in TnI, and 15 min after coronary ligation, it was already 1.7-fold control (P < 0.05), whereas TnI was 1.8-fold control (P < 0.05). However, TnI peaked at the 6 h time point, whereas miR-499-5p exhibited a slower time course and, under our experimental conditions, achieved its peak at the 24 h time point. The effects of MI on miR-122 and -375 plasma levels were minor. The expression level of miR-208a in mouse plasma was also analysed; as recently reported in rats with isoproterenol-induced cardiac toxicity16 and MI,19 miR-208a was undetectable in sham-operated mice (n = 5), whereas in infarcted mice, miR-208a was expressed as early as 30 min after coronary ligation and exhibited a progressive increase up to the 3 h time point when the Ct value was 34.5 ± 0.38 (n = 8). In addition to their circulating levels, the expression of the six selected miRNAs was quantified also in the infarct and in the border areas, 3 and 6 h after coronary artery ligation (Figure 4A and B). Interestingly, miR-1, -133a, -133b, and -499-5p exhibited a decrease which, in the case of miR-499-5p, was consistently found at both time points in the infarct and in the border zone. In contrast, miR-122 and -375 were either at baseline or above control with the most consistent change being a two- to five-fold increase in miR-122 in the infarct area.
Figure 3.
Time course of microRNAs plasma levels and troponin I in mice with acute myocardial infarction. miR-1, -133a, and -133b achieved their peak either at the 6 or the 18 h time point. In contrast, miR-499-5p achieved its peak 24 h after coronary occlusion. Changes in miR-122 and -375 were minor. It is noteworthy that the magnitude of the increase was highest for miR-499-5p and that this microRNA, 15 min to 6 h after coronary occlusion, closely paralleled the increase in troponin I which was monitored in a separate group of animals. Fold changes were calculated against the mean value of the sham at each time point (for microRNAs, at each time point, n = 4–5 both for myocardial infarction and sham-operated mice; for troponin I, n = 5 at each time point from 15 to 180 min and n = 2 at each time point from 6 to 24 h; results for each microRNA and troponin I are reported as mean ± SEM; NS, not significant; *P < 0.01, §P < 0.05 vs. sham-operated control mice.) Red bars indicate sham controls at each time point, arbitrarily set at 1; yellow bars indicate microRNA values at each time point after myocardial infarction; and blue bars indicate troponin I values after myocardial infarction.
Figure 4.
MicroRNAs cardiac levels in mice with acute myocardial infarction. The cardiac expression level of miR-1, -133a, -133b, -499-5p, -122, and -375 was evaluated in the border zone and infarct area of mice, 3 and 6 hr following coronary artery ligation, as well as in the left ventricle of sham-operated mice at the same time points. miR-499-5p exhibited a decrease at both time points, both in the infarct and border zones. miR-1, -133a, and -133b decreased, either in the border area, the infarct, or both, and this response was apparent either 3 or 6 h after coronary occlusion. Interestingly, miR-122 exhibited an increase in the infarct both 3 and 6 h after coronary occlusion. miR-375 exhibited a minor increase in the infarct area which was apparent only at the 3 h time point (at each time point, n = 4–5 for myocardial infarction and n = 4–5 for sham operated; results for each microRNA are reported as mean ± SEM; NS, not significant; *P < 0.01, §P < 0.05 vs. sham-operated control mice.) Control values were arbitrarily set at 1 as indicated by the red bar.
MicroRNAs plasma and skeletal muscle levels in mice with acute hind-limb ischaemia
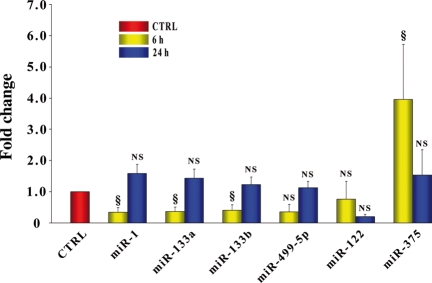
Since miR-1, -133a, and -133b are highly expressed in skeletal muscle as well as in the heart, we examined whether muscle damage, which is well known to occur in the model of hind-limb ischaemia used in the present study,21 had any effect on miRNAs plasma levels. It was found that 6 h following femoral artery dissection, miR-1, -133a, and -133b plasma levels were ∼50–70% lower than in sham-operated mice, whereas miR-375 was enhanced; however, this effect was transient and, at 24 h, they were back to control (Figure 5). In contrast, miR-499-5p and miR-122 plasma levels did not change. Thus, acute hind-limb ischaemia, unlike MI, did not increase the plasma levels of the miRNAs under study.
Figure 5.
MicroRNAs plasma levels in mice with acute hind-limb ischaemia. miR-1, -133a, -133b, -499-5p, -122, and -375 plasma levels were evaluated in mice with acute hind-limb ischaemia, 6 and 24 h after femoral artery dissection. At 6 h, miR-1, -133a, and -133b exhibited a marked decrease, whereas miR-375 increased; all microRNAs were back to control value at 24 h. In contrast, miR-499-5p and miR-122 exhibited no significant change. (n = 5 for hind-limb ischaemia and n = 5 for sham operated; values indicate fold changes of each microRNA vs. sham-operated mice; results for each microRNA are reported as mean ± SEM; NS, not significant; §P < 0.05). Control values were arbitrarily set to 1 as indicated by the red bar.
The expression of these miRNAs in the ischaemic gastrocnemius and adductor skeletal muscles was examined both 6 and 24 h after femoral artery dissection and it was compared with the same muscle in sham-operated mice and in the contralateral, non-ischaemic limb of the same animal (see Supplementary material online, Figure S1). In our analysis, we considered of interest those statistically significant changes which occurred both between the ischaemic and the contralateral normoperfused muscle and between the ischaemic and the normoperfused muscle in sham-operated mice. According to these criteria, acute ischaemia caused a decrease in miR-1, -133a, -133b, and -499-5p skeletal muscle expression within 24 h following surgery.
Discussion
The present study demonstrates that acute MI induces a distinctive circulating miRNAs signature. Similar results were obtained in patients with STEMI and in a mouse model of MI.
MicroRNA levels in humans and mice
In preliminary experiments, it was established that out of 667 miRNAs screened, 259 were present in the plasma of healthy human subjects. During MI, 6 of the 259 miRNAs expressed in the systemic circulation of control subjects were modulated; miR-1, -133a, -133b, and -499-5p increased, whereas miR-122 and -375 were lower than in control. The magnitude of the increase as well as the kinetics of the changes differed among miR-1, -133a, -133b, and -499-5p; taken together, the results from the patients in Groups 1 and 2 indicate that miR133a exhibited the most pronounced fold increase, whereas the smallest change was observed for miR-1. Further, miR-1, -133a, and -133b peak expression occurred at T0, whereas miR-499-5p exhibited a slower time course (Figure 2). Interestingly, miR-122 plasma levels at Days 5 and 30 after MI were persistently lower than in healthy subjects.
Most findings in humans were reproduced in a murine model of acute MI. The remarkable similarities occurred despite species differences, the different type of cardiac injury, i.e. ischaemia/reperfusion injury in humans vs. permanent coronary occlusion in the mouse, and the absence of variables normally found in humans, e.g. drug therapy and risk factors for coronary artery disease (CAD). In the mouse, miR-1, -133a, and -133b plasma levels achieved their peak 6–18 h after coronary occlusion. Further, it was confirmed that miR-499-5p had slower kinetics since, after coronary ligation, it increased progressively and reached its peak only at the 24 h time point. A major difference between human and mouse was the behaviour of miR-122 and -375; in humans with MI, these miRNAs were lower than in control; in contrast, in the mouse, miR-122 and -375 exhibited only minor changes and were not consistently modulated. The reason for this difference is unclear but the possibility needs to be considered that miR-122 and -375 plasma levels in humans with risk factors for CAD, and without acute MI, may be lower than in healthy subjects. Additional studies will be required to address this issue and establish whether any miRNA plasma level is related to CAD risk factors and/or can predict the occurrence of acute coronary syndromes.
A recent study has examined the effect of isoproterenol toxicity on circulating miRNAs and reported a significant increase in miR-208.16 In that study, isoproterenol was injected subcutaneously in rats and the increase in TnI provided evidence of cardiac damage. Cardiac toxicity due to β-adrenergic stimulation differs from MI due to coronary occlusion and the two conditions cannot be directly compared. In another study, which appeared ‘online’ while this manuscript was under review,19 circulating miR-1, -133a, -499, and -208a were quantified in patients with acute MI and their levels were found to increase. In the present work, miR-208a was undetectable in plasma samples from sham-operated mice. In infarcted mice, miR-208a was expressed as early as 30 min after coronary ligation and exhibited a progressive increase up to the 3 h time point; however, its expression was low and the peak average Ct was 34.5. Further, miR-208a was undetectable in plasma samples from healthy humans; it was expressed at low levels in three of nine STEMI patients and was undetectable in the others. In light of the results of the present work, miR-208a does not appear to be an ideal biomarker of MI in humans. Another recent study has shown that miR-1 increased in patients with acute MI and suggested a potential association with a prolonged QRS.18 Those authors also examined miR-133 and, differently from our results in humans and mice, did not find it to vary; no other circulating miRNAs were analysed. The diminution of circulating miRNAs has been reported less frequently, in liver damage,15 acute leukaemia,22 and ovarian cancer.23 Which mechanisms determine the level of circulating miRNAs under physiological conditions and in response to pathological stimuli remains to be elucidated and several possibilities should be taken into consideration: (i) release from dead cells following disruption of the plasma membrane, (ii) release from living cells exposed to stressful conditions, (iii) modulation of miRNAs synthesis and degradation processes which occur in the cell, (iv) modulation of miRNAs degradation in the systemic circulation, and (v) uptake of circulating miRNAs by cells that get in contact with them. These regulatory mechanisms are all plausible and each of them, either alone or in combination, may determine the level of a given miRNA in the bloodstream. It is noteworthy that in the mouse, at the 3 and 6 h time points, miRNAs were quantified both in the plasma and in the heart of the same mice. It was found that the changes in the plasma level of the miRNAs were associated to reciprocal changes in their cardiac expression; miR- 1, -133a, -133b, and -499-5p exhibited a decrease either in the infarct zone, in the border zone, or both. Interestingly, the cardiac expression of miR-499-5p was consistently below control in the infarct and in the border areas, both 3 and 6 h after coronary ligation. These results suggest that at least in the case of miR-1, -133a, -133b, and -499-5p, the increase in the systemic circulation may be due to release from damaged cardiac cells. However, if this was the only mechanism, all miRNAs which are highly expressed in the heart should have increased. This was not the case since miR-24, -26a, -126, and -30c are strongly expressed in the heart24 (Simona Greco, Y.D., M.C.C., and F.M., unpublished observation) and their levels in the bloodstream were not found to increase in the present study.
MicroRNAs specificity and sensitivity in myocardial infarction
MicroRNAs expression varies among tissues; miR-1, -133a, and -133b are strongly expressed in the heart and skeletal muscle, whereas miR-499-5p is regarded as cardiac-specific,25 although in the present study, very low levels of expression were found also in the skeletal muscle. In contrast, miR-122 and -375 are either expressed at very low levels or undetectable in the heart; miR-122 is regarded as liver-specific,26 whereas miR-375 is found in pancreatic islets and appears to play a key role in α- and β-cell homeostasis and glucose metabolism.27 Interestingly, none of the miRNAs under study were found to increase in the plasma after acute hind-limb ischaemia. This result does not exclude that other miRNAs, different from those examined in the present work, may have varied; however, it is noteworthy that miR-1, -133a, and -133b, which are highly expressed in skeletal muscle, failed to increase after acute ischaemia. Interestingly, the same observation was made also after acute coronary occlusion; the plasma level of some miRNAs which are highly expressed in the heart did not vary during MI (see above). These findings suggest that miRNAs release into the systemic circulation may be a relatively selective process, possibly tissue- and miRNA-specific. Further, both the extent and the timing of cell death are expected to modulate miRNAs release into the systemic circulation; these differ markedly between femoral artery dissection and coronary ligation. In fact, the collateral circulation in the limb is well developed, whereas in the rodent's heart, it is extremely poor and this will ultimately determine how many cells die and how rapidly they do so after acute ischaemia. Indeed, we have previously shown that upon hind-limb ischaemia, ∼30% of the capillaries and virtually all arterioles and venules remained perfused.28 It is noteworthy that circulating miR-1, -133a, and -133b have been found to increase in mice with acetaminophen-induced drug toxicity.15 Further, miR-133a has been found to increase in the bloodstream of patients with lung cancer29 and with colorectal cancer.30 In contrast, a decrease in circulating miR-122 and -375 has not been reported in any other medical condition examined to date, neither in humans nor in the animal models of human diseases.
The findings on the present study do not enable us to conclusively establish whether circulating miRNAs may be indicators of cardiac necrosis which are more sensitive than the commonly used TnI. However, this possibility should be considered since, in humans, miR-1, -133a, and -133b achieved their peak before TnI. In contrast, in mice, miR-499-5p appeared to be a more sensitive biomarker than the other miRNAs; the results in Figure 3 show that 15 min to 6 h after coronary occlusion, the increase in TnI and miR-499-5p paralleled each other, whereas miR-1, -133a, -133b, -122, and -375 failed to change at the early time points after coronary ligation, when TnI was already rising.
Potential role of circulating microRNAs
It has been recently shown that exosomes and microparticles containing miRNAs can be released into the extracellular environment and then be internalized by other cells.10,11 Therefore, future studies will need to establish whether, following an acute MI, miRNAs in the bloodstream are contained in exosomes or microparticles, whether they can enter cells and, eventually, which cells and whether they have any biological action. This is an important issue since some of the miRNAs which increase in the bloodstream after MI have effects on the cardiovascular system. miR-133a and -133b are transcribed by different loci, but have an almost identical mature sequence displaying only a one base mismatch at the 3′ terminus. Thus, their biological function is likely very similar or identical. Both miR-1 and -133 seem to play a crucial role in the regulation of cardiac hypertrophy and their down-regulation allows for the de-repression of growth-related genes that are involved in cardiac hypertrophy.6 Intriguingly, miR-1 and -133 play opposing roles in cell fate determination; miR-1 has a pro-apoptotic function31,32 that is rescued by the expression of miR-133 (32). miR-1 and miR-133 also regulate cardiac electrical properties; miR-1 is known to trigger cardiac arrhythmias33 and both miR-1 and -133 have been shown to target the pacemaker current If.34 miR-499-5p is highly expressed in the myocardium25,35 and its up-regulation is associated with cell senescence, suggesting that it may play a role in terminal differentiation.36,37 Further, miR-499 has also been shown to promote the differentiation of cardiac progenitor cells into myocytes;38 therefore, it may play a role in the activation of repair mechanisms following MI. Neither miR-122 nor -375 are known to have any effect on the heart.
Conclusions
The results of the present study show that circulating miRNAs are sensitive markers of MI and open two new areas of investigation: on circulating miRNAs as markers of cardiac ischaemic damage and as potential regulators of cell/organ function and remodelling after MI.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The present study was supported by the following grants to G.P. and M.C.C.: Italian Ministry of Health RC 2008-BIO29; RF 2008; FIRB- MIUR RBLA035A4X-1. Funding to pay the Open Access publication charges for this article was provided by the Centro Cardiologico Monzino.
Conflict of interest: none declared.
Supplementary Material
Acknowledgement
We thank Franco Moro (Centro Cardiologico Monzino—IRCCS) for his valuable technical support to this work.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. doi:10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.miRBase, release 14.0. http://microrna.sanger.ac.uk . (September 2009) [Google Scholar]
- 3.Shan ZX, Lin QX, Fu YH, Deng CY, Zhou ZL, Zhu JN, Liu XY, Zhang YY, Li Y, Lin SG, Yu XY. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem Biophys Res Commun. 2009;381:597–601. doi: 10.1016/j.bbrc.2009.02.097. doi:10.1016/j.bbrc.2009.02.097. [DOI] [PubMed] [Google Scholar]
- 4.Van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. doi:10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. doi:10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW, II, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. doi:10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 7.Divakaran V, Mann DL. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res. 2008;103:1072–1083. doi: 10.1161/CIRCRESAHA.108.183087. doi:10.1161/CIRCRESAHA.108.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greco S, De Simone M, Colussi C, Zaccagnini G, Fasanaro P, Pescatori M, Cardani R, Perbellini R, Isaia E, Sale P, Meola G, Capogrossi MC, Gaetano C, Martelli F. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009;23:3335–3346. doi: 10.1096/fj.08-128579. doi:10.1096/fj.08-128579. [DOI] [PubMed] [Google Scholar]
- 9.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. doi:10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. doi:10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 11.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. doi:10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Kerin MJ. MicroRNAs as novel biomarkers for breast cancer. J Oncol. 2009;2009:950201. doi: 10.1155/2010/950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. doi:10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. doi:10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem. 2009;55:1944–1949. doi: 10.1373/clinchem.2009.125310. doi:10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- 17.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106:1035–1039. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 18.Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, Li K, Yu B, Li Z, Wang R, Wang L, Li Q, Wang N, Shan H, Yang B. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;39:73–77. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 20.Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Müller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac c-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. doi:10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 21.Straino S, Germani A, Di Carlo A, Porcelli D, De Mori R, Mangoni A, Napolitano M, Martelli F, Biglioli P, Capogrossi MC. Enhanced arteriogenesis and wound repair in dystrophin-deficient mdx mice. Circulation. 2004;110:3341–3348. doi: 10.1161/01.CIR.0000147776.50787.74. doi:10.1161/01.CIR.0000147776.50787.74. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Oikawa K, Takanashi M, Kudo M, Ohyashiki J, Ohyashiki K, Kuroda M. Down-regulation of miR-92 in human plasma is a novel marker for acute leukemia patients. PLoS One. 2009;4:e5532. doi: 10.1371/journal.pone.0005532. doi:10.1371/journal.pone.0005532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112:55–59. doi: 10.1016/j.ygyno.2008.08.036. doi:10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 24.Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, Reinhardt F, Liao R, Krieger M, Jaenisch R, Lodish HF, Blelloch R. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009;105:585–594. doi: 10.1161/CIRCRESAHA.109.200451. doi:10.1161/CIRCRESAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy AM, Zheng Y, Jagadeeswaran G, Macmil SL, Graham WB, Roe BA, Desilva U, Zhang W, Sunkar R. Cloning, characterization and expression analysis of porcine microRNAs. BMC Genomics. 2009;10:65. doi: 10.1186/1471-2164-10-65. doi:10.1186/1471-2164-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A. miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol. 2008;48:648–656. doi: 10.1016/j.jhep.2008.01.019. doi:10.1016/j.jhep.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci USA. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. doi:10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, Biglioli P, Giorgio M, Martin-Padura I, Pelicci PG, Capogrossi MC. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–2923. doi: 10.1161/01.CIR.0000129309.58874.0F. doi:10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. doi:10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 30.Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. Published online ahead of print 6 February 2009. doi:10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 31.Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377–387. doi: 10.1536/ihj.50.377. doi:10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, Xiao J, Shan H, Wang Z, Yang B. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–3052. doi: 10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- 33.Terentyev D, Belevych AE, Terentyeva R, Martin MM, Malana GE, Kuhn DE, Abdellatif M, Feldman DS, Elton TS, Györke S. miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ Res. 2009;104:514–521. doi: 10.1161/CIRCRESAHA.108.181651. doi:10.1161/CIRCRESAHA.108.181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X, Lin H, Pan Z, Xiao J, Zhang Y, Lu Y, Yang B, Wang Z. Down-regulation of miR-1/miR-133 contributes to re-expression of pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. J Biol Chem. 2008;283:20045–20052. doi: 10.1074/jbc.M801035200. doi:10.1074/jbc.M801035200. [DOI] [PubMed] [Google Scholar]
- 35.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21:461–469. doi: 10.1016/j.ceb.2009.01.029. doi:10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lafferty-Whyte K, Cairney CJ, Jamieson NB, Oien KA, Keith WN. Pathway analysis of senescence-associated miRNA targets reveals common processes to different senescence induction mechanisms. Biochim Biophys Acta. 2009;1792:341–352. doi: 10.1016/j.bbadis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. doi:10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosoda T, Urbanek K, Carvalho AB, Bearzi C, Bardelli S, Rizzi R, D'Amario D, Carrillo-Infante C, Zheng H, Quaini F, Rota M, Anversa P, Leri A. MicroRNA-499 promotes the differentiation of cardiac progenitor cells into myocytes. Circulation. 2008;118:S_395. (abstr) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.