Abstract
Purpose of review
Sleep–wake problems such as night wakings, excessive crying, or difficulties in falling asleep are frequent behavioral issues during childhood. Maturational changes in sleep and circadian regulation likely contribute to the development and maintenance of such problems. This review highlights the recent research examining bioregulatory sleep mechanisms during development and provides a model for predicting sleep–wake behavior in young humans.
Recent findings
Findings demonstrate that circadian and sleep homeostatic processes exhibit maturational changes during the first two decades of life. The developing interaction of both processes may be a key determinant of sleep–wake and crying behavior in infancy. Evidence shows that the dynamics of sleep homeostatic processes slow down in the course of childhood (i.e., sleep pressure accumulates more slowly with increasing age) enabling children to be awake for consolidated periods during the day. Another current topic is the adolescent sleep phase delay, which appears to be driven primarily by maturational changes in sleep homeostatic and circadian processes.
Summary
The two-process model of sleep regulation is a valuable framework for understanding and predicting sleep–wake behavior in young humans. Such knowledge is important for improving anticipatory guidance, parental education, and patient care, as well as for developing appropriate social policies.
Keywords: adolescence, children, excessive crying, sleep behavior, sleep homeostasis
Introduction
Anticipatory guidance is an important component of health supervision visits in primary care. In more than 70% of these visits, children’s sleep and crying issues are discussed between parents and healthcare professionals [1•]. Epidemiological studies indicate that 30% of the pediatric population has sleep problems, which are frequently associated with significant psychological and social consequences [2]. Furthermore, the classical sleep phase delay from school age through adolescence poses a major challenge to many teenagers and their families attempting to cope with school schedules, academic demands, and extracurricular activities [3••,4•,5,6••]. The high prevalence of sleep issues in primary care highlights the importance of understanding normal sleep–wake behavior during development. Such knowledge is important for improving anticipatory guidance, parental education, and patient care, as well as for developing social policies [7].
Recent work has provided a conceptual framework for understanding and predicting sleep–wake patterns during development. This review summarizes the latest research on children’s sleep–wake mechanisms in the context of the two-process model of sleep regulation and highlights the use of such a model in conceptualizing and treating sleep–wake problems in infants, children, and adolescents.
The two-process model of sleep regulation
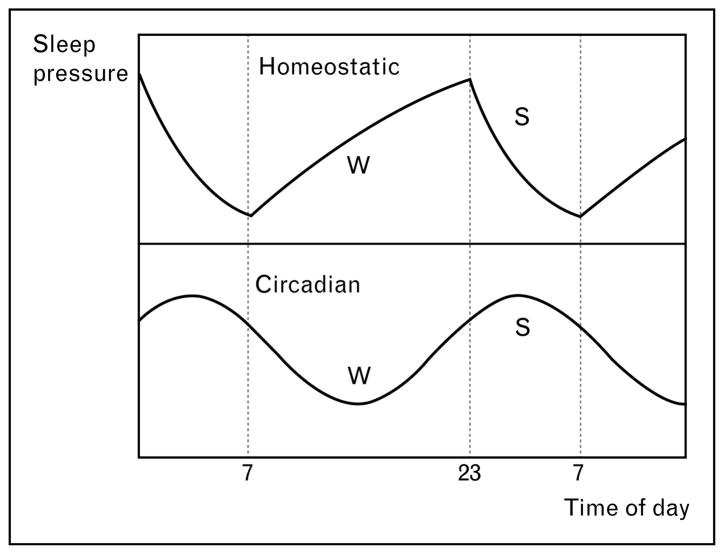
Current theoretical models describe two intrinsic bio-regulatory processes that determine the timing of sleep and waking in humans (first conceptualized in the two-process model of sleep regulation by Borbély in 1982, Fig. 1 [8,9•,10,11]). A sleep–wake dependent homeostatic process accounts for an increase of sleep pressure as waking is extended and for a recovery process occurring during sleep. This homeostatic process interacts with a sleep–wake independent, clock-like circadian process.
Figure 1. Two-process model of sleep regulation.
Homeostatic and circadian modulation of sleep pressure during the day and at night. The circadian process is a clock-like process, whereas the homeostatic sleep–wake dependent process is an hourglass process. Data from Achermann and Borbély [11]. W indicates the wake period and S sleep.
While the circadian process has a distinct anatomical locus in the nucleus suprachiasmaticus of the hypothalamus (‘the internal clock’) [12•], the homeostatic sleep–wake process is less well defined on a neuronal basis. Specific features of the sleep electroencephalogram (EEG), however, have been proposed as markers for sleep homeostasis [8], including stage 4 nonrapid eye movement sleep (slow-wave sleep) and EEG power density in the low-frequency range (0.75–4.5 Hz, delta activity or slow-wave activity). The time course of the homeostatic process delineated from delta activity exhibits an exponential decline during sleep and a saturating rise during wakefulness reflecting the evolution of sleep pressure during the day and at night (Fig. 1). Another marker for sleep homeostatic pressure is the speed of falling asleep (sleep latency) [13•]. For example, sleep deprivation induces a reduction of sleep latency (i.e., faster sleep onsets) indicating higher sleep pressure [13•].
In contrast, the circadian process is essentially independent of prior waking and sleep [9•,14]. The circadian system contributes to the sleep–wake timing by providing clock-like signals interpreted as ‘sleep gates’ (with high-circadian sleep pressure) [15] and ‘wake maintenance zones’ (with low-circadian sleep pressure) [16]. The wake-promoting or alerting signal of the circadian system reaches its maximal levels in the early evening and its trough in the early morning [14] (see also Fig. 2, adapted from [17]).
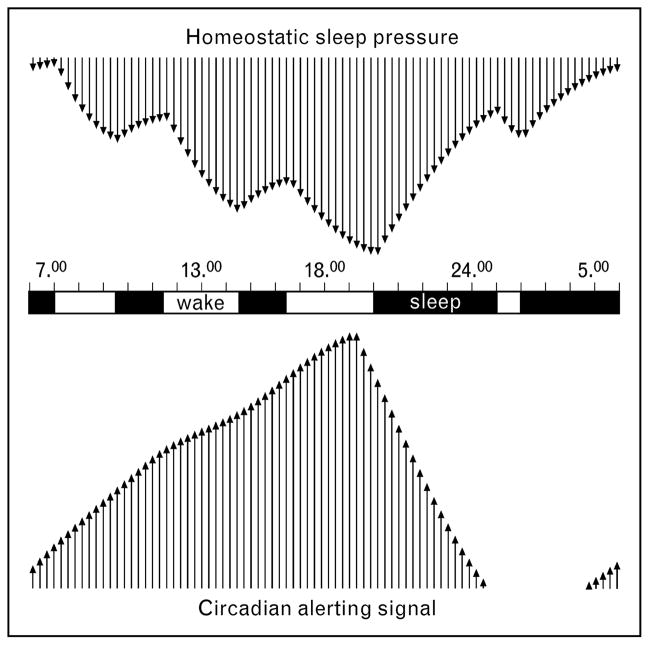
Figure 2. Conceptual model of sleep–wake regulation in early infancy.
Negative upper arrows represent increasing sleep pressure (plotted upside down), while positive lower arrows indicate increasing circadian drive. Black bars represent sleep episodes. Adapted from [17]. The ability of the infant to decrease sleep pressure during the night counteracts the drop in circadian alertness during the night for maintaining a consolidated nocturnal sleep episode. The increase of sleep pressure during the day opposes the circadian alerting signal in the early evening for sustaining a balanced vigilance state during the day.
Circadian and sleep homeostatic mechanisms are well explored in adults. Recent research has examined the ability of both processes to predict sleep–wake behavior in infancy, childhood, and adolescence. Although both mechanisms play an important role, this review focuses more on the homeostatic system. We note that data on circadian processes in children are limited due to methodological challenges in studying the developing ‘internal clock.’
Sleeping and crying behavior in infancy
A recent longitudinal study supported previous surveys that by the end of the fourth month 70% of all infants sleep through the night [18•]. Adams and colleagues [19•] demonstrated that the age at which infants first sleep through the night cannot be exclusively explained by extrinsic factors such as infant and parent characteristics, obstetric variables, parental care or feeding practices, suggesting that the nocturnal sleep consolidation also reflects the maturation of intrinsic bioregulatory processes. In fact, a recent commentary in Behavioral and Brain Sciences proposed a regulatory model for sleeping through the night and crying behavior on the basis of the two-process model of sleep regulation [20•]. The developing interaction of homeostatic and circadian processes may be a key determinant for infant sleep–wake behavior (Fig. 2). The proper alignment of both processes in the first few months after birth results in sleep consolidation at night. In contrast, frequent night wakings may occur when homeostatic and circadian processes are not aligned (misalignment).
Infant crying is characterized by two typical features [21]: (a) crying increases in duration and intensity during the first few weeks after birth reaching a peak at age 6 weeks and then decreasing again and (b) crying occurs primarily during the evening hours. Although crying is apparent in the majority of infants, some babies cry more than expected, either relative to what is normal or to what the caregiver can tolerate (referred as to ‘excessive crying’). Barr and colleagues [22•] recently showed that crying bouts are longer and occur more frequently in excessively crying infants compared to normal controls. An extensive review in Behavioral and Brain Sciences described infant crying from an evolutionary perspective [23•]. Soltis [23•] hypothesized that excessive crying is a signal of vigor that evolved to diminish the risk of a reduction or withdrawal of parental care. Excessive crying, however, may also result in parental maltreatment. A recent Dutch publication in The Lancet [24•] reported that 5.6% of parents have shaken their baby at least once because of infant crying.
Although infant crying behavior has been extensively described as a behavioral phenomenon [21,22•], the origins of crying largely remain unclear. Some authors have hypothesized that disorders of the developing sleep–wake rhythm may drive excessive crying. A recent 24-h polysomnographic sleep study [25•], however, indicated that the total amount of sleep and the proportion of sleep stages do not differ between excessive crying infants and normal healthy controls. This finding does not rule out the role of basic bioregulatory processes. That is, the developing interaction of sleep homeostatic and circadian processes may determine crying behavior in young infants [20•]. The clock-dependent alerting signal reflecting the circadian process may generate a rise in alertness during the day providing the basis for the predicted late afternoon and evening clustering of crying (Fig. 2). The diurnal rhythm of crying becomes progressively more distinct from birth through the first few weeks reflecting the increasing magnitude of the circadian drive, which may underlie the tendency for some infants to express crying behavior preferentially at certain times of day. Several studies [26,27•,28] suggest that sleep homeostasis emerges in the second month of life. Thus, from the second month, homeostatic sleep pressure increases during the course of the day. Homeostatic and circadian processes, however, may not develop at the same rate. Hyperalertness, hyperarousal, and excessive crying may occur, when circadian alertness is not yet opposed by homeostatic sleep pressure. When the infant gains the capacity to increase sleep pressure during the waking day in the second month of life, evening crying diminishes because circadian alertness is opposed by increased sleep pressure. When both processes remain misaligned at age 6 months and excessive crying behavior persists, delayed neurological maturation or even subtle neurological problems must be considered. This view is supported by the recent study of Rao and colleagues [29•] published in Archives of Disease in Childhood demonstrating that excessive crying, which persists beyond the age of 3 months is related to cognitive deficits during childhood. In addition, Wolke and colleagues [30] identified an association between prolonged crying during infancy and hyperactivity when the children were between 8 and 10 years old.
Sleep and sleep problems in early and middle childhood
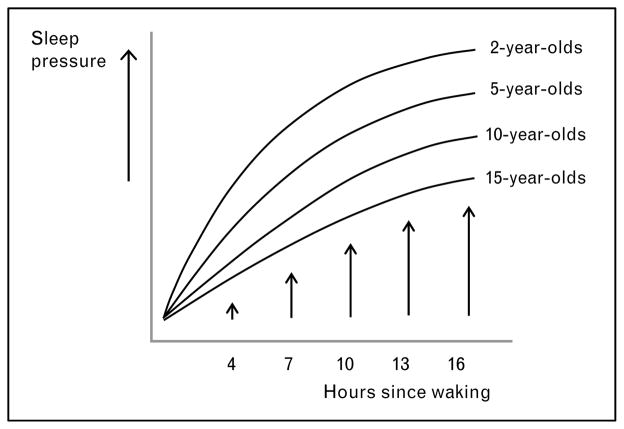
The decline in daytime napping and consolidation of sleep into one period at night is a gradual process that occurs between early and middle childhood. The present state of knowledge on the biphasic-to-monophasic sleep transition is primarily descriptive; however, we propose that the early sleep distribution changes may result from differences in sleep homeostatic control based upon the following results: (a) overall sleep need, as measured by reported sleep duration, declines from about 13 h at 2 years to about 11 h at 6 years [31]; (b) the decline in 24-h sleep duration is primarily attributed to a decrease in daytime sleep [31], nighttime sleep duration remains fairly constant during early childhood [32•]; (c) age-related changes in napping include gradual declines in reported duration and weekly frequency [33•]; and (d) nap sleep-onset latencies increase with age [34]. If early maturational changes in the homeostatic process exist, it is likely that younger children accumulate sleep pressure more quickly during the day than older children do, thereby necessitating daily daytime naps of longer duration and accounting for shorter nap sleep-onset latencies (Fig. 3). Conversely, if homeostatic sleep pressure accumulates more slowly during development, then older children should be able to sustain wakefulness for longer periods, thus, resulting in fewer naps per week, shorter nap durations, and eventually, a consolidated sleep–wake pattern.
Figure 3. The sleep homeostatic process as a function of age.
Proposed developmental changes in accumulation of sleep pressure as a function of time since waking depicted for different ages. Sleep pressure accumulates more slowly during the day with increasing age.
When children should stop napping and get all their sleep at night is a common question posed by parents. Napping is also a current controversy in early childhood settings, where increased emphasis on academic performance has led some school systems to replace afternoon naps with learning activities. Variability in the timing of a monophasic sleep–wake pattern exists [31,33•], and parents and policy makers should consider individual/group differences. For example, while most children stop napping between ages of 4 and 5, some continue to nap until early school age. Additionally, Crosby and colleagues [33•] recently found significant differences in the proportion of White and Black children napping at least one day per week beginning at 3 years and extending until 8 years. Clinical sleep experts suggest that children are ready to stop napping if they stop falling asleep at naptime, take a long time to fall asleep when put down for a nap, or do not appear tired, cranky, or irritable on days when they miss a nap [35•]. Signs such as these are consistent with the hypothesis that the dynamics of the homeostatic process slow down during early childhood (sleep pressure accumulates less rapidly in older children than in younger children; see Fig. 3).
Another application of the model can be derived from two recent publications in Pediatrics. Iglowstein and colleagues [31] showed a delay in bedtime and a decrease of sleep duration between their examined cohorts of the 1970s and the 1990s. A further analysis of the same cohorts revealed that bedtime resistance was significantly lower in the later cohorts compared to the earlier cohort, which tempted these authors to conclude that parents in the 1990s adjusted bedtime more appropriately to children’s individually preferred bedtimes [18•]. Thus, Swiss children were put to sleep later, at a time when they were really tired and therefore demonstrated less resistance in going to bed. This interpretation is in line with recent experimental research by Sadeh and colleagues [36] published in Child Development reporting that delaying bedtime leads to increased sleepiness at bedtime, shorter sleep latency and less night wakings. The sleep regulatory mechanisms for this finding are easy to identify; delaying bedtime enhances the homeostatic pressure to fall asleep and increases sleep intensity and sleep consolidation, and thus, may be an appropriate approach to bedtime struggles or night wakings. A note of caution is indicated, however, when delaying bedtime is used as a behavioral strategy (as in the faded bedtime approach, reviewed in [37•]); delaying bedtime may lead to sleep restriction with negative effects on some children’s behavior and academic performance [38•]. The primary role of parents and childcare professionals should be to determine the optimal amount of sleep for the individual child while taking age and individual sleep need into account. Sadeh and colleagues [36] stated that ‘parents and child-care professionals can explore the appropriate sleep need of an individual child by experimenting with restricting or extending sleep and tracking the changes in the child’s behavior and well being (…), which is done intuitively, if not systematically, by many parents’ (see p. 453). This approach is based on the fact that general recommendations in regard to optimal amount of sleep are problematic because of the large variability of sleep–wake biology among individuals [31]. In fact, individual differences in human sleep (i.e., sleep need, sleep timing, daytime sleepiness, vulnerability to the effects of sleep loss and so forth) should be ‘a leitmotiv for the future research agenda’ (see review by Van Dongen and colleagues in the journal Sleep [39•]).
The adolescent sleep phase delay and its consequences
A recent supplement of the journal Pediatrics [40] on cultural issues and children’s sleep supported the view that sleep phase progressively delays from school age through adolescence in different societies around the world [41–44,45•,46,47]. Until recently, the delay in the sleep phase from school age through adolescence was viewed to originate primarily from shifts in peer culture, family configurations, academic demands, school culture, employment opportunities, and extracurricular activities [6••]. The low variability of sleep patterns across cultures has led some authors to conclude that biological processes, not changes in the psychosocial milieu, drive the sleep phase delay in teens [48•]. In fact, this view has received support from scientific studies performed under strict control of psychosocial influences [5,6••]. Maturational changes in the biology of sleep–wake and circadian regulation were reported, which may provide impetus for the sleep phase delay [5,6••].
Two recent reviews by Carskadon and colleagues [5,6••] reported that several distinct changes of the circadian process that influence the adolescent sleep phase delay may occur during puberty (i.e., delay of intrinsic circadian phase, lengthening of the intrinsic period of the circadian clock, heightened sensitivity to evening light, or decreased sensitivity to morning light). Several studies [49,50••,51] on sleep homeostasis in adolescents were also published in the past 12 months using EEG spectral analysis and mathematical simulations. Briefly, the rise rate of homeostatic sleep pressure during the day is slower in mature adolescents than pre-pubertal or early pubertal children contributing to delay of the sleep phase over the course of puberty (Fig. 3). The nocturnal dissipation of sleep pressure, however, does not differ between prepubertal and mature teenagers. The practical implications of these findings are that mature adolescents may experience a decrease in their sensitivity to extended wakefulness and live under lower sleep pressure at scheduled bedtimes than prepubertal children who live closer to sleep pressure saturation. This interpretation is consistent with a recent study [13•] published in the Journal of Sleep Research demonstrating that mature adolescents manifest less sleep pressure than pre-pubertal children for approximately 4 h following scheduled bedtime. After being awake for 14.5, 16.5, and 18.5 h, sleep tendency assessed by the time to fall asleep (sleep latency) was significantly lower in post-pubertal than prepubertal adolescents independent of circadian phase. The maturational changes of sleep homeostasis in adolescents may decrease their sensitivity to sleep loss and increase the tolerance to sleep pressure, a prerequisite for adult lifestyles. Thus, the physiologically mediated delay in evening bedtimes and sleep onset may ‘open the gate’ for increasing capacities to participate in opportunities in the evening and at night. These bioregulatory changes may, however, also increase the vulnerability of adolescents to specific sleep–wake disorders such as delayed sleep phase syndrome (DSPS) [52•].
As a consequence of the adolescent sleep delay, societal demands can result in the curtailment of sleep that may influence cognitive and emotional functioning, school performance, and mood [3••,38•,53,54,55••]. The changing adolescent sleep–wake system exists in the context of a relatively unforgiving educational structure demanding earlier school attendance in older than younger children. Thus, inadequate and ill-timed sleep is observed in a number of adolescents [42–44,45•,46,47]. Several short-term and long-term strategies that address the epidemic of daytime sleepiness among adolescents have been proposed to improve health and maximize school performance (e.g., delaying school start time, exposure to bright light in the morning [4•]).
Conclusions
The two-process model of sleep regulation may serve as a conceptual framework for understanding sleep–wake behavior and sleep problems not only in adults but also in children. Rosen [7] noted in his editorial in Sleep that ‘the importance of this model is less in the details than in the concept that forces can be understood and predicted’ (see p. 1354). Further application of the model in the clinical setting may finally help us to improve parental education and patient care and to develop appropriate social policies.
Abbreviation
- EEG
electroencephalogram
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 332–333).
- 1•.Olson LM, Inkelas M, Halfon N, et al. Overview of the content of health supervision for young children: reports from parents and pediatricians. Pediatrics. 2004;113:1907–1916. This article highlights the importance of parent–physician discussions about many topics (e.g., sleeping, crying, feeding) that are critical to healthy child development. [PubMed] [Google Scholar]
- 2.Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems in children and adolescents. Lippincott Williams & Wilkins Publishers; 2003. [Google Scholar]
- 3••.Millman RP. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–1786. doi: 10.1542/peds.2005-0772. This author presents on behalf of the Working Group on Sleepiness in Adolescents/Young Adults (AAP Committee on Adolescence) a comprehensive review of the scientific data on normal sleep changes during adolescence, factors related to chronic insufficient sleep, and the effects of poor/insufficient sleep on daytime functioning. The primary sleep disorders associated with excessive daytime sleepiness in this population are also reviewed. [DOI] [PubMed] [Google Scholar]
- 4•.Hansen M, Janssen I, Schiff A, et al. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. This novel study examined the impact of starting school on adolescent sleep (timing, duration, weekend oversleep) and assessed the effects of early morning bright light on sleep and neuropsychological performance. [DOI] [PubMed] [Google Scholar]
- 5.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25:606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 6••.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. This article presents experimental data from adolescents examining EEG markers of sleep homeostasis to evaluate whether sleep homeostatic processes show maturational changes during puberty. This paper also examines maturational alterations of the circadian timing system, including phase, period, melatonin secretory pattern, light sensitivity, and phase relationships. [DOI] [PubMed] [Google Scholar]
- 7.Rosen G. The value of a model. Sleep. 2005;28:1354. [PubMed] [Google Scholar]
- 8.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 9•.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3. Elsevier Saunders; 2005. pp. 405–417. An excellent chapter devoted to the development and evaluation of models of sleep regulation, including the two-process model. [Google Scholar]
- 10.Daan S, Beersma DG, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 11.Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:S683–S693. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- 12•.Van Gelder RN. Recent insights into mammalian circadian rhythms. Sleep. 2004;27:166–171. doi: 10.1093/sleep/27.1.166. This short but excellent paper reviewed recent progress in understanding the mammalian circadian clock. [DOI] [PubMed] [Google Scholar]
- 13•.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–244. doi: 10.1111/j.1365-2869.2005.00467.x. This study investigated sleep onset latency during extended waking in prepubertal and mature adolescents to determine whether sleep pressure is lower near bedtime in the latter group. [DOI] [PubMed] [Google Scholar]
- 14.Dijk D-J, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzischinsky O, Shlitner A, Lavie P. The association between the nocturnal sleep gate and nocturnal onset of urinary 6-sulfatoxymelatonin. J Biol Rhythms. 1993;8:199–209. doi: 10.1177/074873049300800303. [DOI] [PubMed] [Google Scholar]
- 16.Strogatz SH. The mathematical structure of the human sleep–wake cycle. New York: Springer-Verlag; 1986. [DOI] [PubMed] [Google Scholar]
- 17.Cajochen C, Blatter K, Wallach D. Circadian and sleep–wake dependent impact on neurobehavioral function. Psychol Belg. 2004;44:59–80. [Google Scholar]
- 18•.Jenni OG, Zinggeler Fuhrer H, Iglowstein I, et al. A longitudinal study of bedsharing and sleep problems among Swiss children in the first 10 years of life. Pediatrics. 2005;115:233–240. doi: 10.1542/peds.2004-0815E. This longitudinal study assessed age trends and long-term course of bed-sharing practices and sleep problems among 493 Swiss children between 1974 and 2001. [DOI] [PubMed] [Google Scholar]
- 19•.Adams SM, Jones DR, Esmail A, Mitchell EA. What affects the age of first sleeping through the night? J Paediatr Child Health. 2004;40:96–101. doi: 10.1111/j.1440-1754.2004.00317.x. These authors assessed the influence of 61 potential factors on the age at which infants first slept through the night using multivariate survival analysis. [DOI] [PubMed] [Google Scholar]
- 20•.Jenni OG. Sleep–wake processes play a key role in early infant crying. Behav Brain Sci. 2004;27:464–465. This commentary on signal functions of early infant crying proposes that the crying curve across infancy may reflect the developing interaction between circadian and homeostatic processes of sleep regulation. [Google Scholar]
- 21.Barr RG. The normal crying curve: what do we really know? Dev Med Child Neurol. 1990;32:356–362. doi: 10.1111/j.1469-8749.1990.tb16949.x. [DOI] [PubMed] [Google Scholar]
- 22•.Barr RG, Paterson JA, MacMartin LM, et al. Prolonged and unsoothable crying bouts in infants with and without colic. J Dev Behav Pediatr. 2005;26:14–23. This well controlled, longitudinal study of infants with and without colic evaluated the daily amount, frequency, and bout duration of fussing, crying, and unsoothable crying with parental diaries. [PubMed] [Google Scholar]
- 23•.Soltis J. The signal functions of early infant crying. Behav Brain Sci. 2004;27:443–458. [discussion 459–490]. This paper explores infant crying from an evolutionary perspective and reviews evidence suggesting that excessive early infant crying is a signal of vigor that evolved to reduce the risk of a reduction or withdrawal of parental care. [PubMed] [Google Scholar]
- 24•.Reijneveld SA, van der Wal MF, Brugman E, et al. Infant crying and abuse. Lancet. 2004;364:1340–1342. doi: 10.1016/S0140-6736(04)17191-2. These authors present data from a large-scale study of 1–6-month-old infants on detrimental parental reactions to infant crying. [DOI] [PubMed] [Google Scholar]
- 25•.Kirjavainen J, Lehtonen L, Kirjavainen T, Kero P. Sleep of excessively crying infants: a 24-hour ambulatory sleep polygraphy study. Pediatrics. 2004;114:592–600. doi: 10.1542/peds.2003-0651-L. This study examined sleep differences in the sleep of excessively crying infants in comparison to control infants at 6 weeks of age using polygraphy and parental sleep diaries. [DOI] [PubMed] [Google Scholar]
- 26.Salzarulo P, Fagioli I. Post-natal development of sleep organization in man: speculations on the emergence of the ‘S process’. Neurophysiol Clin. 1992;22:107–115. doi: 10.1016/s0987-7053(05)80748-8. [DOI] [PubMed] [Google Scholar]
- 27•.Jenni OG, Borbély AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R528–R538. doi: 10.1152/ajpregu.00503.2003. The authors examined the nocturnal sleep and sleep EEG at five time points across infancy. Results suggest that EEG markers of sleep homeostasis appear in the first postnatal months, and that sleep homeostasis goes through a period of maturation. [DOI] [PubMed] [Google Scholar]
- 28.Peirano P, Algarin C, Uauy R. Sleep–wake states and their regulatory mechanisms throughout early human development. J Pediatr. 2003;143 (4 Suppl):S70–S79. doi: 10.1067/s0022-3476(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 29•.Rao MR, Brenner RA, Schisterman EF, et al. Long term cognitive development in children with prolonged crying. Arch Dis Child. 2004;89:989–992. doi: 10.1136/adc.2003.039198. This study evaluated the prediction that colic/excessive crying beyond 3 months of age is associated with adverse cognitive development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolke D, Rizzo P, Woods S. Persistent infant crying and hyperactivity problems in middle childhood. Pediatrics. 2002;109:1054–1060. doi: 10.1542/peds.109.6.1054. [DOI] [PubMed] [Google Scholar]
- 31.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 32•.Acebo C, Sadeh A, Seifer R, et al. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28:1568–1577. doi: 10.1093/sleep/28.12.1568. These authors reported actigraphic and sleep diary data on sleep patterns and sleep duration during 24-h periods in 1–5-year-old children. [DOI] [PubMed] [Google Scholar]
- 33•.Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics. 2005;115:225–232. doi: 10.1542/peds.2004-0815D. This study examined racial differences (Black and White) in reported napping and nighttime sleep of 2–8-year-old children and explored factors accounting for these differences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dales RJ. Afternoon sleep in a group of nursery-school children. J Genet Psychol. 1941;58:161–180. [Google Scholar]
- 35•.Mindell JA. Sleeping through the night. 2. New York: Harper Collins Publishers; 2005. This book is a comprehensive tool for parents wanting to understand normal sleep–wake behaviors, sleep problems, and available treatments in infants, toddlers, and preschoolers in a better way. [Google Scholar]
- 36.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 37•.Sadeh A. Cognitive–behavioral treatment for childhood sleep disorders. Clin Psychol Rev. 2005;25:612–628. doi: 10.1016/j.cpr.2005.04.006. This author provides a review of existing data on the effectiveness of cognitive–behavioral treatment for childhood sleep disorders and identifies important methodological issues and limitations, as well as areas in need of investigation. [DOI] [PubMed] [Google Scholar]
- 38•.Fallone G, Acebo C, Seifer R, Carskadon MA. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep. 2005;28:1561–1567. doi: 10.1093/sleep/28.12.1561. This 3-week study investigated the experimental effects of sleep restriction on teacher ratings of behavior and academic performance in 6–12-year-olds. [DOI] [PubMed] [Google Scholar]
- 39•.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28:479–496. doi: 10.1093/sleep/28.4.479. This provocative paper reviewed the literature on interindividual variability in human sleep parameters, sleepiness, responses to sleep deprivation, and manifestations of sleep disorders. [DOI] [PubMed] [Google Scholar]
- 40.Owens JA. Introduction: culture and sleep in children. Pediatrics. 2005;115:201–203. doi: 10.1542/peds.2004-0815a. [DOI] [PubMed] [Google Scholar]
- 41.Jenni OG. Sleep onset insomnia during childhood or poor fit between biology and culture. J Sleep Res. 2005;14:195–197. doi: 10.1111/j.1365-2869.2005.00459_1.x. [author reply 197–199] [DOI] [PubMed] [Google Scholar]
- 42.Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–256. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115:241–249. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- 44.Gau SF, Soong WT. The transition of sleep–wake patterns in early adolescence. Sleep. 2003;26:449–454. doi: 10.1093/sleep/26.4.449. [DOI] [PubMed] [Google Scholar]
- 45•.Ohida T, Osaki Y, Doi Y, et al. An epidemiologic study of self-reported sleep problems among Japanese adolescents. Sleep. 2004;27:978–985. doi: 10.1093/sleep/27.5.978. The large-scale, cross-sectional study investigated the prevalence and correlates of sleep problems in Japanese adolescents. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira LR, Fischer FM, de Andrade MM, et al. Sleep patterns of day-working, evening high-schooled adolescents of Sao Paulo. Brazil Chronobiol Int. 2004;21:239–252. doi: 10.1081/cbi-120037808. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Khalek AM. Prevalence of reported insomnia and its consequences in a survey of 5,044 adolescents in Kuwait. Sleep. 2004;27:726–731. doi: 10.1093/sleep/27.4.726. [DOI] [PubMed] [Google Scholar]
- 48•.Jenni OG, O’Connor BB. Children’s sleep: an interplay between culture and biology. Pediatrics. 2005;115:204–216. doi: 10.1542/peds.2004-0815B. This comprehensive review of cross-cultural features of children’s sleep behavior in industrialized and complex modern societies uses the goodness-of-fit model as a framework for understanding sleep problems. [DOI] [PubMed] [Google Scholar]
- 49.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–783. [PubMed] [Google Scholar]
- 50••.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. This well controlled study used 36 h of sleep deprivation in early and late pubertal children to examine aspects of sleep homeostasis. A slower build-up of sleep pressure during wakefulness was observed in late than in early puberty, suggesting maturational changes in homeostatic sleep regulation that are permissive of the sleep phase delay in the course of adolescence. [DOI] [PubMed] [Google Scholar]
- 51.Jenni OG, van Reen E, Carskadon MA. Regional differences of the sleep electroencephalogram in adolescents. J Sleep Res. 2005;14:141–147. doi: 10.1111/j.1365-2869.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- 52•.Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27:1195–1203. doi: 10.1093/sleep/27.6.1195. This paper presented a comprehensive review of DSPS, including conceptualization of the disorder within the context of shared modulation of sleep and wakefulness through sleep homeostatic and circadian systems. [DOI] [PubMed] [Google Scholar]
- 53.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 54.Wolfson AR, Carskadon MA. Understanding adolescent sleep patterns and school performance: a critical appraisal. Sleep Med Rev. 2003;7:491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- 55••.Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: tracking the effects of adolescent sleep loss during the middle school years. Child Dev. 2004;75:84–95. doi: 10.1111/j.1467-8624.2004.00655.x. This is one of the few large-scale, longitudinal studies assessing the influence of sleep patterns on trajectories of depressive symptoms, self-esteem, and grades using latent growth cross-domain models. Results indicated that less sleep across the 3-year span (ages 11–14 years) was predictive of heightened levels of reported depressive symptoms and decreased self-esteem. [DOI] [PubMed] [Google Scholar]