Abstract
Maintenance treatment in ulcerative colitis often fails to prevent flares and long term complications. The first key to maintenance is to use effective therapy, even when patients become asymptomatic. The second key is to communicate the importance of adherence to patients, and to help them achieve long term adherence. Simplified dosing schedules are of some benefit, but the bond between patient and doctor, and the patient's belief in the efficacy of the therapy are essential. Decreased co-pays (a fixed amount paid by patients seeking care that is not reimbursed my medical insurance) have been associated with increased adherence, and incentives for patients may be a cost-effective approach to improving adherence. While the most substantial data on the association between adherence and clinical outcomes is in 5-ASAs, non-adherence can also limit the efficacy of thiopurines and biologics. The third key to maintenance treatment is monitoring and maintaining control of inflammation. Decreased histologic and endoscopic damage to the colon has been associated with decreased risk of colon cancer. The most cost-effective way to monitor smoldering inflammation is not known, but endoscopy, structured symptom indices, and biomarkers may be valuable approaches. The fourth key to maintenance treatment is optimizing immunomodulator therapy with thiopurines, and possibly methotrexate in the future. The fifth key to maintenance treatment in ulcerative colitis is maintaining biologic efficacy by avoiding low trough levels and being vigilant for subclinical inflammation and symptom recurrence at the end of dose intervals. Combination therapy with immunomodulators improves trough levels in Crohn's, and may prove to have benefits for the maintenance of biologic efficacy in ulcerative colitis.
Key Words: Adherence, Maintenance, Ulcerative colitis
Introduction
Life-long maintenance of remission is a critical goal of therapy in ulcerative colitis. Maintenance of remission improves quality of life and work productivity, and reduces the risk of colon cancer. However, this goal can be difficult to achieve for several reasons. First, current medications are not always effective in all patients, and even when initially effective, may not prevent all flares. Second, patients are frequently non-adherent, especially when they have been in remission for some time. Third, flares are difficult to predict, and current practice is not very successful in identifying subclinical inflammation and intervening before clinical flares occur.
This paper will present five keys to maintaining remission in ulcerative colitis: (1) Use effective therapies, and encourage patients to use these rather than various unproven and questionable forms of ‘alternative’ medication. (2) Achieve adherence in patients, or hold an open conversation about adherence and drug holidays. (3) Monitor for subclinical inflammation, which can provide an opportunity to intervene therapeutically. (4) Optimize thiopurines when these are used. (5) Maintain biologic efficacy once biologics are started in ulcerative colitis.
Using Effective Therapies
There are five main proven therapies for ulcerative colitis. These include sulfasalazine, 5-ASA medications, thiopurines, infliximab, and colectomy. Despite the known efficacy of these approaches, and published randomized controlled trials for the medical therapies, patients still pursue much-hyped alternative therapies, including fish oil, probiotics, ‘immune control’ diets, and low-dose naltrexone.
It is important to share with your patients the data on the efficacy of proven therapies, and contrast these with the lack of data for ‘alternative’ therapies. This approach helps patients understand why particular medications are used, how effective they will (and will not) be, and the importance of clinical trials to identify more effective therapies. Many patients assume that prescribed medications should and will be 100% effective in maintaining remission, and when this expectation is not met, their physician and the medical profession loses credibility. It is important to present the limitations of our current medications, which do not maintain remission in all patients, leading to a lifetime colectomy rate of ∼30%.
The data to support the use of sulfasalazine and 5-ASA medications in ulcerative colitis is summarized in the Cochrane review of this topic [1]. Sulfasalazine or 5-ASAs are about twice as likely as placebo to produce global improvement or remission in ulcerative colitis. Adding rectal 5-ASA formulations for patients with left sided disease, particularly during mild flares, can be quite helpful. Financial considerations may move many patients to try sulfasalazine, and third-party payer pressures may require patients to fail sulfasalazine before they can get 5-ASAs.
Thiopurines have been tested for ulcerative colitis in several small studies, and found to clearly be steroid sparing [2], to have a higher rate of remission versus 5-ASAs [3], and to have sustained benefit from continuing azathioprine [4]. Infliximab has been tested in two large trials [5] and been found to be more effective than placebo for induction and maintenance.
Cyclosporine, while used as a rescue therapy, is generally used to bridge patients to thiopurine maintenance. Colectomy is also an important therapeutic option which can dramatically improve quality of life in patients with ulcerative colitis [6]. It is important for clinicians to take the long view of therapy, especially in young patients. It is important not to create a fear of colectomy, as ∼30% of patients will need colectomy at some point.
It is reasonable to present sulfasalazine and 5-ASAs as first-line maintenance in most patients, and either thiopurines, infliximab, or colectomy as second-line therapy. Some patients may choose colectomy early (due to family history of CRC and/or lymphoma), others may prefer thiopurines (convenience of oral therapy), and others may prefer infliximab (largest clinical trials data). It is important to identify an effective maintenance strategy for each patient that they will be willing to adhere to, as they may need to continue on maintenance therapy for more than 60 years.
Achieving Adherence
It is an unfortunate truth that most patients lie to their doctors. The majority of patients with ulcerative colitis will be non-adherent to prescribed therapy, and will not tell their gastroenterologists. Non-adherence is intentional in many patients, and some will not disclose their non-adherence even when they know their urine will be tested for metabolites [7]. Pharmacy records have demonstrated that more than half of patients are non-adherent to their 5-ASAs for ulcerative colitis, and that this significantly increases the risk of flare and increases medical costs [8].
Patients have a number of reasons for not taking their medications, but approximately equal numbers are unintentionally non-adherent and intentionally non-adherent. Patients who are unintentionally non-adherent often forget to take their medications, or to get their refills, have trouble paying for them, or have other demands in their lives that interfere with their ability to meet the organizational challenge of taking pills every day. More difficult are the patients who are intentionally non-adherent. These patients often do not believe the medications work, do not have faith in their doctor's credibility, often adjust the doses of their own medications, think the side effects outweigh the benefits, and do not want the daily reminder that they have a chronic illness.
Why do patients not believe that 5-ASAs or other medications work for their ulcerative colitis? First, they have an incentive not to believe that these medications work. Patients want to be ‘cured’. They want to no longer need medications, to no longer have a chronic illness. They do not want to accept the sick role, and do not want to take medications (and thereby show a flaw) in front of others. This leads them to frequent testing behavior. They test whether they ‘still have ulcerative colitis’, whether they ‘still need the medication’, and whether they can skip doses. The overwhelming majority of ulcerative colitis patients have skipped doses at some point. Unfortunately, due to the waxing and waning nature of the disease, and the slow washout of medications, they often do not suffer any consequences of short-term non-adherence. This convinces patients that (1) they can stop their medications, (2) their doctor, who has been preaching medication adherence, is wrong, or (3) their ulcerative colitis could have gone away. This outcome is consistent with the results of studies by Kane and colleagues [8,9], which showed a low rate of flare in the first three months of non-adherence. Unfortunately, this outcome both reduces the credibility of the physician, and promotes more non-adherence. So what factors affect non-adherence, and what interventions can improve adherence?
Kane has shown that factors that predict non-adherence are: being young, single, male, mentally ill, having no recent flares, heavy pill burden, perception of lack of benefit and feeling uninformed about the effect of medication, and having a high co-pay (an amount a patient seeking care must pay and which is not reimbursed by medical insurance) [9]. Chernew et al. showed that lower co-pays could increase adherence to statins, ACEi, ARBs, and beta blockers [10]. Many interventions have been tried to promote medication adherence in chronic diseases, but most only work in the short term (1–3 months). This short-term benefit may be due to the Hawthorne effect, whereby observation of adherence promotes adherence. Unfortunately, this effect wears off quickly unless new forms of observation or adherence interventions are made frequently.
In the United States healthcare system, one novel strategy that might be effective in ulcerative colitis is to lower co-pays, or even to pay for adherence (P4A). Adherence could be monitored using MEMS caps, which are embedded with a microchip that records when the bottle is opened. For patients with >80% adherence to their prescribed regimen, they could receive an immediate bonus payment of USD 20 when they pick up a refill (rather than a co-pay). Monetary incentives for adherence might, at the right price point, reduce flares and net costs in ulcerative colitis. However, prospective studies of this strategy, including testing of the optimal incentive (or a Las Vegas-style variable), are necessary before this approach can be adopted.
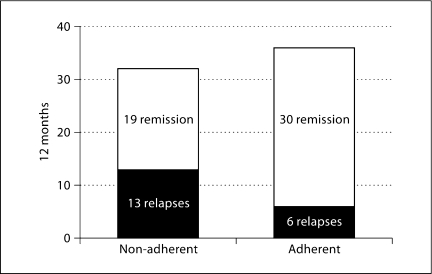
A more immediate approach is to maximize physician credibility and accurately present the data on adherence and outcomes. As seen in figure 1, patients who are non-adherent to their 5-ASAs have a 59% rate of flare at one year. Many patients will perceive this in the reverse manner: I can go off this medication for a year, and still have a 41% chance of not having a flare. This is an important discussion to have with your patients. The risk of flare increases over time, but many patients can go off their medications for months with no ill effects. If the physician does not explain this, and patients discover it for themselves (while physicians are preaching perfect adherence), this can lead to a major loss of credibility.
Fig. 1.
Over a 12-month period, patients who are non-adherent to 5-ASA maintenance therapy have a 41% relapse rate, compared to 17% in adherent patients. Adapted from [8].
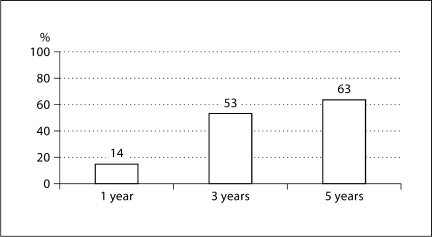
Similar data exist for thiopurines, and Treton et al. advocate sustained thiopurine use because thiopurine withdrawal is associated with eventual flares. However, the rate of flares is relatively low in this selected group of patients with sustained remission after more than three years on thiopurines [11]. Eighty-six percent are still in remission at 1 year after stopping the medication, 47% at 3 years, and 37% after 5 years of non-adherence (fig. 2). It is important to explain this to patients, and to tell them that while adherence is important to reduce flares, steroid use, hospitalizations, costs, and colectomies, non-adherence does not result in immediate negative feedback.
Fig. 2.
Percent Crohn's relapse with azathioprine withdrawal after long remission. Thiopurine non-adherence = 14% relapse at 1 year. Adapted from [11].
It is important to ask your patients to be honest with you about their non-adherence. They are often greatly encouraged to be open and honest if you disclose the results of the Kane and Treton studies, as these often match their experience of trying to stop their own medications. It helps patients to disclose their non-adherence if you ask questions in a way that gives them permission to be non-adherent. For example, preface questions about non-adherence with, ‘most patients miss doses of their medications’, or ‘very few patients take all of their medications on time’. It can also be helpful to overestimate non-adherence. For example, ask ‘Have you missed more than half of your doses this month?’ will help a patient disclose that they have only missed 30% of their doses. This identifies a problem that patients often have great difficulty disclosing to their doctors.
It can help build credibility to predict the future for patients. Tell them that they will test whether they really need their meds, and that they will not flare immediately, but that eventually they will flare. Be honest with them about the limitations of current medications, and that short-term non-adherence may not lead to rapid flares. You should then ask them to be honest with you, and tell you if and when they will change their medication dosing or adherence, and to discuss it with you so that you can monitor them more closely when they are off medications. There are important risks of serious flares, but these can be reduced if they are recognized and treated early. It is critical to ask patients to commit to notifying their physician as soon as they start to have symptoms of a flare if they are on a ‘drug holiday’. Only very reliable patients willing to have close monitoring for inflammation are appropriate for ‘drug holidays’. A realistic approach to maintenance in ulcerative colitis advocates for adherence, but recognizes that patients will be non-adherent, tries to maintain physician credibility, and negotiates for honesty, full disclosure, and immediate notification of the physician when symptoms occur while off medications.
Monitoring for Subclinical Inflammation
The third key to maintenance in ulcerative colitis is to carefully monitor patients for subclinical inflammation. This is in order to prevent future flares and reduce the risk of future colorectal cancer. The options for monitoring patients include: clinical symptoms, biomarkers, endoscopy, and imaging.
When monitoring clinical symptoms, patients are rarely forthright and concrete. Many patients tend to minimize symptoms, or state that they are doing ‘the same’, assuming that their doctor can assess the status of their colon from that statement. It is important to obtain a structured, detailed description of symptoms that is consistent at each visit, so that subtle changes in symptoms can be detected. The number of bowel movements (liquid or solid) in 24 h, the form of the bowel movements, the contents (including blood and mucus) and quantities of blood are all important. The effects on energy level, sleep, naps taken, and functioning at work and in social situations are important for assessing general health. Appetite, changes in weight and stamina are important for assessing chronic changes. Rectal function can be assessed with questions about urgency (in minutes), tenesmus, detection and control of rectal gas, and incontinence. Abdominal versus rectal pain, bloating, and discomfort, while non-specific, are important to patients and can suggest a change in disease activity. A structured instrument like the Simple Clinical Colitis Activity Index [12] can be self-administered by the patient and can start a detailed, concrete conversation with the physician about symptoms.
More objective measures are often used, and at times the complete blood count, mean corpuscular volume, albumin, C-reactive protein, and sedimentation rate can be valuable. However, these have relatively few prospective studies of their value for predicting future flares, and have poor correlation with endoscopic or histologic inflammation. Endoscopy and imaging can be helpful, but they are invasive and expensive, making them difficult to use to monitor patients frequently. Endoscopy is often reserved for patients not responding to therapy or before big decisions (change in drug class, colectomy) are made.
Recent data have provided a basis for using fecal biomarkers for monitoring subclinical inflammation. Fecal calprotectin and lactoferrin have a high correlation with endoscopic findings (0.7–0.8), and recent data have suggested that they have predictive value for future clinical flares in the short term. Gisbert showed that for patients with ulcerative colitis or Crohn's disease, the negative predictive value of a fecal calprotectin <167 was 100% at 12 weeks [13]. Being able to state that a patient will not flare in the next 12 weeks is a powerful prognostic ability that is new in ulcerative colitis, and may affect how we discuss maintenance and adherence with patients. This potentially could allow monitoring of inflammation regularly, like glucose levels and the hemoglobin A1c in diabetes mellitus. It could help identify patients who can safely take 12-week drug holidays, or even lead to intermittent, as-needed therapy directed by levels of pre-clinical inflammation. This approach would require prospective validation, and patients who are reliable and willing to handle stool. Monitoring of subclinical inflammation does provide a new opportunity to intervene before clinical symptoms occur. This strategy can be effective for reliable patients, given inexpensive, non-invasive tests with a durable negative predictive value for a future flare.
Optimizing Thiopurines
Given the limited second-line options for ulcerative colitis, it is important to maximize the value and durability of each option. Thiopurines require careful management because of their limited therapeutic index. A measurement of thiopurine methyltransferase (TPMT) phenotype is recommended for each patient prior to initiating therapy. This is helpful to identify both patients with low TPMT (<4), who may have significant bone marrow suppression in the first eight weeks of therapy, and those with high TPMT (>25), who are less likely to respond to thiopurine therapy.
Once the medication is started, optimizing the dose can be done in two ways. Many practitioners have tried to use metabolite levels of 6-TGN and 6-MMP to guide dosing, but have found them disappointing. This is illustrated by Osterman et al.'s meta-analysis, which found that metabolites have a sensitivity of 62% and a specificity of 72% for clinical response [14]. More traditional approaches use patterns in the complete blood count and chemistry panel. Our group has found that these patterns can be identified with machine learning, and that this approach has an accuracy of 86% for clinical response, compared to 59% accuracy of metabolites in the same patients [15]. This approach is being prospectively tested as a way to guide dosing, and could be available as an internet-based calculator in the future.
For patients with high TPMT levels, adjunctive allopurinol has been used to shift the metabolism of thiopurines to produce more 6-TGN. When combined with significant dose reduction of thiopurines (i.e. 50 mg azathioprine or 25 mg 6-MP daily) this can produce substantial clinical improvement [16,17], but may lead to increased risk of infection [18]. Our group recently reported a series of infections, including two cases of shingles, and cases of viral meningitis, Epstein Barr virus, and pneumocystis pneumonia in 27 subjects with IBD who had used a combination of thiopurines and allopurinol. A lowered absolute lymphocyte count (median 200 per mm3) was associated with infectious outcomes.
In order to optimize thiopurines in ulcerative colitis, it is important to: know your patient's TPMT enzyme level and adjust dosing accordingly, optimize dosing using patterns in standard laboratory tests, and consider very limited use of allopurinol in only carefully selected cases. We are still learning how to use allopurinol with thiopurines, and it may be best evaluated in clinical trials with very close monitoring.
Maintaining Biologic Efficacy
Since infliximab is currently the only approved biologic for ulcerative colitis, it is critical that patients on infliximab derive the maximum benefit for the longest duration possible. As a general rule in Crohn's disease, more than 60% of patients who start a biologic will have discontinued that medication within two years. Similar results are seen in ulcerative colitis. It is believed that most treatment failures are due to rapid drug clearance. This is accelerated with intermittent therapy, and patients must understand the dangers of canceling or delaying infusions. This process is illustrated in figure 3. Patients begin to have an immune reaction to the drug, start to clear the drug more rapidly after each infusion, develop low trough levels, then develop subclinical inflammation, and eventually develop clinical symptoms at the end of their infliximab interval.
Fig. 3.
Pathway to biologic failure.
The key question is whether we can identify these patients early, and intervene to produce better outcomes. There is a paucity of data in this area, but it may be reasonable to start with whether we can detect these patients before they develop clinical symptoms. Without a doubt, clinical symptoms in the week before infusion, or earlier blood and stool biomarkers can detect subclinical inflammation in ulcerative colitis and predict future flares, so that is a possible point of intervention. A dose increase, decreased interval, or addition of a thiopurine (if not already used) concomitantly with the infliximab could be helpful in avoiding or delaying a clinical flare. The minimum dose for preventing rapid infliximab clearance is unknown, but it may be less than the therapeutic dosing of 2.5 mg/kg.
We might be able to identify problems at an earlier step in the pathway, by monitoring trough levels, and acting on these. A 3T (Treat To Trough) strategy, in which dosing would be adjusted to achieve a target trough level, before clinical symptoms occur, needs prospective testing, but has promise. These approaches could help maintain a therapeutic level of biologic in more patients, and preserve the durability of response.
A more speculative approach to this problem earlier in this pathway would be to use biologic mechanisms to induce tolerance to the biologic, and pre-empt the rapid clearance process. This might be done with daily nasal sprays of low doses of biologic combined with a cytokine that would induce a tolerogenic response (i.e. interleukin 10 or transforming growth factor β).
Conclusions and Future Directions
The five keys to maintenance in ulcerative colitis are to use effective therapies, to achieve or negotiate adherence with each patient, to monitor closely for subclinical inflammation, to optimize thiopurines, and to maximize the durability of biologics. In the future, we may have better prognostic tools to predict flares, and may be able to plan immunosuppressant drug holidays with our patients. These could provide opportunities for optimal vaccination, and for immune surveillance of dysplasia before resumption of immune suppression before clinical flares occur. New strategies, which may include closer monitoring of preclinical inflammation and trough levels, are needed to sustain the durability of biologic efficacy. Above all, we need to share the data we have with our patients, and explain the limitations of our current therapies, so that patients do not have over-inflated expectations, and so that they understand why further clinical trials are needed.
Disclosure Statement
P.D.R. Higgins receives research grants from the NIH, Genentech, Centocor, Abbott, UCB, and Shire. He serves on the speakers’ bureau of Abbott and Warner Chilcott.
References
- 1.Sutherland L, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2003;3 doi: 10.1002/14651858.CD000543. CD000543. [DOI] [PubMed] [Google Scholar]
- 2.Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47–53. doi: 10.1136/gut.2005.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mate-Jimenez J, Hermida C, Cantero-Perona J, Moreno-Otero R. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227–1233. doi: 10.1097/00042737-200012110-00010. [DOI] [PubMed] [Google Scholar]
- 4.Hawthorne AB, Logan RF, Hawkey CJ, Foster PN, Axon AT, Swarbrick ET, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20–22. doi: 10.1136/bmj.305.6844.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 6.Muir AJ, Edwards LJ, Sanders LL, Bollinger RR, Koruda MJ, Bachwich DR, et al. A prospective evaluation of health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol. 2001;96:1480–1485. doi: 10.1111/j.1572-0241.2001.03801.x. [DOI] [PubMed] [Google Scholar]
- 7.Cerveny P, Bortlik M, Kubena A, Vlcek J, Lakatos PL, Lukas M. Nonadherence in inflammatory bowel disease: results of factor analysis. Inflamm Bowel Dis. 2007;13:1244–1249. doi: 10.1002/ibd.20189. [DOI] [PubMed] [Google Scholar]
- 8.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114:39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 9.Kane SV. Strategies to improve adherence and outcomes in patients with ulcerative colitis. Drugs. 2008;68:2601–2609. doi: 10.2165/0003495-200868180-00006. [DOI] [PubMed] [Google Scholar]
- 10.Chernew ME, Shah MR, Wegh A, Rosenberg SN, Juster IA, Rosen AB, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff. 2008;27:103–112. doi: 10.1377/hlthaff.27.1.103. [DOI] [PubMed] [Google Scholar]
- 11.Treton X, Bouhnik Y, Mary JY, Colombel JF, Duclos B, Soule JC, et al. Azathioprine withdrawal in patients with Crohn's disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. 2009;7:80–85. doi: 10.1016/j.cgh.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gisbert JP, Bermejo F, Perez-Calle JL, Taxonera C, Vera I, McNicholl AG, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–1198. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 14.Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047–1053. doi: 10.1053/j.gastro.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 15.Waljee AK, Joyce JC, Wang S, Saxena A, Hart M, Zhu J, et al. Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol. 2010;8:143–150. doi: 10.1016/j.cgh.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Sparrow MP, Hande SA, Friedman S, Cao D, Hanauer SB. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol. 2007;5:209–214. doi: 10.1016/j.cgh.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, et al. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005;22:441–446. doi: 10.1111/j.1365-2036.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 18.Govani SM, Higgins PDR: Combination of thiopurines and allopurinol: adverse events and clinical benefit in IBD. J Crohns Colitis DOI: 10.1016/j.crohns.2010.02.009. [DOI] [PMC free article] [PubMed]