Abstract
Hypertension is associated with mild decrements in cognition. Additionally, regional cerebral blood flow responses during memory processing are blunted in parietal and thalamic areas among untreated hypertensive adults, who compared to normotensives, manifest greater correlation in blood flow response across task-related brain regions. Here, we test whether pharmacologic treatment of hypertension normalizes regional cerebral blood flow responses and does so differentially according to drug class. Treatment with lisinopril, an angiotensin converting enzyme blocker, known to enhance vasodilative responsivity, was compared to treatment with atenolol, a beta blocker. Untreated hypertensive volunteers, n=28, were randomized and treated for one year. Whole brain and regional cerebral flow response to memory processing and acutely administered acetazolamide, a vasodilator, was assessed pre- and post-treatment. Peripheral brachial artery dilation during reactive hyperemia was also measured. Quantitative blood flow measures showed no difference in the magnitude of regional cerebral blood flow responses pre-and post-treatment to either memory tasks or acetazolamide injection. Brachial artery flow-mediated dilation increased with treatment. No differences between medications were observed. In brain regions active in memory processing, however, regional cerebral blood flow responses were more highly correlated after treatment. Specificity of cerebral blood flow to different regions appears to decline with treatment of hypertension. This greater correlation among active brain regions, which is present as well in untreated hypertensive relative to normotensive volunteers, may represent compensation in the face of less region-specific responsivity in individuals with hypertension.
Keywords: Hypertension, Cerebral Blood Flow, Magnetic Resonance Imaging, Positron Emission Tomography, Beta-blocker, Angiotensin Converting Enzyme Inhibitor
Mild-to-moderate hypertension is associated with minor deficits in cognition1–4. Brain structure or function might account for these deficits5–8. Cognitive processing elicits a regional redistribution of blood flow providing metabolic support to active neural areas9. Interference with this redistribution, blunting of regional blood flow, or structural loss due to poor perfusion might underlie the deficits of hypertensive individuals. Limited existing evidence supports all these possibilities5–8, 10. We demonstrated that, compared to normotensive individuals, those with hypertension have damped regional cerebral blood flow (rCBF) responses in parietal and thalamic areas (regions of interest, ROI) during working memory tasks and greater correlation among rCBF responses across task-related regions7. Both observations might be due to a cerebrovascular adaptation to hypertension—most particularly the remodeling of walls of small arteries, that is, thickening of the medial layer with or without change in lumen diameter7.
Anti-hypertensive medications have established influences on peripheral vascular function and may have similar effects on cerebrovascular function. Through its endocrine and paracrine actions, angiotensin promotes vascular remodeling and hypertrophy and also attenuates cerebral vasodilatory responses11. Medications reducing angiotension, e.g., angiotensin-converting enzyme inhibitors (ACE-I’s), generally reverse the peripheral vascular remodeling that occurs with hypertension, particularly when compared to beta-blockers12–15. Not all human studies, however, show reversal of remodeling with ACE I’s 14–16 and less is known about the impact of antihypertensive medications on cerebral circulation17–19.
In the current investigation, we tested the hypothesis that treatment of hypertension with an ACE-I, lisinopril, as compared to a beta-blocker, atenolol, alters remodeling and vasodilative capability and normalizes rCBF responses to working memory. Neither medication penetrates the blood brain barrier, thus vascular effects are tested rather than any direct neural influence20. Treatment effects on cerebral and peripheral vasodilatory reserve were assessed by acute administration of acetazolamide during quantitative cerebral blood flow measurement and with brachial artery flow-related dilation, respectively21,22.
METHODS
Participants
A community sample was recruited from the greater Pittsburgh area via radio, television, newspaper, and health fairs. Participants were between 35 and 65 years of age, and required to have an average diastolic (5th phase) blood pressure (BP) of 90–109 mm Hg, systolic BP of 140–179 mm Hg, or both. The restriction to middle-aged, mild to moderately hypertensive individuals was dictated by risk of our procedures and the likelihood that the reversibility of vascular remodeling might be higher in a relatively young, less hypertensive sample. BP was assessed after at least 5 minutes rest using the ausculatory technique with a mercury manometer. The average of the last 2 of 3 readings done on two occasions defined BP. Participants had either no prior pharmacologic treatment for hypertension or no more than 6 months of BP medication within the past 5 years with no BP medication taken in 6 months preceding enrollment. All participants provided informed consent and the study was approved as ethical by the Institutional Review Board of the University of Pittsburgh.
Physician-administered medical history and standard blood chemistry established medical eligibility. Participants were excluded for current use of cardiovascular or psychotropic medications, or contraindication for use of an ACE -I or beta blocker, neurological disorders, coronary heart disease (myocardial infarction or revascularization) or reported stroke, angina pectoris (determined by Rose questionnaire), a history of insulin-dependent diabetes, chronic renal insufficiency (serum creatinine > 1.8 mg/dl), heavy alcohol consumption (24 or more standard drinks per week), consistent use of illegal drugs, inability to abstain from coffee, nicotine, or alcohol for a minimum of 4 hours prior to testing, or absence of literacy in the English language (determined by self-report and ability to read consent documents). Serum thyroid stimulating hormone level was assessed in any participant reporting a history of thyroid abnormality. Those with known allergy to sulfa-containing antibiotics were included but did not undergo acetazolamide testing. These procedures excluded those with secondary hypertension due to renal failure, alcohol abuse, BP raising drugs, and untreated thyroid disorders.
Design
Figure 1 illustrates the research protocol, participant flow, and participant withdrawal. Initial BP assessment, medical screening, self-report questionnaires, and detailed consent followed phone screening. The next session included a second BP assessment and a neuropsychological examination. Separate sessions followed for brachial artery ultrasound, MRI, and PET examinations. All examinations were repeated after 1 year except for the screening and self-report instruments. This report focuses on 28 participants with complete quantitative rCBF measures. Participants were similar to non-completing individuals in age, education, and personality factors; they differed in that continuing participants were significantly (Chi-square p<.05) more likely to be male, Caucasian, and married.
Figure 1.
Study Design and Participant flow. Initial consent to participate was obtained from 81 volunteers. During screening and initial testing attrition occurred due to lack of interest (n=14), medical exclusion (n=18) or claustrophobia in MRI (n=4). After randomization to a drug treatment, one participant from each drug group failed to meet treatment expectations within the 1-year treatment and was withdrawn from the study. Due largely to participant refusal, arterial catheterization was not performed to obtain quantitative blood flow on 15 of the completing participants. Among those with complete blood flow data, 6 did not receive acetazolamide due either to sulfide sensitivity or inability to complete the PET session, e.g., due to back pain.
The University of Pittsburgh Institutional Review Board approved all procedures as consistent with ethical principles.
Medication Procedures
Patients were treated for 1 year within a randomized, double-blind design employing either lisinopril (from 10 mg up to 40 mg daily) or atenolol (from 25 mg up 100 mg daily). Medications were contained in unembossed capsules to ensure blinding. During a 6-week titration phase, drug dosage was gradually increased by 10 mg for lisinopril and 25 mg for atenolol. Participants were seen every 2-weeks for dosage adjustment and determination of BP, compliance, and side effects. Upward titration stopped if a participant’s BP had fallen to 135/85 mm Hg or less, or if resting pulse fell below 50 beats per minute. If a participant’s BP remained greater than 140/90 mm Hg on the full dose treatment, 12.5 mg hydrochlorothiazide was added (4 in lisinopril group and 7 in beta-blocker group). Participants were withdrawn from the study if BP averaged > 160/95 on two consecutive visits during the maintenance phase (trimonthly visits, Week 6 through the final visit at Week 52). During visits, physical symptoms and quality of life were assessed by administering the Bulpitt Hypertension Questionnaire 23 and compliance was estimated based upon returned pill counts.
Ultrasound Measurement of Endothelial Function
The influence of treatment on capability for dilation of peripheral vessels was assessed with flow-mediated dilation of the brachial artery following standard methods24,25. Brachial artery diameter was measured before and after reactive hyperemia induced by brachial occlusion for 4 minutes using a cuff inflated to 50 mm Hg above systolic BP. Brachial artery flow-related dilation assesses endothelium dependent dilation related to the nitric oxide synthase, and is impaired by hypertension25,26.
Structural MRI Measures
All subjects underwent a structural MRI using a GE Signa 1.5 Tesla scanner (Milwaukee, WI) to provide a structural image for mapping PET results and for assessment of white matter hyperintensities. T2, fast fluid-attenuated inversion recovery, and spoiled gradient-recalled images were obtained (please see details http://hyper.ahajournals.org), Severity of white matter hyper-intensities was graded using a 10-point scale by two trained, experienced raters using standards developed for the Cardiovascular Health Study27. Raters showed 90% agreement and consensus ratings were analyzed. Participants with significant lacunar or other infarcts (n=2) were excluded from participation. These were identified from the MRI films by a board certified neuroradiologist. Significant infarcts were those judged to potentially influence functional images and/or influence cognitive processing.
Functional PET Measures
Nine PET scans were performed to test rCBF responses during information processing (working memory tasks) and response to acetazolamide. (please see details http://hyper.ahajournals.org). Each of the memory tasks used the same display and two button responses. The Control Memory task required the subject to respond with the thumb to any letter appearing on the left of the screen and with the index finger for a letter appearing on the right of the screen. The One-back Memory task required the subject to respond with the thumb if the spatial position of the letter currently appearing matched the spatial position which appeared immediately before it; otherwise press with the index finger. The Two-back Memory task required the subject to respond with the thumb if the spatial position of the letter currently appearing matched the spatial position which appeared two times back. Rest and a Checkerboard/Response task (annular reversing checkerboard flickering at 8Hz with right finger tapping) provided comparison conditions. Each task lasted 5 minutes and started 2 minutes prior to tracer injection. An inter-task interval of approximately 5 minutes provided a break for the subject and permitted time for the preparation and delivery of the tracer. Checkerboard and rest tasks were presented only once and randomized to be either the initial or final task of the cognitive battery, i.e., scans 1 and 8. Each of the other tasks was presented twice with the order randomized, i.e., scans 2 through 7. The cognitive battery was followed by the intravenous injection of a 13 mg/kg dose of acetazolamide, and the post-absorption scan followed after 20 minutes, i.e., scan 928 Acetazolamide induces an extracellular acidosis and a resultant large, if not maximal, cerebral vasodilatory response acting through cyclooxygenase; the blood flow response to acetazolamide is impaired by hypertension29–31`
Quantitative CBF
The [15O]water data were analyzed using a one-tissue compartment model32,33. Model parameters corresponded to clearance of water from blood to brain (K1; mL/min/mL), brain-to-blood transfer (k2; min−1), and an arterial input function timing delay (Δt). The three parameters were simultaneously determined using iterative least-squares curve fitting on a regional basis. K1 is a clearance parameter that is directly proportional to flow and K1 × 100 was used as a blood flow measure, mL/min/100mL
ROIs were defined based on areas of differential participant/control activation from our initial study7. The ROIs were regionally defined on the SPGR MRI for each participant and manually drawn using a version of the Imagetool software (CTI PET Systems, Knoxville, TN). ROI placement for dorso and ventrolateral prefrontal, parietal, amygdala/hippocampus, thalamic, cingulate, insula, and inferior frontal areas are shown in Figure S1 at http://hyper.ahajournals.org.
Analysis
We tested the hypothesis that rCBF responses during memory processing would be enhanced by antihypertensive treatment with a repeated measures analysis of variance employing the general linear model program within Statistica (StatSoft, Inc., Tulsa, OK). Pre- and post-treatment was a within-subjects factor and medication group was a between-subjects factor. Covariates were employed to control for factors possibly influencing treatment response; due to the small sample size, age and gender were the only covariates routinely included in all analyses. Higher order interactions with gender were not interpreted due to the small number of women per cell. Other possible confounders were added singly to check on their influence. The hypothesis of a greater impact on rCBF responses following treatment with lisinopril was tested with the pre-post treatment by medication group interaction. We tested the hypothesis that correlations between activated brain areas would change with treatment by first determining the correlations of rCBF responses to working memory within prefrontal, posterior parietal, and amygdala/ hippocampal ROI’s before and after treatment. We then tested the difference between correlations using the Fisher z approach as implemented in Statistica. Two-tailed tests were uniformly used.
RESULTS
Participant Characteristics
The participants as classified by their assignment to medication were comparable, showing no statistically significant differences although numerical differences were present for gender, race, education and smoking history (Table 1). Heart rate, BP, and vascular distensibility for the two groups were comparable pre-treatment (Table 2). F-values from the analysis of variance are presented for baseline differences between groups, pre-post, and pre-post by medication group factors. All variables changed with treatment, but only heart rate changed differentially as a function of treatment group. Atenolol lowered heart rate more than lisinopril.
Table 1.
Demographic characteristics of completing participants.
| Medication Group: | Lisinopril (ACE-Inhibitor) n=20 | Atenolol (Beta-blocker) n=23 |
|---|---|---|
| Age | 53.9 (5.6) | 51.3 (7.4) |
| Gender (% male) | 70 | 83 |
| Race (% Caucasian) | 80 | 96 |
| Body Mass Index (kg/m2) | 30.5 (4.9) | 29.5 (4.7) |
| Education (%>high school) | 80 | 96 |
| Married (%) | 55 | 57 |
| Total Cholesterol (mg/dl) | 203 (34) | 214 (31) |
| Glucose (mg/dl) | 98 (20) | 96 (30) |
| Drink alcohol more than 1 or 2 times per year (% of group) | 55 | 48 |
| Average Number of Cigarettes/day | 2 (5) | 1(4) |
| Sulcus size rating | 3.0 (.8) | 2.8 (.9) |
| Ventricle size rating | 2.2 (.6) | 2.2 (1.1) |
| White Matter rating | 1.3 (.8) | 1.2 (.6) |
| Medication Compliance (%) | 97 (8) | 98 (2) |
Arithmetic mean and (standard deviation). Groups compared by independent t-tests or chi-square, no statistically significant differences were observed between medication groups.
Table 2.
Initial and treated mean values (standard error) of BPs, heart rate, and peripheral vascular (Brachial artery) dilation and analysis of variance results (see text for description of analyses, age and gender were used as covariates in these analyses).
| Measure | Lisinopril Group (n=12) |
Atenolol Group (n=16) |
Statistical Pre- Treatment: Group |
Results Pre to Post Treatment |
Group by Pre-Post Treatment |
||
|---|---|---|---|---|---|---|---|
| Pre- Treatment |
Post- Treatment |
Pre- Treatment |
Post- Treatment |
F-value p: | F-value p: |
F-value p: | |
| SBP (mmHg) | 148. 7(3.2) | 127.0 (3.6) | 147.7(2.7) | 124.0 (3.1) | .1 ns | 72.2 <.001 | .3 ns |
| DBP (mmHg) | 93.3(2.6) | 79.3(2.7) | 98.3 (2.3) | 80.9 (2.4) | 1.2 ns | 78.5 <.001 | .8 ns |
| Heart Rate (bpm) | 71.4 (2.8) | 66.8 (2.7) | 76.0 (2.5) | 62.0(2.4) | 1.9 ns | 27.6 <.001 | 6.01 <.01 |
| Percent brachial artery dilation | 4.4 (1.0) | 7.1 (1.0) | 4.5 (.8) | 7.0 (.8) | .1 ns | 7.6 <.01* | .0 ns |
F (1,20)=7.4, p=.01 for percent vascular dilation uncorrected for initial baseline.
CBF and rCBF
Treatment of hypertension with either drug maintained pre-treatment values for estimated CBF, rCBF, and changes in these measures elicited by the working memory task (Table 3). In contrast to our hypothesis, the working memory tasks did not elicit a greater rCBF response following 1 year of antihypertensive treatment. The results tabled are for the posterior parietal ROI; in our prior work this area was shown to have decreased rCBF response in hypertensives relative to normotensives. This area is representative of all the ROI results, including overall CBF. The posterior parietal results show an effect of increasing memory load (F(2,46)=18.1, p<.001), but no treatment effect (F(1,23)=1.1, ns) or treatment by medication group interaction (F(1,23)= 0.0, ns). Cerebral dilation in response to acetazolamide was unaffected by treatment (F(1,11) = 0.0, ns) and did not show a treatment by medication interaction (F(1,11)= .7, ns). Essentially the same results were obtained if patients receiving supplementary hydrochlorothiazide were excluding from the analyses. Similarly, the addition of covariates individually failed to alter the statistical significance of any of the terms in the analysis and also failed to show significant covariate effects. Those tested were: white matter load, smoking, alcohol use, education level, body mass index, estimated intelligence, depression, and systolic blood pressure response to treatment.
Table 3.
rCBF estimates (mL/min/100mL) within the posterior parietal ROI for the different task conditions pre and post drug treatment. Means and standard errors are given; confidence intervals for 95% of the possible means may be approximated assuming normality by multiplying the standard error by + and − 2. There are no statistical differences between medication groups or pre-post treatment, see text.
| Medication: | Lisinopril Group (n=12) |
Atenolol Group (n=16) |
||
|---|---|---|---|---|
| Condition | Pre-Treatment | Post- Treatment |
Pre-Treatment | Post- Treatment |
| Rest | 48.0 (2.7) | 47.2 (2.6) | 44.2 (1.8) | 39.9 (1.7) |
| Checkerboard | 49.0 (4.0) | 51.1 (3.7) | 42.6 (2.6) | 42.0 (2.4) |
| Control Memory | 44.2 (2.6) | 44.5 (2.3) | 43.0 (2.4) | 41.5 (2.1) |
| 1-Back | 46.7 (3.0) | 44.4 (2.7) | 44.4 (2.7) | 42.5 (1.9) |
| 2-Back | 49.2 (3.3) | 46.4 (2.9) | 46.4 (2.9) | 43.8 (1.8) |
| Acetazolamide* | 57.5 (4.2) | 53.4 (4.2) | 59.6 (3.2) | 59.6 (3.0) |
Note.
n for acetazolamide data is to 9 for lisinopril group and 13 for atenolol group.
Peripheral Vasodilative Effects
An analysis of maximal brachial artery dilation after occlusion, covarying initial artery diameter, indicated an increase in dilation following treatment for both medications (See Table 2)34. No difference between medications was observed. The increase in percent dilation following 1 year of treatment was inversely correlated with change with treatment in the rCBF response during working memory in all of the ROI’s (r’s −.50 to −.68, p’s =or <.01). Changes with treatment in rCBF dilation response to acetazolamide were not correlated with change in brachial artery dilation.
Correlation of Regional Responses
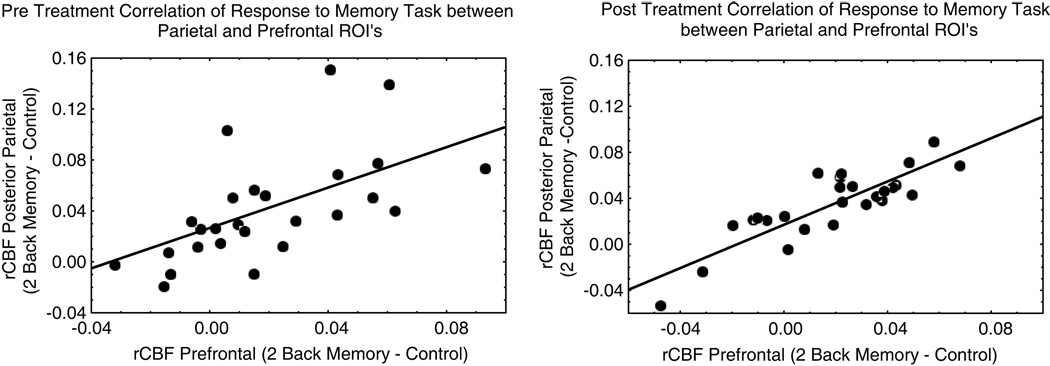
Finally, we examined correlations between rCBF responses in regions known to be involved in working memory processing. The magnitude of the correlations increased significantly following one year of antihypertensive treatment (Table 4, Figure 2). Correlations did not differ between medication groups.
Table 4.
Correlation of rCBF responses to 2-back working memory task among pre-frontal, parietal, and amygdala/hippocampal ROI’s.
| Correlation | Pre-Treatment N=28 |
Post-Treatment N=28 |
Significance Of Difference |
|---|---|---|---|
| Pre-Frontal to Parietal | .62* | .94* | P=.006 |
| Pre-Frontal to Amygdala/Hippocampus | .58* | .84* | P=.05 |
| Parietal to Amygdala/Hippocampus | .42* | .80* | P=.03 |
p< or =.01. Pre-treatment correlations show a similar pattern when participants with PET data only for the pre-treatment are added to the calculation (to yield n=43) and the significance of the pre-frontal to parietal correlation difference remains (p=.002). Removal of one participant with extreme change scores reduces the amygdala/hippocampus differences to a trend but the difference between prefrontal/parietal correlations pre and post remains significant (p=.04).
Figure 2.
Scatter diagrams of the relationship between dorsolateral prefrontal and posterior parietal areas before and after treatment. Data from one extreme participant is omitted, pre treatment correlation =.61; post treatment correlation =.86.
DISCUSSION
This experiment had two important outcomes. The first outcome was the absence of any difference in cerebrovascular treatment effects between ACE-I and beta-blocker medication. We assumed that ACE-I treatment would be superior to beta blocker treatment for reversing any remodeling of cerebral vessels, restoring cerebral vasodilative responsivity and enhancing greater specificity of response among brain areas during cognitive processing. Our results suggested that these assumptions were oversimplifications. The second outcome was an increase in correlation of rCBF responses between memory-related regions after successful blood pressure treatment. We had previously found that rCBF increases during memory in different activated brain regions were more highly correlated among hypertensive relative to normotensive individuals—standard antihypertensive treatment increased these correlations7.
The increase in correlation among brain areas after treatment indicates altered cerebrovascular support for memory processing, i.e., rCBF response during working memory in one region, e.g. prefrontal cortex, can be predicted more accurately from rCBF response in another region, e.g. posterior parietal cortex, after relative to before treatment. Posterior parietal, dorsolateral prefrontal, and amygdala/hippocampal areas were tested because: a) these areas showed differences in correlation between hypertensive and normotensive groups in our initial study7, b) rCBF in these areas changes during memory performance—increasing in dorsolateral and posterior parietal areas and decreasing in amygdala/hippocampus in our work 7 and that of others35,36, and c) posterior parietal and amygdala/hippocampus rCBF change are related to memory performance35,36. Different cognitive tasks typically elicit rCBF responses that redistribute existing CBF to brain regions whose activation is associated with successful performance of the particular task9. We previously interpreted increased concomitance of rCBF response across regions among hypertensive relative to normotensive individuals as a compensation for less robust rCBF response among hypertensive individuals in their thalamic and posterior parietal regions7. Treatment appeared to facilitate or, at least, not alter such compensation because correlations of rCBF responses across brain regions increased with treatment. Note that our findings are for responsivity of rCBF to cognitive load. Indeed, regional correlations of glucose metabolism at rest between brain regions have been shown to be lower in long-term treated, older hypertensive relative to normotensive participants37.
Observations supporting a compensation interpretation are that: a) concurrent activation of disparate, but task-relevant brain regions characterizes compensation38,39, b) compensatory changes within hippocampal circuits are documented in the animal literature40, and c) enhanced post-treatment correlations were not maladaptive in that cognitive performance was maintained or increased across our treatment period (data not shown). Our measures lack the resolution, however, to specifically relate our results to those in the animal literature (in which compensation can more readily be demonstrated). Longitudinal research with larger samples will be required to assess whether the observed changes are compensatory or, perhaps, even deleterious for some functions.
Our interpretation must be tentative absent further knowledge of the mechanism inducing increased correlation among regions after treatment. Our results show that quantitative, global CBF was maintained despite a significant fall in BP. Presumably, this reflects cerebral autoregulation with flow held constant via a decrease in vascular resistance41. This possibility is consistent with prior observations, e.g., Lipsitz et al. 42, of ACE-I treatment heightening both CBF velocity and carotid artery distensibility. The increased correlation between the rCBF responses in activated regions with treatment may be a consequence of dilated resistance vessels that are less responsive to variations in metabolic debt in the different regions active in a task43. This interpretation seems reasonable as our measures show an absence of vasodilative responsivity of cerebral vessels, heightened brachial artery dilative responsivity post-treatment and an inverse correlation between these measures. However, clearly different dilative mechanisms and vessel types are probed by brachial artery flow-mediated dilation and dilation induced by cognitive performance and acetazolamide22,26,29,31. Thus, our interpretation is limited by differences between vasodilative mechanism in peripheral and central circulations, differences in sensitivities of these mechanisms within small and large vessels, and the variety of vasodilative mechanisms in general34,44–47.
This complexity of vasodilative mechanisms is highlighted by our incorrect initial assumption of a greater cerebrovascular efficacy of ACE-I’s relative to beta-blockers. ACE-I’s are known to successfully reverse hypertensive vascular hypertrophy and remodeling48. An increase in vasodilative capacity of the brain after ACE-I treatment, however, has been an equivocal finding 49,50. Our results showed antihypertensive treatment increased vasodilative response in brachial artery, but not in cerebrovascular vessels. The increase in endothelium-dependent vasodilation in the peripheral circulation after successful blood pressure lowering, irrespective of type of medication, has been reported although typically ACE-I are superior to other medications17,51,52. The failure to show a parallel influence in the cerebral circulation is consistent with observations that a) acetazolamide acts through cyclooxgenase rather than the nitric oxide synthase pathway induced by flow-induced dilation, and b) dilation via this pathway has been reported to be unaltered in hypertension and uninfluenced by its treatment in animal models45,47. Assessment of vasodilative changes with quantitative blood flow measures is a strength of our study, but we did not assess velocity measures, other measures of flow or blood volume5,42. Our understanding of the cerebrovascular response to hypertension treatment requires greater attention to vessel characteristics, vasodilative mechanisms as well as converging measurement approaches that were not feasible in the current investigation. Finally, the role of eutrophic versus hypertrophic remodeling and the importance of reversing this in the cerebral circulation also requires further consideration, particularly, given suggestions that vasodilative capacity and characteristics of remodeling are unrelated47.
Our study has significant limitations. Sample size was modest, reflecting the invasiveness of our measures and difficulty recruiting untreated hypertensive participants. Statistical power was sufficient only to detect relatively large, clinically significant effects. Furthermore, women withdrew from testing more than men. This threatens generalization from our study. We only studied 2 medications; others might act differently. In particular, an angiotensin II receptor blocker might be more effective than the current ACE-I in altering cerebral function14. Severe cerebrovascular disease appears to alter the coupling between blood flow and neural activation43. If this coupling is also disrupted at moderate levels of hypertension, our interpretation of rCBF as an indicant of neural activation is threatened53. Peripheral BP response to our memory tasks might directly alter the rCBF signal, but this appears unlikely given the current, modest magnitude of the peripheral BP response54,55. Finally, the absence of an untreated or placebo control group means that we cannot directly assess the possibilities of influence of our results due to time, a continuing hypertensive disease process, or practice effects.
Perspective
Loss of specificity of rCBF with hypertension that is further magnified by treatment may have implications for vulnerability to stroke and cognitive decline. Individuals with low rCBF to one area have a greater tendency to show low rCBF to all active processing areas after treatment. Future work will be required to test whether this creates a vulnerability to future vascular and neuropsychiatric disease or is an appropriate compensatory adjustment to the minimal loss of cognitive function associated with hypertension.
Supplementary Material
Acknowledgements
We thank Dr. Christopher Ryan and Ms. Michelle Geckle for their assistance with the neuropsychological testing and Michael Eddy and Carl Becker for their technical assistance.
Source of Funding. The National Heart, Lung, and Blood Institutes grant 057529 supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosure. None.
References
- 1.Elias MF, Sullivan LM, Elias PK, D'Agostino RB, Sr, Wolf PA, Seshadri S, Au R, Benjamin EJ, Vasan RS. Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham offspring. Hypertension. 2007;49:439–445. doi: 10.1161/01.HYP.0000256361.68158.24. [DOI] [PubMed] [Google Scholar]
- 2.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003;5:255–261. doi: 10.1007/s11906-003-0029-6. [DOI] [PubMed] [Google Scholar]
- 3.Muldoon MF, Waldstein SR, Jennings JR. Neuropsychological consequences of antihypertensive medication use. Exp Aging Res. 1995;21:353–368. doi: 10.1080/03610739508253990. [DOI] [PubMed] [Google Scholar]
- 4.Waldstein SR, Manuck SB, Ryan CM, Muldoon MF. Neuropsychological correlates of hypertension: review and methodologic considerations. Psychol Bull. 1991;110:451–468. doi: 10.1037/0033-2909.110.3.451. [DOI] [PubMed] [Google Scholar]
- 5.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 6.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- 8.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 9.Raichle ME. Functional brain imaging. In: Edvinsson L, Krause DN, editors. Cerebral Blood Flow and Metabolism. 2nd ed. Philadelphia: Lipincott Williams & Wilkins; 2002. pp. 413–419. [Google Scholar]
- 10.Raz N. The Aging Brain Observed in Vivo:Differential Changes and their Modifiers. In: Cabez R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. Oxford, UK: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- 11.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 12.Barenbrock M, Spieker C, Hoeks AP, Zidek W, Rahn KH. Effect of lisinopril and metoprolol on arterial distensibility. Hypertension. 1994;23:I161–I163. doi: 10.1161/01.hyp.23.1_suppl.i161. [DOI] [PubMed] [Google Scholar]
- 13.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 14.Dupuis F, Atkinson J, Liminana P, Chillon J-M. Comparative effects of the angiotensin II receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive rats. J Hypertens. 2005;23:1061–1066. doi: 10.1097/01.hjh.0000166848.95592.a5. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand ME. Provision of cardiovascular protection by ACE inhibitors: a review of recent trials. Curr Med Res Opin. 2004;20:1559–1569. doi: 10.1185/030079904X4185. [DOI] [PubMed] [Google Scholar]
- 16.Erzen B, Gradisek P, Poredos P, Sabovic M. Treatment of essential arterial hypertension with enalapril does not result in normalization of endothelial dysfunction of the conduit arteries. Angiology. 2006;57:187–192. doi: 10.1177/000331970605700208. [DOI] [PubMed] [Google Scholar]
- 17.Fu C-H, Yang CCH, Kuo TBJ. Effects of different classes of antihypertensive drugs on cerebral hemodynamics in elderly hypertensive patients. Am J Hypertens. 2005;18:1621–1625. doi: 10.1016/j.amjhyper.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arterioles in rats. Hypertension. 1999;33:856–861. doi: 10.1161/01.hyp.33.3.856. [DOI] [PubMed] [Google Scholar]
- 19.Heinke W, Zysset S, Hund-Georgiadis M, Olthoff D, von Cramon DY. The effect of esmolol on cerebral blood flow, cerebral vasoreactivity, and cognitive performance: a functional magnetic resonance imaging study. Anesthesiology. 2005;102:41–50. doi: 10.1097/00000542-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Tan J, Wang JM, Leenen FHH. Inhibition of brain angiotensin-converting enzyme by peripheral administration of trandolapril versus lisinopril in Wistar rats. Am J Hypertens. 2005;18:158–164. doi: 10.1016/j.amjhyper.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Bruhn H, Kleinschmidt A, Boecker H, Merboldt KD, Hanicke W, Frahm J. The effect of acetazolamide on regional cerebral blood oxygenation at rest and under stimulation as assessed by MRI. J Cereb Blood Flow Metab. 1994;14:742–748. doi: 10.1038/jcbfm.1994.95. [DOI] [PubMed] [Google Scholar]
- 22.Bickler PE, Litt L, Banville DL, Severinghaus JW. Effects of acetazolamide on cerebral acid-base balance. J Appl Physiol. 1988;65:422–427. doi: 10.1152/jappl.1988.65.1.422. [DOI] [PubMed] [Google Scholar]
- 23.Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jenkins CD, Klerman GL, Williams GH. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986;314:1657–1664. doi: 10.1056/NEJM198606263142602. [DOI] [PubMed] [Google Scholar]
- 24.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. International Brachial Artery Reactivity Task F. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen KE, Celermajer DS, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, Deanfield JE. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995;74:247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 27.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 28.Boles Ponto LL, Schultz SK, Leonard Watkins G, Hichwa RD. Technical issues in the determination of cerebrovascular reserve in elderly subjects using 15O-water PET imaging. Neuroimage. 2004;21:201–210. doi: 10.1016/j.neuroimage.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 29.Molina C, Sabin JA, Montaner J, Rovira A, Abilleira S, Codina A. Impaired cerebrovascular reactivity as a risk marker for first-ever lacunar infarction: A case-control study. Stroke. 1999;30:2296–2301. doi: 10.1161/01.str.30.11.2296. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Paulson OB, Lassen NA. Effect of nitric oxide blockade by NG-nitro-L-arginine on cerebral blood flow response to changes in carbon dioxide tension. J Cereb Blood Flow Metab. 1992;12:947–953. doi: 10.1038/jcbfm.1992.131. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Paulson OB, Lassen NA. Indomethacin abolishes cerebral blood flow increase in response to acetazolamide-induced extracellular acidosis: a mechanism for its effect on hypercapnia? J Cereb Blood Flow Metab. 1993;13:724–727. doi: 10.1038/jcbfm.1993.92. [DOI] [PubMed] [Google Scholar]
- 32.Price JC, Drevets WC, Ruszkiewicz J, Greer PJ, Villemagne VL, Xu L, Mazumdar S, Cantwell MN, Mathis C. Sequential [15O]water PET studies in baboons: Pre- and post-Amphetamine. J Nucl Med. 2002;43:1090–1100. [PubMed] [Google Scholar]
- 33.Iida H, Higano S, Tomura N, Shishido F, Kanno I, Miura S, Murakami M, Takahashi K, Sasaki H, Uemura K. Evaluation of regional differences of tracer appearance time in cerebral tissues using [15O] water and dynamic positron emission tomography. J Cereb Blood Flow Metab. 1988;8:285–288. doi: 10.1038/jcbfm.1988.60. [DOI] [PubMed] [Google Scholar]
- 34.Buus NH, Jorgensen CG, Mulvany MJ, Sorensen KE. Large and small artery endothelial function in patients with essential hypertension--effect of ACE inhibition and beta-blockade. Blood Press. 2007;16:106–113. doi: 10.1080/08037050701343688. [DOI] [PubMed] [Google Scholar]
- 35.Jennings JR, van der Veen FM, Meltzer CC. Verbal and spatial working memory in older individuals: A positron emission tomography study. Brain Res. 2006;1092:177–189. doi: 10.1016/j.brainres.2006.03.077. [DOI] [PubMed] [Google Scholar]
- 36.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 37.Mentis MJ, Salerno J, Horwitz B, Grady C, Schapiro MB, Murphy DGM, Rapoport SI. Reduction of functional neural connectivity in long-term treated hypertension. Stroke. 1994;25:601–607. doi: 10.1161/01.str.25.3.601. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 39.Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 40.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu G-Q, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chillon JM, Baumbach GL. Autoregulation: Arterial and Intracranial Pressure. In: Edvinsson L, Krause DN, editors. Cerebral Blood Flow and Metabolism. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 395–412. [Google Scholar]
- 42.Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Kiely DK, Sorond F, Serrador J, Cheng DM, Babikian V, Cupples LA. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–221. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]
- 43.Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol. 2005;98:151–159. doi: 10.1152/japplphysiol.00471.2004. [DOI] [PubMed] [Google Scholar]
- 44.Moore MA, Schiffrin EL. Small artery remodeling in hypertension: can it be corrected? Am J Med Sci. 2001;322:7–11. doi: 10.1097/00000441-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Yang ST, Faraci FM, Heistad DD. Effects of cilazapril on cerebral vasodilatation in hypertensive rats. Hypertension. 1993;22:150–155. doi: 10.1161/01.hyp.22.2.150. [DOI] [PubMed] [Google Scholar]
- 46.Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arteriolar dilatation in hypertensive rats. Hypertension. 2001;37:1388–1393. doi: 10.1161/01.hyp.37.6.1388. [DOI] [PubMed] [Google Scholar]
- 47.Hajdu MA, Baumbach GL. Mechanics of large and small cerebral arteries in chronic hypertension. Am J Physiol. 1994;266:H1027–H1033. doi: 10.1152/ajpheart.1994.266.3.H1027. [DOI] [PubMed] [Google Scholar]
- 48.Iadecola C, Gorelick PB. Hypertension, angiotensin, and stroke: beyond blood pressure. Stroke. 2004;35:348–350. doi: 10.1161/01.STR.0000115162.16321.AA. [DOI] [PubMed] [Google Scholar]
- 49.Oku N, Kitagawa K, Imaizumi M, Takasawa M, Piao R, Kimura Y, Kajimoto K, Matsumoto M, Hori M, Hatazawa J. Hemodynamic influences of losartan on the brain in hypertensive patients. Hypertens Res. 2005;28:43–49. doi: 10.1291/hypres.28.43. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami N, Yamashita T, Nakano S, Ishihara H, Kitahara T, Nakashima K, Kashiwagi S, Ito H. Effect of angiotensin converting enzyme inhibitor on chronic ischemic patients. Acta Neurol Scand Suppl. 1996;166:93–95. doi: 10.1111/j.1600-0404.1996.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 51.von zur Muhlen B, Millgard J, Lind L. Divergent effects of different beta-blocking agents on endothelium-dependent vasodilatation in the human forearm. Blood Press. 2000;9:287–292. doi: 10.1080/080370500448687. [DOI] [PubMed] [Google Scholar]
- 52.von zur Muhlen B, Kahan T, Hagg A, Millgard J, Lind L. Treatment with irbesartan or atenolol improves endothelial function in essential hypertension. J Hypertens. 2001;19:1813–1818. doi: 10.1097/00004872-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 54.Liu H, Rainey C, Lauer KK, Piacentine L, Bloom A, Risinger R, Ward BD, Stein E, Li S-J. Peripheral blood pressure changes induced by dobutamine do not alter BOLD signals in the human brain. Neuroimage. 2006;30:745–752. doi: 10.1016/j.neuroimage.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 55.Wang R, Foniok T, Wamsteeker JI, Qiao M, Tomanek B, Vivanco RA, Tuor UI. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage. 2006;31:1–11. doi: 10.1016/j.neuroimage.2005.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.