Abstract
Chronic infection with cagA-positive Helicobacter pylori is the strongest risk factor for the development of gastric adenocarcinoma. The cagA gene product CagA is injected into gastric epithelial cells and disturbs cellular functions by physically interacting with and deregulating a variety of cellular signaling molecules. RUNX3 is a tumor suppressor in many tissues, and it is frequently inactivated in gastric cancer. In this study, we show that H. pylori infection inactivates the gastric tumor suppressor RUNX3 in a CagA-dependent manner. CagA directly associates with RUNX3 through a specific recognition of the PY motif of RUNX3 by a WW domain of CagA. Deletion of the WW domains of CagA or mutation of the PY motif in RUNX3 abolishes the ability of CagA to induce the ubiquitination and degradation of RUNX3, thereby extinguishing its ability to inhibit the transcriptional activation of RUNX3. Our studies identify RUNX3 as a novel cellular target of H. pylori CagA and also reveal a mechanism by which CagA functions as an oncoprotein by blocking the activity of gastric tumor suppressor RUNX3.
Keywords: CagA, degradation, H. pylori, RUNX3, ubiquitination
Introduction
Infection with Helicobacter pylori is the strongest risk factor for gastric carcinoma (Peek and Blaser, 2002; Hatakeyama, 2004; Polk and Peek, 2010). The H. pylori cag pathogenicity island is a strain-specific locus that encodes a type IV secretion system that injects the bacterial virulence factor CagA into host epithelial cells (Hatakeyama, 2004). Infection by cagA-positive H. pylori strains is associated with an increased risk for gastric cancer compared with infection by cagA-negative strains (Blaser et al., 1995; Parsonnet et al., 1997; Huang et al., 2003), implying an important role for CagA in H. pylori-associated gastric diseases.
Upon injection into epithelial cells, intracellular CagA targets host proteins which regulate various cellular responses, including actin–cytoskeletal rearrangements, cell scattering and inflammation, all of which are believed to be involved in H. pylori-mediated gastric carcinogenesis (Hatakeyama, 2004; Peek, 2005). For example, CagA associates with and activates the cytoplasmic protein tyrosine phosphatase SHP-2, resulting in cytoskeletal reorganization, cell elongation and scattering (Higashi et al., 2002a, b). CagA also interacts with E-cadherin and destabilizes E-cadherin/β-catenin complexes, leading to the activation of β-catenin, and induces intestinal transdifferentiation in gastric epithelial cells (Franco et al., 2005; Murata-Kamiya et al., 2007). Therefore, H. pylori CagA, through its association with various host proteins, is actively involved in H. pylori-mediated gastric carcinogenesis. Furthermore, transgenic expression of CagA in mice induces hyperplasia in the gastric mucosa and polyps in the glandular stomach, highlighting the oncogenic potential of CagA in gastric cancer (Ohnishi et al., 2008).
RUNX3 is a transcription factor that regulates lineage-specific gene expression in developmental processes and is involved in the formation of a variety of cancers (Ito, 2004). RUNX3 is expressed in glandular stomach epithelial cells, and loss of expression of RUNX3 is causally related to the genesis and progression of gastric cancer and correlates with differentiation, metastasis and poor prognosis of gastric cancer (Li et al., 2002; Wei et al., 2005; Sugiura et al., 2008; Hsu et al., 2009). RUNX3 is frequently inactivated in gastric cancers by hemizygous deletion, hypermethylation of its promoter or protein mislocalization. The inactivation of RUNX3 appears to occur both in the early stages and throughout the progression of gastric cancer (Li et al., 2002; Ito et al., 2005). RUNX3 elicits its tumor suppressor functions by controlling the expression of many genes involved in the growth, apoptosis and differentiation of gastric epithelial cells (Chi et al., 2005; Yamamura et al., 2006; Yano et al., 2006), as well as genes involved in angiogenesis and cell junction formation (Peng et al., 2006; Chang et al., 2009). Although emerging evidence suggests that RUNX3 is a tumor suppressor, the inactivation of which is involved in the initiation and progression of gastric cancer, the trigger for RUNX3 inactivation within gastric cells is largely unknown.
In this study, we demonstrate that the H. pylori virulence factor, CagA, specifically associates with RUNX3 and downregulates its expression in gastric epithelial cells. CagA targets RUNX3 for ubiquitination and proteasome-mediated degradation. The identification of RUNX3 as a novel cellular target of CagA will help in better understanding the role of CagA as an oncogene and RUNX3 as a tumor suppressor in gastric carcinogenesis.
Results and discussion
H. pylori infection downregulates the expression of RUNX3 in a CagA-dependent manner
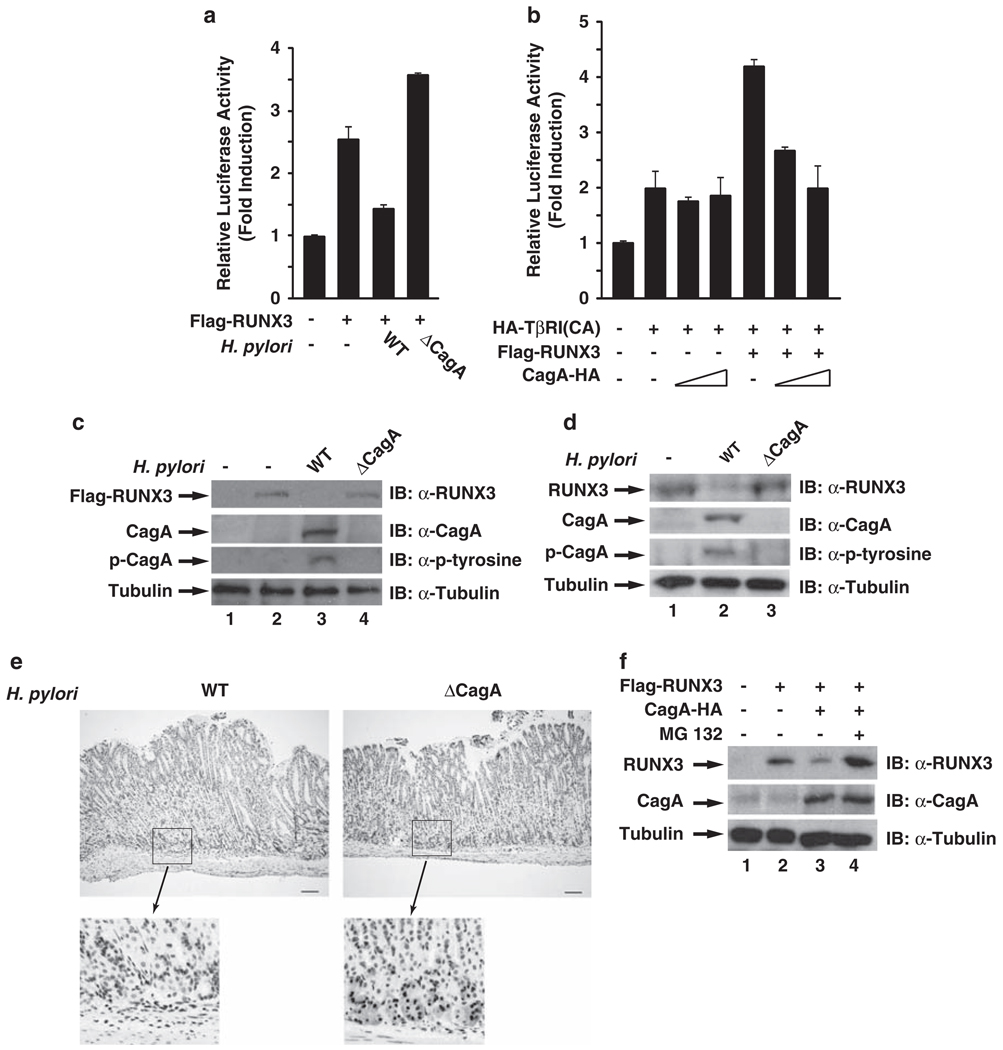
To explore the possibility that H. pylori infection might lead to the inactivation of RUNX3, we first investigated the effect of H. pylori infection on the transcriptional activity of RUNX3. RUNX3 activates the TβRE luciferase reporter, which contains three RUNX binding sites (Hanai et al., 1999), in the presence of constitutively activated TGF-β type I receptor in AGS cells (Figure 1a). However, the transcriptional activity of RUNX3 was inhibited by infection with wild-type (WT) H. pylori, but not with its cagA-deficient isogenic mutant (Figure 1a), indicating that H. pylori downregulates the transcriptional activity of RUNX3 and that CagA is essential for the inhibition.
Figure 1.
H. pylori infection downregulates the expression of RUNX3 in a CagA-dependent manner. (a) CagA inhibits the transcriptional activity of RUNX3. AGS cells were transfected by Lipofectamine 2000 with TβRE reporter plasmid, together with expression vectors for RUNX3 and constitutively activated TβRI(CA) (Hanai et al., 1999; Ito et al., 2005) for 24 h. In each experiment, cells were also co-transfected with Renilla luciferase reporter plasmid as an internal control. Luciferase activity was measured 24 h after the transfected cells were infected with WT or cagA-deficient H. pylori. Results represent the average of three independent experiments ± s.d. H. pylori NCTC11637 strain and its cagA-deficient isogenic mutant were cultured as previously described (Lamb et al., 2009). H. pylori was added to AGS cells for infection at an MOI of 50–100. (b) CagA is sufficient to inhibit the transcriptional activity of RUNX3. CagA, RUNX3 and TβRI(CA) were co-transfected with the TβRE reporter plasmid into AGS cells as indicated. 48 h after transfection, luciferase activity was measured as per procedure (a). (c) CagA is essential for the reduced expression of RUNX3 in response to H. pylori infection. AGS cells transiently expressing RUNX3 were infected with WT and cagA-deficient H. pylori for 24 h, and levels of RUNX3, CagA and phosphorylated CagA were detected by immunoblotting whole-cell extracts with anti-RUNX3, anti-CagA, anti-phosphotyrosine or anti-tubulin antibodies. Antibodies against CagA, phosphotyrosine and RUNX3 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Antibodies against tubulin were purchased from Sigma (St Louis, MO, USA). (d)MKN-45 cells were infected with WT and cagA-deficient H. pylori for 48 h, and levels of RUNX3, CagA and phosphorylated CagA were detected as described in (c). (e) RUNX3 expression is decreased within mouse gastric mucosa early after infection with H. pylori NCTC11637CagA-positive strain. Immunohistochemistry for RUNX3 was performed as previously described (Ito et al., 2008) on gastric mucosa harvested from INS-GAS mice infected with WT or cagA-deficient NCTC11637 H. pylori strains 48 h after inoculation. Representative staining of RUNX3 is shown for WT (left panel) or cagA-deficient mutant (right panel)-infected mice. The bars are equal to 100 µm. Animal experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. (f) CagA is sufficient to induce the proteasome-mediated degradation of RUNX3. AGS cells were transfected with expression vectors for Flag-RUNX3 and CagA-HA. At 24 h after transfection, cells were treated or not with MG-132 (10 µm) for 6 h. Whole-cell lysates were immunoblotted for the expression of RUNX3, CagA and tubulin as indicated.
To further test whether CagA is sufficient to downregulate the transcriptional activity of RUNX3, we examined the effect of CagA on the transcriptional activity of RUNX3 in the same TβRE luciferase reporter assay. Although co-transfection of CagA did not inhibit the basal activity induced by TβRI in AGS cells, it inhibited the transcriptional activity of RUNX3 in a dose-dependent manner (Figure 1b). A similar inhibitory effect for CagA on RUNX3 was also observed in HEK293T epithelial cells, wherein RUNX3 showed higher transcription activity than in AGS cells, likely because of the higher transfection efficiency in these cells (Supplementary Figure 1). These data demonstrate that CagA alone is sufficient to inhibit the transcriptional activity of RUNX3.
Interestingly, examining the cellular levels of RUNX3 in H. pylori-infected AGS cells revealed that the expression of RUNX3 was downregulated by the infection in a CagA-dependent manner (Figure 1c), indicating that CagA is involved in decreasing RUNX3 expression. To further confirm this, we examined the expression of RUNX3 in H. pylori-infected MKN-45 cells, which express endogenous RUNX3. Infection of MKN-45 cells with WT but not cagA-deficient H. pylori also decreased the expression of RUNX3 in MKN-45 cells (Figure 1d). More importantly, when we investigated the expression of RUNX3 in H. pylori-infected mice, we found that expression of RUNX3 in gastric mucosa was significantly reduced 48 h after infection with WT H. pylori (Figure 1e, left panel) compared with the expression either after infection with cagA-deficient H. pylori (Figure 1e, right panel) or in uninfected controls (data not shown). This time point precedes the development of gastric inflammation, further supporting a role of direct microbial effects in carcinogenesis. These in vivo data further support the conclusion that H. pylori reduces the expression of RUNX3 in a CagA-dependent manner. This is also supported by the observation that co-expression of CagA with RUNX3 decreased the cellular levels of RUNX3 (Figure 1f). The decreased expression of RUNX3 was due to the degradation of RUNX3, as treatment of the cells with proteasome inhibitor MG-132 reversed the decreased expression of RUNX3 (Figure 1f) and co-expression of CagA or infection with H. pylori did not alter the RUNX3 mRNA levels (Supplementary Figures 2 and 3). Collectively, these findings demonstrate that H. pylori CagA induces proteolytic degradation of RUNX3.
The RUNX3 PY motif interacts with the CagA WW domain
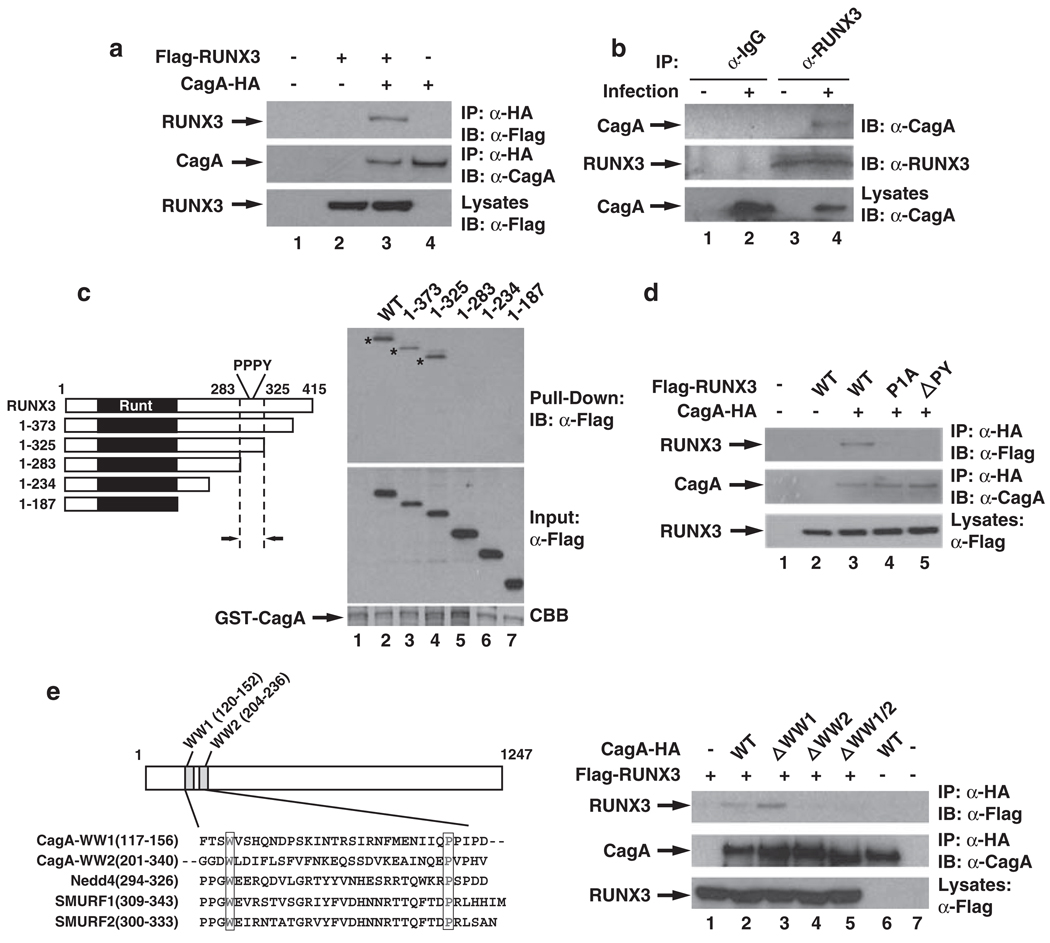
The CagA-dependent degradation of RUNX3 by H. pylori infection prompted us to examine whether CagA might directly target RUNX3. Immunoprecipitation of CagA from transfected HEK293T cells co-immunoprecipitated RUNX3 (Figure 2a). The interaction between CagA and RUNX3 was further confirmed with the in vitro GST pull-down (Supplementary Figure 4), indicating a direct interaction between RUNX3 and CagA. Furthermore, when the physical interaction between CagA and the endogenous RUNX3 was investigated in H. pylori-infected RUNX3-expressing MKN-45 gastric cancer cells, RUNX3 co-immunoprecipitated CagA after H. pylori infection (Figure 2b). These data demonstrate that CagA associates with RUNX3 in vitro and in vivo in response to H. pylori infection.
Figure 2.
RUNX3 interacts with CagA through the PY motif of RUNX3 and the WW2 domain of CagA. (a) CagA interacts with RUNX3 in transfected cells. HEK293T cells were transfected by calcium phosphate with Flag-RUNX3 and HA-tagged CagA as indicated. The transfected HEK293T cell lysates were incubated with anti-HA antibody-conjugated agarose beads (Sigma) for 2 h at 4 °C. CagA-HA immunoprecipitates from whole-cell lysates were immunoblotted for Flag-tagged RUNX3. Antibodies against Flag and HA were purchased from Santa Cruz Biotechnology Inc. (b) CagA interacts with RUNX3 after H. pylori infection. MKN-45 gastric cancer cells were infected with H. pylori for 0 or 24 h. Endogenous RUNX3 was immunoprecipitated from H. pylori-infected MKN-45 cells with either anti-IgG or anti-RUNX3 antibodies, and immunoblotted for associated CagA with anti-CagA antibodies. (c) The region of RUNX3 from amino acids 283–325 is important for its interaction with CagA. Left: Schematic showing the domain structure of RUNX3 and the position of the PY motif. Right: GST-CagA was incubated with cell lysates containing Flag-tagged RUNX3 or its various deletion mutants as indicated and precipitated with glutathione agarose beads. The recovered materials were immunoblotted with anti-Flag antibodies. (d) PY motif of RUNX3 is essential for its interaction with CagA. HEK293T cells were transfected with HA-tagged CagA and WT Flag-RUNX3 or its PY motif mutants as indicated. CagA-HA immunoprecipitates were immunoblotted for the associated RUNX3 WT or PY motif mutants. (e)WW domain 2 of CagA is critical for its interaction with RUNX3. Left: Schematic showing the relative location of the two WW domains of CagA and the sequence alignment of the WW domains of CagA with other known WW domains. Right: HEK293T cells were transfected with HA-tagged CagA or its WW domain deletion mutants and Flag-RUNX3 as indicated. CagA-HA immunoprecipitates were immunoblotted for the associated RUNX3.
To define the region of RUNX3 responsible for its interaction with CagA, we performed an in vitro GST pull-down assay using cell lysates containing various deletion mutants of RUNX3 (Figure 2c). Full-length RUNX3 and two of its C-terminal deletion mutants (deleted to amino acid 325) were able to associate with CagA (Figure 2c). Further deletion to amino acid 283 abolished RUNX3’s interaction with CagA (Figure 2c), indicating that the region from amino acids 283–325 of RUNX3 is critical for its interaction with CagA.
Examining the sequence of amino acids 283–325 revealed that this region contains a PPxY sequence (known as the PY motif), which can be recognized by a WW domain within an interacting partner protein (Sudol et al., 1995). The WW domain is defined by a conserved tryptophan residue and an invariant proline residue placed approximately 20–22 amino-acid residues apart (Sudol et al., 1995). Interestingly, in examining the sequence of CagA, we identified two WW domain-like sequences within the N-terminal region of CagA (designated as WW1 and WW2, respectively; Figure 2e). We then explored the possibility that the PY motif of RUNX3 and the WW domains of CagA might be involved in their interaction. We generated various mutants of RUNX3 or CagA by mutation or deletion of the PY motif or the WW domains and examined their interaction. When the first proline (P) of the PPPY sequence was mutated to alanine (A) (designated as RUNX3-P1A) or the PPPY sequence was deleted (designated as RUNX3-ΔPY), the interaction of CagA with RUNX3 was significantly impaired (Figure 2d), indicating that the PY motif is critical for the interaction of RUNX3 with CagA. As for the CagA WW domain deletion mutants, deletion of WW1 of CagA barely affected its interaction with RUNX3 (Figure 2e). However, deletion of WW2 domain alone or together with WW1 completely abolished its interaction with RUNX3, confirming that the interaction between RUNX3 and CagA is through the specific recognition of the PY motif by the WW domain.
Interaction between CagA and RUNX3 reduces the stability of RUNX3
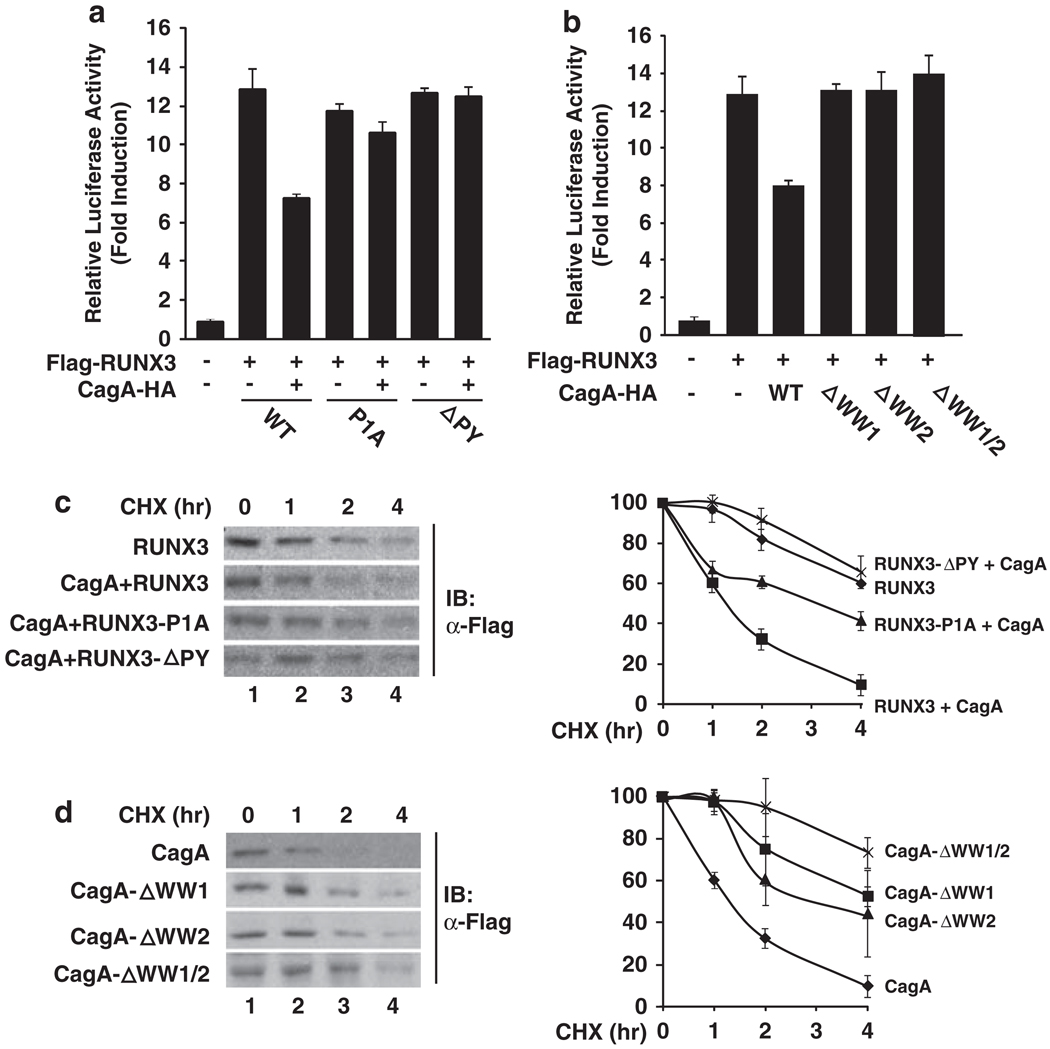
Having identified that CagA interacts with RUNX3, we next investigated whether the recognition of the PY motif by the WW domain of CagA was required for the downregulation of the transcriptional activity of RUNX3. When the effect of CagA on the activity of RUNX3 WT or PY motif mutants was examined, we found that RUNX3-P1A and RUNX3-ΔPY showed resistance to CagA-induced inhibition (Figure 3a). Furthermore, when the capabilities of the CagA WW domain deletion mutants to down-regulate RUNX3 were tested in the TβRE reporter assay, CagA with the deletion of WW1, WW2 or both barely inhibited the activity of RUNX3 (Figure 3b). These results demonstrate that the interaction with CagA is critical for the inactivation of the transcriptional activity of RUNX3.
Figure 3.
CagA reduces the stability of RUNX3 through specific interactions. (a) RUNX3 with PY motif mutations is resistant to CagA-mediated downregulation of its activity. WT RUNX3 or its PY motif mutants, CagA and TβRI(CA), were co-transfected with the TβRE reporter plasmid into HEK293T cells as indicated. Luciferase activity was measured as described in Figure 1b. (b) WW domains of CagA are essential for the downregulation of the transcriptional activity of RUNX3. WT or WW domain deletion mutants of CagA, RUNX3 and TβRI(CA) were co-transfected with the TβRE reporter plasmid into HEK293T cells as indicated. Luciferase activity was measured as described in (a). (c) The PY motif is important for the CagA-induced degradation of RUNX3. COS-7 cells were transfected by calcium phosphate with WT RUNX3 with or without CagA, or with RUNX3-P1A or RUNX3-ΔPY and CagA as indicated. At 24 h after transfection, cells were treated with 100 µg/ml of cycloheximide (CHX) for the indicated time points, and immunoblotted for the expression of Flag-RUNX3. A representative result from three independent experiments is shown in the left panels. Quantification of the results is shown in the right panel. Data represent the average of three independent experiments ± s.d. (d) WW domains of CagA are critical for CagA-induced degradation of RUNX3. COS-7 cells were transfected with RUNX3 together with WT CagA or its WW domain deletion mutants as indicated. At 24 h after transfection, cells were treated with CHX for indicated time points, immunoblotted for the expression of Flag-RUNX3, and levels were quantified as described in (c).
Further, we determined whether the interaction between RUNX3 and CagA is essential for the degradation of RUNX3. We first compared the effect of WT CagA on the stability of RUNX3 by measuring the half-life of WT RUNX3 or its PY motif mutants. Consistent with the notion that CagA promotes the degradation of RUNX3, the half-life of RUNX3 was reduced from >4h to ~1.5 h when CagA was co-expressed with RUNX3 (Figure 3c). Conversely, RUNX3 PY motif mutants were more resistant to CagA-induced degradation, with much longer half-lives (>3 h for RUNX3-P1A and >4h for RUNX3-ΔPY) (Figure 3c), indicating that the PY motif is important for the CagA-induced degradation of RUNX3.
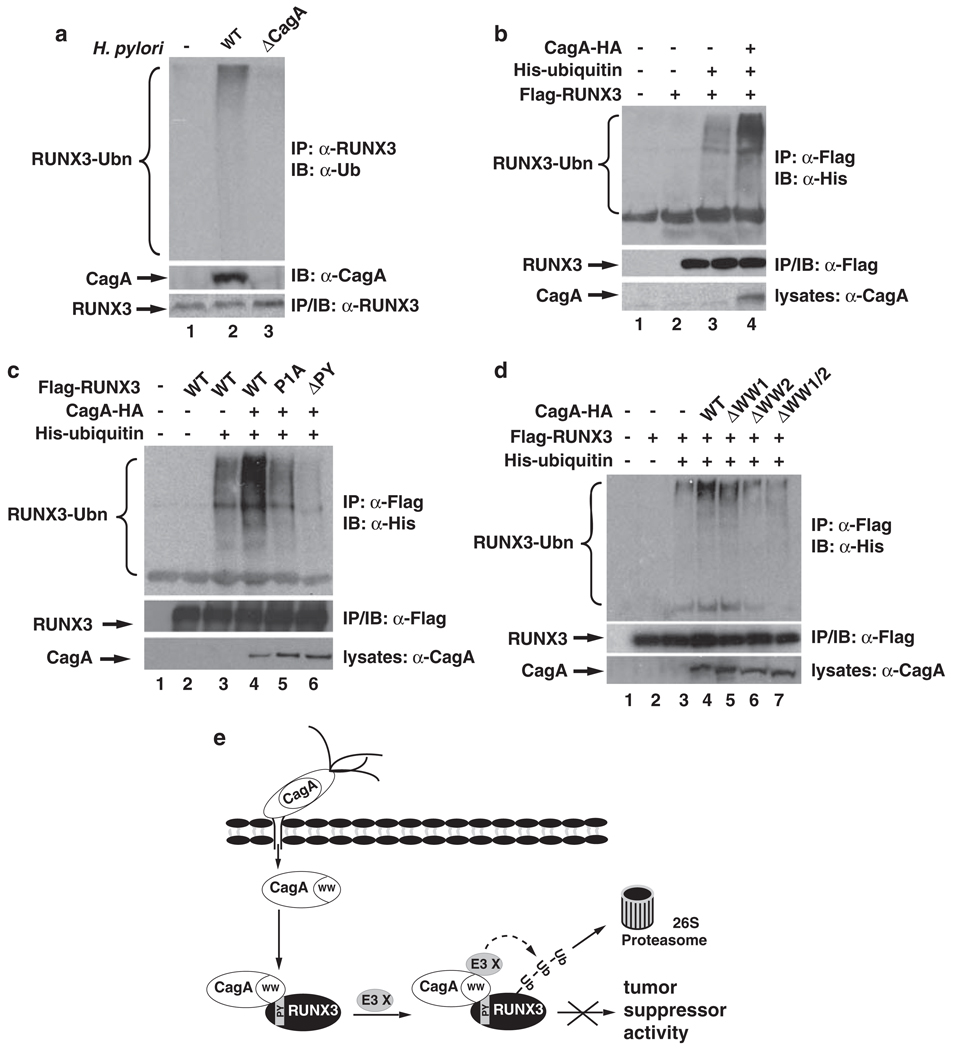
In addition, when the half-life of RUNX3 was measured with CagA WW domain deletion mutants, we found that the half-life of RUNX3 was moderately reduced when co-transfected with either WW1 or WW2 deletion mutants (Figure 3d). CagA with the deletion of both WW1 and WW2 domains failed to affect the half-life of RUNX3 (Figure 3d). Collectively, these data support the idea that CagA reduces the stability of RUNX3 through the specific recognition of the PY motif by the WW domain of CagA. CagA stimulates the ubiquitination of RUNX3 in vivo As CagA stimulates the degradation of RUNX3 (Figure 1), we next investigated whether CagA promoted the ubiquitination of RUNX3, an event that is required for proteasome-mediated degradation. When we examined the ubiquitination of endogenous RUNX3 in MKN-45 cells in response to H. pylori infection, we found that WT, but not cagA-deficient H. pylori, induced the ubiquitination of endogenous RUNX3 (Figure 4a), suggesting that CagA is essential for H. pylori-induced ubiquitination of RUNX3. When RUNX3 was co-transfected with ubiquitin and the ubiquitination of RUNX3 was measured, we found that co-expression of CagA significantly enhanced ubiquitination of RUNX3 (Figure 4b), indicating that CagA is sufficient to promote ubiquitination of RUNX3. However, it has to be noted that CagA by itself is not an E3 ligase, as no cysteine residue, the essential amino acid for an E3 ligase to form a thioester bond with ubiquitin (Pickart, 2001), is found within CagA. In addition, recombinant CagA does not ubiquitinate RUNX3 in vitro (data not shown). Thus, it is likely that CagA functions as a scaffold protein to recruit an E3 ligase for the ubiquitination of RUNX3, but the identity of this ligase remains to be determined, and we are currently exploring this possibility.
Figure 4.
CagA stimulates the ubiquitination of RUNX3. (a) H. pylori infection induces the ubiquitination of RUNX3 in a CagA-dependent manner. MKN-45 cells were infected with WT H. pylori NCTC11637 and its cagA− isogenic mutant. At 24 h after infection, cells were treated with MG-132 (10 µm) for another 24 h. Endogenous RUNX3 immunoprecipitates were immunoblotted for ubiquitination with anti-Ub antibodies (upper panel). Levels of CagA and RUNX3 are shown in the lower two panels. (b) CagA enhances ubiquitination of RUNX3 in vivo. HEK293T cells were transfected with the indicated combination of plasmids expressing Flag-RUNX3, His-ubiquitin and CagA-HA. RUNX3 immunoprecipitates were immunoblotted for ubiquitination with anti-His antibodies (upper panel). Levels of RUNX3 and CagA are shown in the lower two panels. (c) PY motif of RUNX3 is critical for the CagA-induced ubiquitination of RUNX3. HEK293T cells were transfected with the indicated combination of plasmids expressing Flag-RUNX3 or its PY motif mutants with His-ubiquitin and CagA-HA. Ubiquitination of RUNX3 was detected as described in (a). (d) WW domains of CagA are important for enhanced ubiquitination of RUNX3 by CagA. HEK293T cells were transfected with the indicated combination of plasmids expressing Flag-RUNX3, His-ubiquitin, and CagA-HA or its WW domain deletion mutants. Ubiquitination of RUNX3 was detected as described in (a). (e) Schematic model for the role of CagA in H. pylori infection-induced degradation of RUNX3. After injection into cells, CagA associates with the PY motif of RUNX3 via its WW2 domain. Binding of CagA to RUNX3 recruits an unidentified E3 ligase (E3 X) and initiates the ubiquitination and proteasome-mediated degradation of RUNX3, resulting in the inactivation of its tumor suppressor activity in gastric epithelial cells.
We next assessed roles for the PY motif of RUNX3 and the WW domains of CagA in CagA-induced ubiquitination of RUNX3. First, we examined the ubiquitination of RUNX3 PY motif mutants. Compared with WT RUNX3, the CagA-induced ubiquitination of RUNX3-P1A and RUNX3-ΔPY was significantly impaired, with RUNX3-ΔPY showing greater reduction in the levels of ubiquitination (Figure 4c). These data are consistent with the findings that the PY motif is required for CagA-mediated degradation of RUNX3 (Figure 3d). When the WW domain mutants of CagA were examined for their abilities to induce the ubiquitination of RUNX3, we observed that deletion of WW1 moderately reduced CagA-induced ubiquitination of RUNX3 (Figure 4d). However, with the deletion of WW2 or deletion of both WW1 and WW2, CagA failed to enhance the ubiquitination of RUNX3. Although both WW domains are involved in the downregulation of RUNX3 (Figures 3 and 4), only WW2 is required for the specific interaction with the PY motif (Figure 2d), suggesting that the two WW domains might possess different functions. It is possible that CagA utilizes its WW2 to associate with the PY motif of RUNX3 and its WW1 to recruit the ubiquitin E3 ligase for the degradation of RUNX3, because deletion of WW1 impairs the ubiquitination of RUNX3 (Figure 4d).
Loss of RUNX3 expression is associated with gastric carcinogenesis (Li et al., 2002), and hypermethylation of the RUNX3 promoter or mislocalization of the RUNX3 protein largely attributes to the inactivation of RUNX3 in gastric cancer cells and gastric tumors (Li et al., 2002; Ito et al., 2005). H. pylori infection has been shown to be an independent risk factor for methylation of the promoter of RUNX3 (Kitajima et al., 2008; Katayama et al., 2009). However, CagA appears to not be important for this epigenetic regulation (Kitajima et al., 2008). Therefore, the CagA-induced degradation of RUNX3 in gastric epithelial cells identified in this study provides another mechanism for the inactivation of RUNX3 and might represent one mechanism through which H. pylori initiates gastric cancer. Although H. pylori-induced degradation of RUNX3, which is CagA dependent, could represent an immediate cellular response to infection, chronic infection with H. pylori might subsequently induce the methylation of the RUNX3 promoter, which is CagA independent and leads to sustained inactivation of RUNX3.
CagA interacts with various host cellular proteins to trigger distinct signaling pathways in a tyrosine phosphorylation-dependent and -independent manner (Peek, 2005; Hatakeyama, 2008, 2009). Downregulation of RUNX3 by CagA seems to be phosphorylation-independent, because a phosphorylation-deficient mutant of CagA was still able to induce the ubiquitination and degradation of RUNX3 (Supplementary Figures 5 and 6). The specific recognition of the PY motif of RUNX3 by a WW domain of CagA defines a novel interaction of a host protein with CagA, and the identification of RUNX3 as a novel target for CagA provides a mechanism for the carcinogenic effect of CagA, in which CagA inhibits the function of the gastric tumor suppressor RUNX3 by inducing its degradation (Figure 4e). Regulation of the interaction between RUNX3 and CagA might be a potential target for the prevention of H. pylori-induced gastric cancer.
Supplementary Material
Acknowledgements
We thank Dr Hatakeyama (University of Tokyo, Japan) for providing the expression vector for CagA-HA and CagA-PR-HA. This work is supported in part by ICR provided by UIUC and NIH Grants DK-085158 (to LFC) and DK-58587, CA-77955 and CA-116087 (to RMP). AL is a recipient of the CMB-TG. YHT is an A*STAR-Illinois Partnership fellow.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- Chang TL, Ito K, Ko TK, Liu Q, Salto-Tellez M, Yeoh KG, et al. Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells. Gastroenterology. 2009;138:255–265. doi: 10.1053/j.gastro.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, Li QL, et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperation. Mol Cell Biol. 2005;25:8097–8107. doi: 10.1128/MCB.25.18.8097-8107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai J, Chen LF, Kanno T, Ohtani-Fujita N, Kim WY, Guo WH, et al. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Calpha promoter. J Biol Chem. 1999;274:31577–31582. doi: 10.1074/jbc.274.44.31577. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol. 2008;11:30–37. doi: 10.1016/j.mib.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239–248. doi: 10.1007/s00535-009-0014-1. [DOI] [PubMed] [Google Scholar]
- Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA. 2002a;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002b;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- Hsu PI, Hsieh HL, Lee J, Lin LF, Chen HC, Lu PJ, et al. Loss of RUNX3 expression correlates with differentiation, nodal metastasis, and poor prognosis of gastric cancer. Ann Surg Oncol. 2009;16:1686–1694. doi: 10.1245/s10434-009-0428-2. [DOI] [PubMed] [Google Scholar]
- Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–1644. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, Chuang LS, et al. RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell. 2008;14:226–237. doi: 10.1016/j.ccr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, Ida H, et al. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743–7750. doi: 10.1158/0008-5472.CAN-05-0743. [DOI] [PubMed] [Google Scholar]
- Ito Y. Oncogenic potential of the RUNX gene family: ‘overview’. Oncogene. 2004;23:4198–4208. doi: 10.1038/sj.onc.1207755. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Takahashi M, Kuwayama H. Helicobacter pylori causes runx3 gene methylation and its loss of expression in gastric epithelial cells, which is mediated by nitric oxide produced by macrophages. Biochem Biophys Res Commun. 2009;388:496–500. doi: 10.1016/j.bbrc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kitajima Y, Ohtaka K, Mitsuno M, Tanaka M, Sato S, Nakafusa Y, et al. Helicobacter pylori infection is an independent risk factor for Runx3 methylation in gastric cancer. Oncol Rep. 2008;19:197–202. [PubMed] [Google Scholar]
- Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, et al. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–1249. doi: 10.1038/embor.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek RM., Jr Orchestration of aberrant epithelial signaling by Helicobacter pylori CagA. Sci STKE. 2005;2005:pe14. doi: 10.1126/stke.2772005pe14. [DOI] [PubMed] [Google Scholar]
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- Peng Z, Wei D, Wang L, Tang H, Zhang J, Le X, et al. RUNX3 inhibits the expression of vascular endothelial growth factor and reduces the angiogenesis, growth, and metastasis of human gastric cancer. Clin Cancer Res. 2006;12:6386–6394. doi: 10.1158/1078-0432.CCR-05-2359. [DOI] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M, Chen HI, Bougeret C, Einbond A, Bork P. Characterization of a novel protein-binding module–the WW domain. FEBS Lett. 1995;369:67–71. doi: 10.1016/0014-5793(95)00550-s. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Mori Y, et al. Decreased expression of RUNX3 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep. 2008;19:713–719. [PubMed] [Google Scholar]
- Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang L, et al. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005;65:4809–4816. doi: 10.1158/0008-5472.CAN-04-3741. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281:5267–5276. doi: 10.1074/jbc.M512151200. [DOI] [PubMed] [Google Scholar]
- Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, Inoue K, et al. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2006;26:4474–4488. doi: 10.1128/MCB.01926-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.