Abstract
Disaggregated embryonic yolk sac cells and circulating peripheral blood cells were obtained from normal murine day 9 embryos, prior to the formation of the fetal liver. These cells were microinjected transplacentally into days 11-15 W mutant anemic fetuses, when the fetal liver was the major hemopoietic organ. In a small proportion of the recipient animals examined after birth, long-term repopulation by the embryonic donor hemopoietic cells was observed. The donor hemopoietic stem cells proliferated and differentiated in the hosts as evidenced by the presence of donor hemoglobins in the growing recipient host animals. Some mothers of the pups were also repopulated by the donor stem cells. These results provide direct evidence that, during early murine embryogenesis, there are functional hemopoietic stem cells which are capable of colonizing the adult hemopoietic organs and probably the fetal liver and spleen to initiate hemopoiesis in these tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Campbell A. S. New murine globin bands on electrophoresis may not be new globin chains. Hemoglobin. 1982;6(5):517–522. doi: 10.3109/03630268209083764. [DOI] [PubMed] [Google Scholar]
- Barker J. E. Development of the mouse hematopoietic system. I. Types of hemoglobin produced in embryonic yolk sac and liver. Dev Biol. 1968 Jul;18(1):14–29. doi: 10.1016/0012-1606(68)90020-1. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG M. L., RUSSELL E. S. A DEVELOPMENTAL CHANGE IN HEMOGLOBINS CORRELATED WITH AN EMBRYONIC RED CELL POPULATION IN THE MOUSE. Dev Biol. 1964 Oct;10:191–201. doi: 10.1016/0012-1606(64)90040-5. [DOI] [PubMed] [Google Scholar]
- Cooper S. M., Franklin E. C., Frangione B. Molecular defect in a gamma-2 heavy chain. Science. 1972 Apr 14;176(4031):187–189. doi: 10.1126/science.176.4031.187. [DOI] [PubMed] [Google Scholar]
- Fantoni A., Bank A., Marks P. A. Globin composition and synthesis of hemoglobins in developing fetal mice erythroid cells. Science. 1967 Sep 15;157(3794):1327–1329. doi: 10.1126/science.157.3794.1327. [DOI] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Fleischman R. A., Mintz B. Development of adult bone marrow stem cells in H-2-compatible and -incompatible mouse fetuses. J Exp Med. 1984 Mar 1;159(3):731–745. doi: 10.1084/jem.159.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman R. A., Mintz B. Prevention of genetic anemias in mice by microinjection of normal hematopoietic stem cells into the fetal placenta. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5736–5740. doi: 10.1073/pnas.76.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., McFarland E. C., Russell E. S. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981 Feb;97(2):337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Moore M. A. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975 Dec 25;258(5537):726–728. doi: 10.1038/258726a0. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Matsuda H., Hatanaka K. Clonal nature of mast-cell clusters formed in W/Wv mice after bone marrow transplantation. Nature. 1979 Sep 13;281(5727):154–155. doi: 10.1038/281154a0. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970 Mar;18(3):279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Murphy E. D., Harrison D. E., Roths J. B. Giant granules of beige mice. A quantitative marker for granulocytes in bone marrow transplantation. Transplantation. 1973 May;15(5):526–530. [PubMed] [Google Scholar]
- Niewisch H., Hajdik I., Sultanian I., Vogel H., Matioli G. Hemopoietic stem cell distribution in tissues of fetal and newborn mice. J Cell Physiol. 1970 Aug;76(1):107–115. doi: 10.1002/jcp.1040760115. [DOI] [PubMed] [Google Scholar]
- Perah G., Feldman M. In vitro activation of the in vivo colony-forming units of the mouse yolk sac. J Cell Physiol. 1977 May;91(2):193–199. doi: 10.1002/jcp.1040910205. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A., Chui D., Epler H. An ultrastructural study of early morphogenetic events during the establishment of fetal hepatic erythropoiesis. J Cell Biol. 1969 Feb;40(2):343–365. doi: 10.1083/jcb.40.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. S., Bernstein S. E. Proof of whole-cell implant in therapy of W-series anemia. Arch Biochem Biophys. 1968 May;125(2):594–597. doi: 10.1016/0003-9861(68)90617-6. [DOI] [PubMed] [Google Scholar]
- Seller M. J. The presence of donor-type immunoglobulins in anaemic mice of the W-series transplanted with allogeneic foetal liver cells. Immunology. 1973 Feb;24(2):249–252. [PMC free article] [PubMed] [Google Scholar]
- Thakur M. L., Segal A. W., Louis L., Welch M. J., Hopkins J., Peters T. J. Indium-111-labeled cellular blood components: mechanism of labeling and intracellular location in human neutrophils. J Nucl Med. 1977 Oct;18(10):1022–1026. [PubMed] [Google Scholar]
- Whitney J. B., 3rd Simplified typing of mouse hemoglobin (Hbb) phenotypes using cystamine. Biochem Genet. 1978 Aug;16(7-8):667–672. doi: 10.1007/BF00484723. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Chui D. H., Eaves C. J. Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3851–3854. doi: 10.1073/pnas.83.11.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Reicheld S. M., Chui D. H. Hemoglobin switching during murine embryonic development: evidence for two populations of embryonic erythropoietic progenitor cells. Blood. 1986 Mar;67(3):716–721. [PubMed] [Google Scholar]
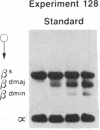
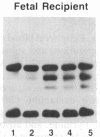
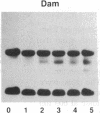
- Wong P. M., Chung S. W., White J. S., Reicheld S. M., Patterson M., Clarke B. J., Chui D. H. Adult hemoglobins are synthesized in murine fetal hepatic erythropoietic cells. Blood. 1983 Dec;62(6):1280–1288. [PubMed] [Google Scholar]
- Wong P. M., Clarke B. J., Carr D. H., Chui D. H. Adult hemoglobins are synthesized in erythroid colonies in vitro derived from murine circulating hemopoietic progenitor cells during embryonic development. Proc Natl Acad Sci U S A. 1982 May;79(9):2952–2956. doi: 10.1073/pnas.79.9.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]