Abstract
In order for site-directed polymer ultrasound contrast agents (UCAs) to provide acoustic enhancement at disease sites to distinguish normal tissue from diseased tissue, the surface of these agents must be functionalized with mixtures of graftedpolymers. Here a combination of longer liganded polyethylene glycol (PEG)-lipids and shorter unliganded PEG-lipids were introduced into the oil phase of a modified solvent evaporation double emulsion method for preparing UCAs. UCAs with different lengths of both liganded and unliganded lipids were imaged under 7.5 MHz ultrasound. The B-mode image brightness of the mixed PEG-lipid UCAs was within 1 dB the brightness of the unliganded surface. After 15 min of continuous insonation, 70% of the contrast signal remained. The peptide Arginine-Glycine-Aspartic Acid (RGD) was added to the surface of these UCAs through a biotin-avidin linkage and binding was assessed under static and shear conditions. Binding was significant after 30 min of static incubation and the adherence of the UCA increased under shear flow from 3 UCA/cell (static) to 5 UCA/cell (shear).
Keywords: Ultrasound contrast agent, Polymer, Binding, In vitro, PEG-lipid
Targeting ultrasound contrast agents (UCAs) to specific areas of disease through site-specific ligand receptor binding has the potential to enhance the ability to delineate diseased tissue from healthy tissue (Bloch et al. 2004; Hughes et al. 2003; Klibanov 2005). A targeted contrast agent should be mechanically stable enough to transit the circulation for 30-60 min to survive multiple passes through the circulatory system as only a small amount of agent will pass through the binding site in one pass (Ferrara et al. 2007). Consequently, the targeted contrast agent accumulates at the binding site and increases signal intensity at the site for the duration of the imaging exam (Ophir and Parker 1989).
In general, UCAs are small (<8 μm), shell-stabilized, intravenously injectable microbubbles (McCulloch et al. 2000) Polymers are well-suited for targeted UCAs as their shells are more durable than lipid or protein shells (Chen et al. 2003; Ferrara et al. 2007; Raisinghani and DeMaria 2002). Polylactic acid (PLA), a biocompatible and biodegradable polymer, has been suggested as a shell material as it is an FDA approved polymer for alternative uses and has gained widespread use in biomedical applications (Albertsson 2002; Arshady 1990). A number of UCAs at various stages of development have been reported using PLA for the shell (Cui et al. 2005; Diaz-Lopez et al. 2009; El-Sherif and Wheatley 2003; Narayan and Wheatley 1999; Pisani et al. 2008).
Predictive theoretical models have shown that optimal binding and protein rejection are obtained by using mixtures of longer ligand attached polymers and shorter free end polymers (Chen and Dormidontova 2005; Longo and Szleifer 2005). UCA surfaces that include short polyethylene glycol (PEG) and longer ligand-coupled PEG groups, have two expected advantages for improving in vivo targeting efficacy. An intravenously administered site-targeted UCA must be decorated with targeting ligands to bind specifically to a disease site as well as contain molecules that block nonspecific protein adsorption that minimize uptake by the mononuclear phagocyte system. First, these surfaces promote specific ligand-receptor binding and prevent nonspecific binding of proteins (Bedu-Addo et al. 1996; Chen and Dormidontova 2005; Gref et al. 1995). Second, the PEG layer increases ligand accessibility for binding by preventing the tethered ligands from bending in towards the UCA surface (Chen and Dormidontova 2005; Klibanov 2005). Therefore, we use this so-called tiered surface architecture expecting it to be more effective than a surface of purely tethered ligands.
Two strategies are currently being investigated for functionalization of PLA UCAs. PLA UCAs chemically conjugated to GRGDS, a peptide sequence that targets αvβ3 and αvβ5 integrins, bind to MDB breast cancer epithelial cells (Lathia et al. 2004) under static binding conditions. A more convenient functionalization technique typically used to functionalize lipid-based UCAs was recently implemented to functionalize of polylactic glycolic (PLGA) and PLA microspheres (Duncanson et al. 2007; Fahmy et al. 2005) with the tiered architecture. Similarly, Diaz-Lopez (Diaz-Lopez et al. 2009) characterized binding of biotinylated PLGA UCAs functionalized with this strategy. Still, with these advances in functionalized PLA UCAs, the effective binding tiered surface architecture has only been investigated for lipid-based UCAs (Borden et al. 2008) and polymer microspheres (Duncanson et al. 2007) with biotin-avidin model systems. The versatility and ease of the incorporation strategies should be further investigated for polymer UCAs beyond biotin avidin model systems towards more physiologically relevant conditions.
Thus, the purpose of this study is to employ the versatile lipid incorporation strategy to functionalize a PLA UCA with tiered surface architecture to achieve binding under shear flow conditions. Here, we extend our mixed phospholipid incorporation studies (Duncanson et al. 2007) with PLA microspheres to double emulsion PLA UCAs and assess their performance as UCAs. Furthermore, we coat the PLA UCA’s surface using biotin-avidin bridging to RGD and quantify binding to cells under both static and shear flow conditions.
Materials and Methods
We prepare functionalized PLA UCAs using a modified double emulsion solvent evaporation technique* by directly incorporating a mixture of phospholipids stored in chloroform into the oil phase of the emulsion (Duncanson et al. 2007). The phospholipids are used in a 5 mol% concentration of biotinylated lipid to PEGylated lipid with 1,2-Diacyl-sn-Glycero-3-Phosphoethanolamine-N -[Methoxy(Polyethylene glycol)-1000] (m1000), and 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Biotinyl(Polyethylene Glycol)2000] (b2000) (Avanti Polar Lipids, Alabaster, AL). Five mg of Oregon green 488 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (Invitrogen, Carlsbad, CA) is used for quantification. The PLA UCAs are formed using either a combination of short (MW: 1000 Da) lipid-methoxyPEG (mPEG) tethers and long (MW: 2000 Da) biotin-lipid-PEG tethers (m1000b2000), or only short lipid-mPEG tethers and OG-DHPE (m1000), [Fig 1A-1B]. Briefly, chloroform is evaporated from lipids using a stream of argon gas (Airgas, Salem, NH) and is further removed under desiccation. Next, 500 mg of PLA (Lakeshore Biomaterials, Birmingham, AL) and 10 mL methylene chloride are added to the lipids in the beaker. The organic phase is covered with parafilm and mixed on a stir plate. After 15 min of stirring, 1 mL of 0.5 M ammonium carbamate is added to the polymer lipid solution. The solution is sonicated for 1 s (Vibracell, VCX 500 (500 watts), tapered tip 3/16”, Sonics& Materials, Newtown, CT) at 20% amplitude power. The first water-in-oil emulsion is poured into 50 mL of 4°C polyvinyl alcohol (MW ~25,000, Polysciences, Warrington, PA) and is homogenized for 5 min at 9500 rpm (High Shear Laboratory Mixer, L4RT-A, Silverson, Chesham, UK.). One hundred mL of 2% isopropyl alcohol (Sigma, St. Louis, MO) is poured into the second emulsion which is stirred for 1.5 hr to evaporate off the organic solvent. The UCAs are then pelleted by centrifugation at 5000g for 5 min at 25°C (Sorvall RC-5C PLUS, Thermo Fisher Scientific Inc, Waltham, MA). The pellet is washed three times with hexane to further extract the organic solvent and to accelerate the UCA hardening. After the hexane dries, the UCAs are washed with deionized water and repelleted. The pellet is frozen at −80°C and lyophilized for 48 hours to sublime off the ammonium carbamate and to dry the capsules.
Fig1.
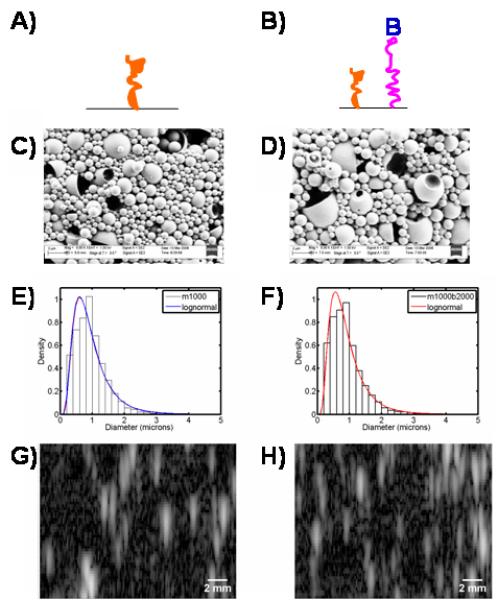
Schematic of surface architecture for m1000 (A) and m1000b2000 (B). Representative FESEM micrographs of the m1000 (C) and m1000b2000 (D) UCAs show a heterogeneous population of spheres (whole and dimpled), and shells. The diameters of the m1000 particles (E) and the m1000b2000 particles (F) are lognormally distributed with a mean diameter of 1.0 ± 0.5 μm and 0.9 ± 0.5 μm respectively. The B-mode images show the echo strength converted to brightness of the m1000 (G) and m1000b2000 (H) UCAs.
Lyophilized microbubbles are fixed to aluminum sample stubs with double-sided carbon tape and viewed with a field emission scanning electron microscope (FESEM) (Zeiss, Supra 40 VP, Thornwood, NY). Diameters of water hydrated UCAs are obtained from analysis of optical images (100X, Axiovert 25, Zeiss, Maple Grove, MN) with ImageJ software (NIH).
The image brightness (echogenicity) of UCAs embedded in polyacrylamide gels is measured using a diagnostic ultrasound imaging system (Terason 2000, Terason, Burlington, MA). Briefly, 8 × 105 UCAs/mL are embedded in 10 % polyacrylamide gels which are placed in a sample holder in a water tank. A B-mode image of the gels is obtained with a 128 element phased array transducer with a bandwidth from 5-10 MHz (L10-5, Terason, Burlington, MA). The raw echo waveforms are envelope detected by means of a Hilbert transform and then log compressed to yield an intensity level in decibels (dB). A region of interest (ROI) 20 × 1.2 mm is extracted from the B-mode image and the average signal intensity of the ROI is calculated and normalized to the average signal intensity of a reference phantom (Model 539, ATS, Bridgeport, CT) with the same ROI.
Dose response and time response curves of the UCAs are determined using a previously described technique (El-Sherif and Wheatley 2003). Briefly, UCAs are added to filtered phosphate buffered saline (PBS) at 37°C and the solution is continually stirred in a small tank and insonated with a 5 MHz spherically focused transducer (Panametrics, Waltham, MA) operating in pulse-echo mode at a pulse repetition frequency of 100 Hz, pulse length of 1 μs, peak positive pressure amplitude of 0.69 MPa, and peak negative pressure amplitude of 0.45 MPa. The dose response curve is measured by increasing the number of UCAs in the solution and measuring the echo signal of the transducer. Time response curves are acquired by maintaining a steady state dosage in the continually stirred PBS solution and collecting and averaging sixty acoustic waveforms every min for 15 min. The time response curve is plotted as normalized echogenicity as a function of time.
Oregon green 488 Neutravidin (OG-NA) (Invitrogen, Carlsbad, CA) is coupled to the biotin on the surface of the UCAs and detected by flow cytometry. To avoid the RGD mimic of Streptavidin ((Alon et al. 1990), neutravidin is used to coat the UCA surface For both m1000b2000 and m1000 UCAs, the agents are suspended at 10 mg/mL in 2% Bovine serum album (Sigma, St. Louis, MO) in Dulbecco’s PBS (DPBS, Invitrogen/Gibco, Carlsbad, CA) and incubated for 15 min at room temperature. OG-NA is added to the suspension of the m1000b2000 UCAs such that the surface biotins are saturated (Supplementary Information). After washing the UCAs in DPBS by centrifugation, the custom synthesized Biotin-C6-RGD (purity 89%, Mimotopes, Melbourne, Australia) is added to the m1000b2000 UCAs in a ~90:1 ratio of biotin:neutravidin (RGD-m1000b2000 UCAs).
Human Umbilical Vein Endothelial Cells (HUVECs) (Lonza, Hopkinton, MA) are maintained with the recommended EGM-2 bullet kit (Lonza Biologics, Portsmouth, NH) in a humidified tissue culture incubator at 37°C under 5% CO2. The 35 mm glass coverslips (Glycotech, Gaithersburg, MD) are sterilized in aluminum foil (Tuttnauer 2540E, Hauppauge, NY) for 3 min at 134°C. The sterilized coverslips are then incubated with 1% w/v gelatin in DPBS at 37°C for 30 min. The gelatin-coated coverslips are incubated in 0.5% v/v glutaraldehyde (Polysciences, Warrington, PA) at room temperature for 25 min. The glutaraldehyde is washed with 0.1 M glycine in DPBS. The glutaraldehyde cross-linked gelatin coated coverslips are rinsed in DPBS followed by a serum-free culture media wash and placed in 60 mm tissue culture treated dishes. The cells are seeded onto the coverslips at a density of ~10,000/cm2 and are used after having grown to confluence.
A Glycotech circular flow chamber (Glycotech, Gaithersburg, MD) is used to study the adhesion of UCAs under static conditions with a controlled shear wash rate. A syringe pump (Orion M362, Thermo Electron Corporation, Waltham, MA) connected to the flow chamber with silastic tubing (Glycotech, Gaithersburg, MD) controls the manually specified rates ranging from 0.05 mL/min to 1.5 mL/min to achieve laminar flow shear rates of 46 s−1, 62 s−1or 108 s−1 of fluid flow in the chamber. The cell-coated coverslip seals to the flow chamber deck through a vacuum seal.
For static adhesion experiments, serum-free media with UCAs (0.5 mg/mL) is perfused across the HUVECs for 2 min at a shear rate of 46 s−1. After the flow stops, the UCAs are incubated with the cells for time points of 5, 15, and 30 min. Following the incubation period, the unbound particles are removed with DPBS at a steady shear rate of 46 s−1 for 2 min. For experiments to assess binding of UCAs to HUVECs under dynamic conditions the UCA solution in serum-free media flows for 30 min at 46 s−1, 62 s−1or 108 s−1 followed by a DPBS wash at a shear rate of 46 s−1 for 2 min. The unbound UCAs are removed in a DPBS rinse at 46 s−1. Each coverslip is exposed to one time point or shear rate. After the final DPBS rinse the coverslips are removed from the chamber by releasing the vacuum seal and are then fixed and stained.
Cells are fixed with 4% v/v glutaraldehyde in DPBS. The coverslips are washed with cold DPBS and the cells are permeabilized in 0.5% Triton X-100 (Sigma, St. Louis, MO) in DPBS for 15 min. To reduce glutaraldehyde-related background autofluorescence, coverslips are washed in DPBS, then incubated with borohydride (0.5 mg/mL), and finally washed in DPBS. Selected coverslips are incubated with rhodamine phalloidin (Sigma, St. Louis, MO) (1:200) in 1% BSA in DPBS for 2 hr at 37°C to stain the actin filaments and then washed with 1% BSA. The cell nuclei are stained with DAPI (Vectashield Mounting Medium ,Vector Labs, Burlingame, CA) on all coverslips. The stained coverslips are mounted on 48 × 65 mm coverslips (VWR, West Chester, PA) and sealed with nail polish for imaging with confocal microscopy.
Results are presented as mean ± standard deviation for a sample size of three groups. Comparisons for echogenicity data are made using a Student’s t-test and comparisons for size data are made using a Z-score test. Ten images containing at ~20-30 cells are analyzed for each n. Statistical significance is defined as p< 0.05.
Results & Discussion
To tailor a UCA surface idealized for in vivo binding (Klibanov 2005), we employ a noncovalent instead of a covalent approach. Ligand functionalized UCAs without PEG are useful for in vitro evaluation of binding (Lathia et al. 2004), yet impractical in vivo due to the rapid clearance caused by immediate opsonin adsorption(Gref et al. 1995) Thus, UCA microparticles with mixed surface functionality are required. These surfaces can be obtained by covalent methods (Gref et al. 2003); however, a covalent approach requires additional complex copolymer synthesis, and does not offer the ease with which commercially available lipid-conjugates can be incorporated. Thererfore, the UCA surface presented here is functionalized with a ratio of PEGylated and biotinylated lipids that has been shown to facilitate binding under flow conditions (Duncanson et al. 2007).
The surface morphology of the fabricated tiered surface UCAs is characterized by FESEM. The FESEM images show that both populations contain whole, dimpled, and shelled UCAs (Figure 1C-D): shapes that are consistent with UCAs formed using the double emulsion technique (Wheatley et al. 2007). The UCA diameters, obtained through optical microscopy, are lognormally distributed about the means of 1.0± 0.6 μm and 0.9 ± 0.6 μm for the m1000, and m1000b2000 UCAs, respectively (Fig 1E-F). Analysis of the B-mode ultrasound images (Fig 1G-H) indicates that the m1000 and m1000b2000 have equivalent brightness - the difference in the images is less than 1 dB. Figure 2 shows the time response of the UCAs under ultrasound insonation. It can be seen that both populations retain 70% of their original signal after 15 min. The implication of these results is that the addition of the b2000 ligand does not affect the morphology or ultrasound contrast of the agents. Further, the stability of the UCA signal is sufficient for the duration of an ultrasound imaging exam.
Fig2.
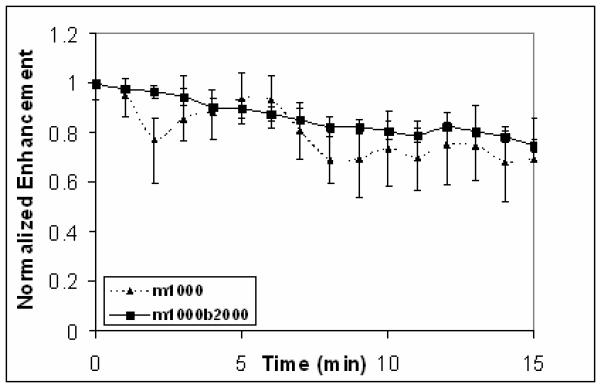
The time response curves of m1000 (triangle-dash) and m1000b2000 (square-solid) UCAs show the echogenicity only decays to 70% of the original signal after 15 min of insonation at 5 MHz .
Figure 3A shows the binding of the UCAs under static conditions. The number of UCAs/cell for the RGD-m1000b2000 UCAs is significant at t=30 min with 3 UCAs/cells relative to the m1000 particles with ~ 1.25 UCAs/cell. There is no significant attachment of the RGD-m1000b2000 UCAs or the m1000 UCAs (striped) after 5, or 15 min of static incubation with the cells (Fig 3A); however RGD-m1000b2000 UCA binding (solid) is significant at 30 min as seen in Fig 3B-C. These results for static binding studies of tiered ligand architecture RGD-m1000b2000 UCAs at 30 min show strong agreement with the results of ~ 3 RGD-m1000b2000 UCAs bound per MDB cell (Lathia et al. 2004; Wheatley et al. 2007) The limited adherence of m1000 UCAs to HUVEC indicates that the components of the lipid shell have minimal affinity to HUVEC.
Fig3.
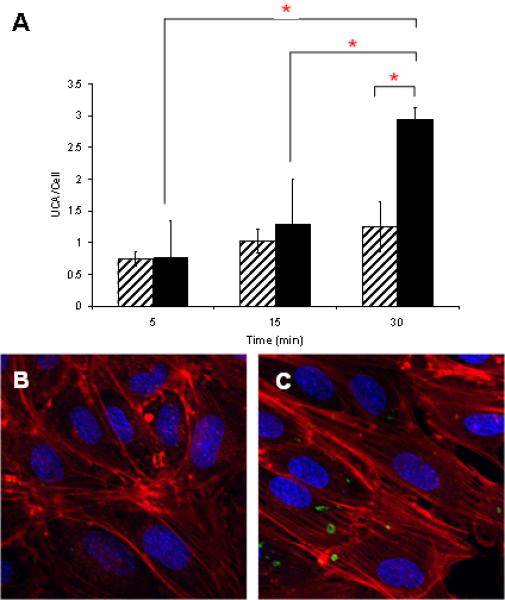
Attachment UCAs under static conditions to HUVEC is shown as a function of the three timepoints. Adherence of m1000 UCAs (striped) to HUVECs is significantly lower than RGD-m1000b2000 UCAs (solid) after 30 min of static incubation (A) (‘*’ indicates significant differences) . From the DAPI-stained nuclei (blue), rhodamine phalloidin labeled actin filaments (red), and Oregon green labeled microbubbles (green) in the confocal micrographs, we see the minimal adhesion of m1000 UCAs (B) and the significant binding of RGD-m1000b2000 UCAs.
A surface architecture similar to the m1000b2000 surface studied in static binding studies by Borden et al. (2008) showed one bound UCA per cell after 5 min of exposure while there is no significant binding observed for our PLA UCA microbubbles. Because buoyancy is known to affect affinity, Borden (Borden et al. 2008) inverted their chamber to accommodate the buoyancy of lipid microbubbles. Our results may have been comparable to these if we had inverted the chamber.
Figure 4 shows the binding performance of these targeted agents under shear flow. Here results are only shown for the 30 min time point. It can be seen that RGD-m1000b2000 UCAs for the lowest shear rate (46 s−1) exhibit less binding than in the static study. However, as shear rate increases the binding increases with equivalent binding to the static results at 62 s−1 and enhanced binding of approximately 5 UCA/cell occurs at 108 s−1. The increase in binding with increased shear rate may be explained by the increased delivery of UCAs at high flow rates (Takalkar et al. 2004) . For the m1000 UCAs, the presence of flow reduces the binding, relative to the static case, for all shear rates studied.
Fig 4.
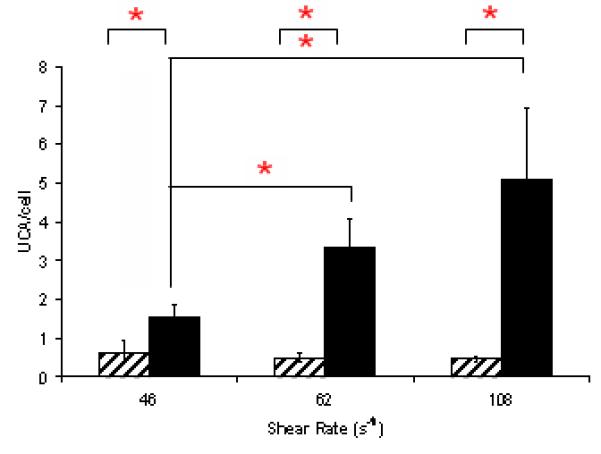
The bar graph displays the attachment of UCAs to HUVECs as a function of shear rate after 30 min of UCA perfusion. Binding of the RGD-m1000b2000 UCAs increases from 46 s−1 to 62 s−1 and 108 s−1 with shear rate from ~1.5 UCAs/cell to ~5 UCAs/cell. The binding of m1000 (striped) was insignificant (‘*’ indicate significant differences) at all shear rates with less than one UCA/cell.
Conclusions
In this work, we modified PLA UCAs by physically incorporating short unliganded PEG lipid with long liganded PEG lipids. The UCAs were imaged with B-mode ultrasound and the presence of the liganded PEGs change the brightness by less than 1 dB. These PLA UCAs were then further modified with RGD to study their binding to HUVECs under static and dynamic conditions. RGD-m1000b2000 UCAs adhere to HUVECs significantly after 30 min of static incubation. The shear rates investigated in these studies are particularly relevant for carotid atherosclerosis which is characterized by low shear stress (Nighoghossian et al. 2005). In flow studies, adherence of PLA UCAs increases under shear flow. This is consistent with others (Ham et al. 2009; Ottoboni et al. 2006; Takalkar et al. 2004) that have shown initial increased adherence up to critical shear stress of (~ 4 Dyn/cm2 (~400 s−1)) followed by decreased adherence. Binding studies at shear rates that span those of the vasculature (3-1100s−1 (Wu et al. 2004)) will be useful for determining the trends in binding with increased shear rate and critical binding shear rate. Additionally, protein adsorption studies will give more insight into the blocking of non-specific interactions due to the shorter methoxy capped PEGs in these tiered surface architecture PLA UCAs. Ultimately, these tiered ligand architecture PLA UCAs may be useful for site-targeted molecular imaging of disease.
Supplementary Material
Acknowledgments
We would like to thank Dr. Mark Grinstaff for generously donating HUVECs for our cell studies. This work was supported in part by a supplement to a R01 Award to JYW (NIH NHLBI HL72900) the National Institutes of Health National Heart Blood and Lung Institute and by CenSSIS, the Gordon Center for Subsurface Sensing and Imaging Systems, under the Engineering Research Centers Program of the National Science Foundation (Award Number EEC-9986821).
Footnotes
Oum K. 2004. Polymeric Ultrasound Contrast Agents Targeted to Malignancies: Optimization of Contrast Agent Composition and Cellular Attachment. Master’s Thesis Philadelphia: Drexel University.
References
- Albertsson A-C. Degradable aliphatic polyesters. Springer; Berlin London: 2002. p. viii.p. 179. [Google Scholar]
- Alon R, Bayer EA, Wilchek M. Streptavidin contains an RYD sequence which mimics the RGD receptor domain of fibronectin. Biochem Biophys Res Commun. 1990;170(3):1236–41. doi: 10.1016/0006-291x(90)90526-s. [DOI] [PubMed] [Google Scholar]
- Arshady R. Microspheres and Microcapsules, a Survey of Manufacturing Techniques: Part III: Solvent Evaporation. Polymer Engineering and Science. 1990;30(15):915–924. [Google Scholar]
- Bedu-Addo FK, Tang P, Xu Y, Huang L. Interaction of polyethyleneglycol-phospholipid conjugates with cholesterol-phosphatidylcholine mixtures: sterically stabilized liposome formulations. Pharm Res. 1996;13(5):718–24. doi: 10.1023/a:1016043431778. [DOI] [PubMed] [Google Scholar]
- Bloch SH, Dayton PA, Ferrara KW. Targeted imaging using ultrasound contrast agents. Progess and opportunities for clinical and research applications. IEEE Eng Med Biol Mag. 2004;23(5):18–29. doi: 10.1109/memb.2004.1360405. [DOI] [PubMed] [Google Scholar]
- Borden MA, Zhang H, Gillies RJ, Dayton PA, Ferrara KW. A stimulus-responsive contrast agent for ultrasound molecular imaging. Biomaterials. 2008;29(5):597–606. doi: 10.1016/j.biomaterials.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Dormidontova EE. Architectural and structural optimization of the protective polymer layer for enhanced targeting. Langmuir. 2005;21(12):5605–15. doi: 10.1021/la047109v. [DOI] [PubMed] [Google Scholar]
- Chen WS, Matula TJ, Brayman AA, Crum LA. A comparison of the fragmentation thresholds and inertial cavitation doses of different ultrasound contrast agents. Journal of the Acoustical Society of America. 2003;113(1):643–651. doi: 10.1121/1.1529667. [DOI] [PubMed] [Google Scholar]
- Cui W, Bei J, Wang S, Zhi G, Zhao Y, Zhou X, Zhang H, Xu Y. Preparation and evaluation of poly(L-lactide-co-glycolide) (PLGA) microbubbles as a contrast agent for myocardial contrast echocardiography. J Biomed Mater Res B Appl Biomater. 2005;73(1):171–8. doi: 10.1002/jbm.b.30189. [DOI] [PubMed] [Google Scholar]
- Diaz-Lopez R, Tsapis N, Libong D, Chaminade P, Connan C, Chehimi MM, Berti R, Taulier N, Urbach W, Nicolas V. Phospholipid decoration of microcapsules containing perfluorooctyl bromide used as ultrasound contrast agents. Biomaterials. 2009;30(8):1462–72. doi: 10.1016/j.biomaterials.2008.11.032. others. [DOI] [PubMed] [Google Scholar]
- Duncanson WJ, Figa MA, Hallock K, Zalipsky S, Hamilton JA, Wong JY. Targeted binding of PLA microparticles with lipid-PEG-tethered ligands. Biomaterials. 2007;28(33):4991–9. doi: 10.1016/j.biomaterials.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherif DM, Wheatley MA. Development of a novel method for synthesis of a polymeric ultrasound contrast agent. J Biomed Mater Res. 2003;66A(2):347–55. doi: 10.1002/jbm.a.10586. [DOI] [PubMed] [Google Scholar]
- Fahmy TM, Samstein RM, Harness CC, Saltzman W Mark. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials. 2005;26(28):5727–36. doi: 10.1016/j.biomaterials.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–47. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- Gref R, Couvreur P, Barratt G, Mysiakine E. Surface-engineered nanoparticles for multiple ligand coupling. Biomaterials. 2003;24(24):4529–37. doi: 10.1016/s0142-9612(03)00348-x. [DOI] [PubMed] [Google Scholar]
- Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. The Controlled Intravenous Delivery of Drugs Using Peg-Coated Sterically Stabilized Nanospheres. Advanced Drug Delivery Reviews. 1995;16(2-3):215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R, Miralles G, Dellacherie E. Polyoxyethylene-coated nanospheres: effect of coating on zeta potential and phagocytosis. Polymer International. 1999;48:251–256. [Google Scholar]
- Ham AS, Klibanov AL, Lawrence MB. Action at a Distance: Lengthening Adhesion Bonds with Poly(ethylene glycol) Spacers Enhances Mechanically Stressed Affinity for Improved Vascular Targeting of Microparticles. Langmuir. 2009;25(17):10038–44. doi: 10.1021/la900966h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MS, Lanza GM, Marsh JN, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy: a brief review. Medicamundi. 2003;47(1):66–73. doi: 10.1016/j.cpcardiol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Klibanov AL. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconjug Chem. 2005;16(1):9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Leodore L, Wheatley MA. Polymeric contrast agent with targeting potential. Ultrasonics. 2004;42(1-9):763–8. doi: 10.1016/j.ultras.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Longo G, Szleifer I. Ligand-receptor interactions in tethered polymer layers. Langmuir. 2005;21(24):11342–51. doi: 10.1021/la051685p. [DOI] [PubMed] [Google Scholar]
- McCulloch M, Gresser C, Moos S, Odabashian J, Jasper S, Bednarz J, Burgess P, Carney D, Moore V, Sisk E. Ultrasound contrast physics: A series on contrast echocardiography, article 3. J Am Soc Echocardiogr. 2000;13(10):959–67. doi: 10.1067/mje.2000.107004. others. [DOI] [PubMed] [Google Scholar]
- Narayan P, Wheatley MA. Preparation and characterization of hollow microcapsules for use as ultrasound contrast agents. Polymer Engineering and Science. 1999;39(11):2242–2255. [Google Scholar]
- Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke. 2005;36(12):2764–72. doi: 10.1161/01.STR.0000190895.51934.43. [DOI] [PubMed] [Google Scholar]
- Ophir J, Parker KJ. Contrast agents in diagnostic ultrasound. Ultrasound Med Biol. 1989;15(4):319–33. doi: 10.1016/0301-5629(89)90044-6. [DOI] [PubMed] [Google Scholar]
- Ottoboni S, Short RE, Kerby MB, Tickner EG, Steadman E, Ottoboni TB. Characterization of the in vitro adherence behavior of ultrasound responsive double-shelled microspheres targeted to cellular adhesion molecules. Contrast Media Mol Imaging. 2006;1(6):279–90. doi: 10.1002/cmmi.115. [DOI] [PubMed] [Google Scholar]
- Pisani E, Tsapis N, Galaz B, Santin M, Berti R, Taulier N, Kurtisovski E, Lucidarme O, Ourevitch M, Doan BT. Perfluorooctyl Bromide Polymeric Capsules as Dual Contrast Agents for Ultrasonography and Magnetic Resonance Imaging. Advanced Functional Materials. 2008;18(19):2963–2971. others. [Google Scholar]
- Raisinghani A, DeMaria AN. Physical principles of microbubble ultrasound contrast agents. Am J Cardiol. 2002;90(10A):3J–7J. doi: 10.1016/s0002-9149(02)02858-8. [DOI] [PubMed] [Google Scholar]
- Takalkar AM, Klibanov AL, Rychak JJ, Lindner JR, Ley K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J Control Release. 2004;96(3):473–82. doi: 10.1016/j.jconrel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Wheatley MA, Lathia JD, Oum KL. Polymeric ultrasound contrast agents targeted to integrins: importance of process methods and surface density of ligands. Biomacromolecules. 2007;8(2):516–22. doi: 10.1021/bm060659i. [DOI] [PubMed] [Google Scholar]
- Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging. 2004;19(2):188–93. doi: 10.1002/jmri.10441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


