Abstract
The peripheral T cell lymphomas (PTCL) carry a worse prognosis compared to B cell non-Hodgkin lymphoma. There is no uniform standard therapy for PTCL, and autologous hematopoietic cell transplant (AHCT) is often offered as consolidation in first remission or at relapse because of the poor outcomes with conventional therapy. We conducted a retrospective review of patients who underwent AHCT for PTCL from 1989 to 2006. Fifty-three cases were identified consisting of systemic anaplastic large cell (n = 18), PTCL unspecified (n = 17), angioimmunoblastic (n = 9), nasal type extranodal NK/T (n = 7), hepatosplenic (n = 2), and adult T cell leukemia/lymphoma (n = 1). Fifteen patients were transplanted in first complete or partial response (CR1/PR1), 32 in second or beyond CR or PR (CR2/PR2+), and 11 with primary refractory disease (REF). With a median follow-up was 5 years (range: 1.0–11.5), the 5-year progression-free survival (PFS) and overall survival (OS) were 25% and 48%, respectively. Disease status at AHCT had a significant impact on PFS and OS. The 5-year PFS for patients in CR1/PR1, CR2/PR2+, and REF was 51%, 12%, and 0%, respectively, and the corresponding figures for OS were 76%, 40%, and 30%, respectively. The pretransplant factors that impacted survival were disease status and the number of prior regimens. Histology, age, sex, stage, B symptoms, bone marrow involvement, and duration of first response did not significantly affect PFS or OS. Based on these results, AHCT as consolidation therapy in first complete or partial response may offer a durable survival benefit. However, AHCT with conventional salvage chemotherapy has minimal durable benefit in patients with relapsed or refractory PTCL, and thus novel strategies and/or allogeneic HCT should be more aggressively explored in lieu of AHCT for relapsed/ refractory PTCL.
Keywords: Lymphoma, Autologous transplantation, Peripheral T cell lymphoma
INTRODUCTION
The peripheral T cell lymphomas (PTCL) are a rare and heterogeneous group of disorders. PTCL accounts for approximately 10% of all lymphomas, and the most common types of PTCL are PTCL unspecified (PTCL-U), systemic anaplastic large cell lymphoma (ALCL), and angioimmunoblastic T cell lymphoma (AITL), although nasal type extranodal NK/T cell lymphoma (nNK/T) is more frequent in East Asia. Excluding anaplastic lymphoma kinase expressing (ALK+) ALCL, PTCL has a poor prognosis, with a 5-year disease free survival (DFS) of <30% [1]. Compared to the aggressive B cell lymphomas, the prognosis of patients with PTCL is considerably inferior.
To improve on this poor outcome, autologous hematopoietic cell transplantation (AHCT) has been offered to patients with PTCL as consolidation in first remission and for relapsed or refractory disease (REF). However, most studies have been limited in sample size and duration. Variable response rates have been reported, which probably reflect interstudy differences in disease status, risk factors, types of PTCL, and duration of follow-up. Recent retrospective studies in the relapsed and refractory setting have reported an estimated DFS or progression-free survival (PFS) ranging from 18%–34% at 5 years following AHCT [2–4]. As an upfront consolidation strategy, results from prospective studies have been contradictory regarding benefit [5,6]. We conducted a retrospective review and report our single center experience of patients who underwent AHCT for PTCL at Stanford University.
METHODS
Study Population
Patients who underwent AHCT from 1988 to 2006 for PTCL were identified from the Stanford University Blood and Marrow Transplantation database. Patients with lymphoblastic lymphoma, primary leukemias, and primary cutaneous disease were excluded. Response criteria were defined according to the International Harmonization Project guidelines with the addition of REF, which was defined as a response less than partial remission (PR) to induction therapy as multiple induction regimens were allowed [7]. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of <2 and had adequate cardiac, hepatic, and renal function prior to transplant. International prognostic index (IPI) and prognostic index for PTCL-U (PIT) were not included in this analysis because of missing data for lactate dehydrogenase at diagnosis and at the time of transplant on most patients. All pathology was reviewed by the Stanford Pathology Department to confirm diagnosis.
Transplant Procedures
Patients underwent hematopoietic cell collection either by bone marrow harvest or leukapheresis following cyclophosphamide (4 gm/m2) and filgastrim (10 μg/kg) mobilization. Grafts were T cell purged either by negative selection with monoclonal antibody (mAb) and complement [8] or by CD341-positive selection using the Isolex® immunomagnetic column [9]. Assessment of residual tumor in the purged grafts was not performed. The high-dose chemotherapy regimen consisted of carmustine, etoposide, and cyclophosphamide as previously described [10]. The total body irradiation (TBI)-based regimen consisted of TBI, etoposide, and cyclophosphamide. Formal response assessments with radiologic imaging and bone marrow biopsies were performed at 3, 6, and 12 months post-HCT and annually thereafter, unless an earlier time point was clinically indicated.
Statistics
Overall survival (OS) and PFS were estimated according to the Kaplan-Meier method. The variables of interest were disease status at AHCT, number of prior regimens, histologic subtype, sex, age, advanced stage, B symptoms, bone marrow involvement, duration of CR1 for patients with relapsed disease, and CR status at day 1 90 post-AHCT. Variables of interest were compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. Multivariate and day 190 posttransplant statistics utilized a landmark analysis at day 190. Statistics were calculated using the R program (R Foundation for Statistical Computing, version 2.6.2, 2008, Vienna, Austria).
RESULTS
Patient Characteristics
Patient characteristics are listed in Table 1. The median age at AHCT was 45 years (range: 18–73), and 57% were male. Fifteen patients were transplanted in first complete or partial response (CR1/PR1), 26 in second complete or partial response or beyond (CR2/PR2+), and 10 with primary REF. Twelve of 15 patients transplanted in first response were in CR with 3 patients in PR. Six of 15 patients transplanted in CR1/PR1 required 2 or more regimens to achieve first response. Of the 2 patients with ALCL transplanted in first response, 1 had ALK- ALCL, and the other with ALK1 ALCL required 3 different induction regimens to reach PR1. Of the 26 patients transplanted in CR2/PR21, 16 were in CR. The autologous graft source was mobilized peripheral blood in 86% and bone marrow in 14% of all cases. The graft was T cell purged in 86% of all cases. The median follow-up of surviving patients was 4.9 years (range: 1.0–11.5).
Table 1.
Patient Characteristics
| Disease Status at AHCT | CR1/PR1 | CR2/PR2+ | REF |
|---|---|---|---|
| Number of cases | 15 | 28 | 10 |
| Age (range) | 49 (18–63) | 46 (24–67) | 38 (25–73) |
| Sex (M/F) | 5/10 | 19/9 | 5/5 |
| Histology | |||
| Unspecified | 2 | 10 | 4 |
| ALCL | 2 | 13 | 3 |
| AITL | 4 | 4 | 1 |
| nNK/T | 5 | 0 | 2 |
| Other* | 2 | 1 | 0 |
| Number prior regimens (range) | 1 (1–4) | 2 (1–5) | 3 (1–3) |
| Stage at initial diagnosis | |||
| I–II | 3 | 9 | 1 |
| III–IV | 12 | 19 | 9 |
| B symptoms | 10 | 11 | 3 |
| Marrow involvement | 8 | 5 | 6 |
| Median months from diagnosis to AHCT (range) | 7 (3–17) | 20 (9–192) | 11 (4–17) |
| Conditioning regimen | |||
| Chemotherapy only | 13 | 24 | 7 |
| TBI based | 2 | 4 | 3 |
| T cell depletion | 13 | 22 | 9 |
AHCT indicates autologous hematopoietic cell transplant; ALCL, anaplastic large cell lymphoma; AITL, angioimmunoblastic T cell lymphoma; nNK/T, nasal type extranodal NK/T cell lymphoma; TBI, total body irridiation; CR1/PR1, first complete or partial response.
Other: hepatosplenic (N = 2), adult T cell leukemia/lymphoma (N = 1).
Forty-four of 53 patients received CHOP (cyclophosphamide, daunorubicin, vincristine, prednisone) as initial induction therapy. Thirty-six patients received additional regimens either during induction or at relapse. Of these, 28 patients received a platinum and cytarabine-based regimen, such as DHAP (dexamethasone, cytarabine, cisplatin) or ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin), and 4 received a methotrexate-based regimen. No patients received targeted therapy, novel agents, purine analogues, or gemcitabine prior to AHCT.
Clinical Outcomes
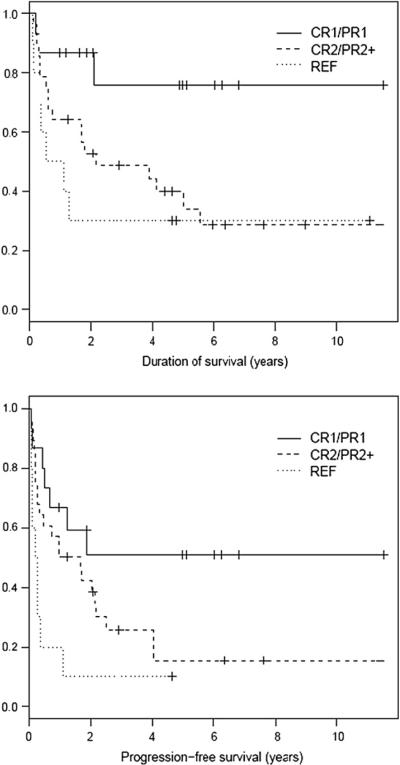
For all patients, the estimated OS and PFS at 5 years were 48% and 25%, respectively. The PFS at 5 years for patients in CR1/PR1, CR2/PR21, and REF were 51%, 12%, and 0, respectively. Corresponding figures for OS at 5 years were 76%, 40%, and 30%, respectively. Disease status at time of AHCT had a significant impact on both OS (P = .03) and PFS (P = .01) (Figure 1). The median PFS has not been reached for CR1/PR1 patients and is 1.3 years and 0.3 years for CR2/PR21 and REF patients, respectively. The median OS has not been reached for patients in CR1/PR1 and is 2.2 years and 0.8 years for the CR2/PR2 and REF patients, respectively. Of the 34 patients who developed progressive disease, the median time to progression was 0.4 years (range: 0.1–4.0). Progressive disease was the major cause of treatment failure yielding a relapse mortality of 49% (n = 26). Interestingly, 7 patients who relapsed after AHCT had achieved a subsequent CR at last follow-up. Three of these patients underwent reduced intensity allogeneic HCT, and 4 patients received salvage therapy outside of Stanford (details not available). Nonrelapse mortality (NRM) was 4% (n = 2), and both patients expired from treatment-related acute myelogenous leukemia (AML) at 4 and 48 months post-HCT.
Figure 1.
OS (P = .03) and PFS (P < .01) based on disease status at time of transplant.
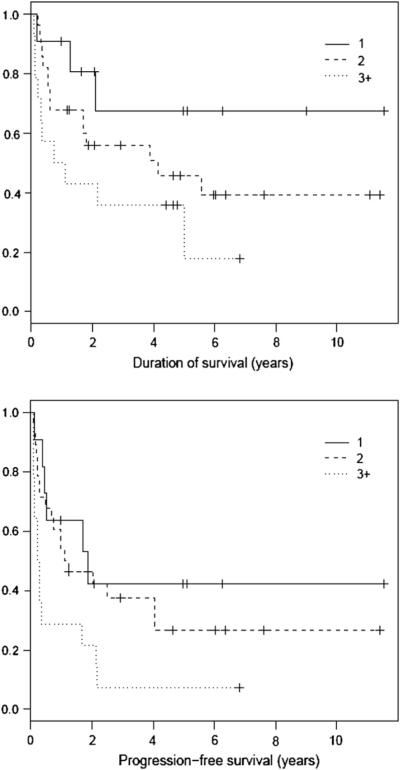
By univariate analysis, disease status at the time of AHCT was a significant factor for both OS and PFS (Figure 1). Apart from disease status, the number of prior regimens also was a significant pre-HCT predictor for PFS and showed a trend toward significance for OS (Figure 2). In contrast, survival outcomes were not affected by type of PTCL, disease stage, bone marrow involvement, B symptoms, sex, age, and duration of first response for patients transplanted in CR2/PR21 (Table 2). After AHCT, CR status at day 190 post-HCT was predictive for PFS and OS by both univariate and multivariate analysis. Nine of 14 patients with relapsed disease in partial response (PR2 or PR3) and 2 of 10 patients with primary REF converted to CR at day 190 post-HCT assessment. Of these 11 patients who converted to CR, only 3 patients remain in continuous remission at last follow-up.
Figure 2.
OS (P = .09) and PFS (P = .01) based on number of prior regimens.
Table 2.
Univariate Analysis of Prognostic Factors
| Variable | OS | PFS |
|---|---|---|
| Disease status at AHCT | .03 | <.01 |
| Number prior regimens | .09 | .01 |
| Histology subtype | .87 | .72 |
| Sex | .21 | .95 |
| Age >50 | .87 | .15 |
| Stage III or IV | .87 | .15 |
| B symptoms | .85 | .87 |
| Bone marrow involvement | .90 | .29 |
| CR1 >1 year * | .77 | .98 |
| CR at day +90 post AHCT | <.01 | <.01 |
AHCT indicates autologous hematopoietic cell transplant; OS, overall survival; PFS, progression-free survival; CR, complete remission.
Restricted to patients with relapsed disease.
DISCUSSION
Despite high initial response rates, patients with PTCL typically fare poorly after conventional chemotherapy. With the goal of overcoming the dismal outcome seen in this patient population, intensive regimens such as AHCT are offered as consolidation of first remission and at relapse. This report summarizes our retrospective review of patients with PTCL who underwent AHCT at Stanford University since 1988. Among the 53 patients in this report, 15 patients underwent AHCT while in CR1/PR1, whereas the other 38 patients were in second remission or beyond or had REF. Approximately half of patients transplanted in CR1/PR1 had a durable PFS. In contrast, only a small minority of patients transplanted with relapsed disease had prolonged PFS, and no patients with primary REF experienced a sustained benefit.
Unlike other published series of AHCT in PTCL, the patients in our study received purged autografts to reduce potential tumor cell contamination. Autografts were manipulated using monoclonal antibody and complement through 2001, and CD34+ selection of the graft was performed after 2001. Despite the infusion of a T cell purged graft, we did not observe a significant increase in infections, and none of our patients died of infectious complications. However, the high relapse rate seen in our study was comparable to other series where purging was not done. Therefore, tumor cell depletion via ex vivo T cell purging cannot be routinely recommended in this patient population.
We did not find a survival difference by type of PTCL, although there were not sufficient numbers to stratify by type and disease status. The City of Hope series also did not find a survival difference based on histology [11]. Some retrospective series have reported improved survival in ALCL, but this likely reflected inclusion of relatively more cases of ALK+ ALCL [12,13]. In our series, 4 of 13 patients with ALCL in CR2/PR2+ are in continuous remission; 2 had ALK negative disease, and the ALK status of the other 2 cases is unknown. Early disease progression was common, and the median time to progression was only 0.4 years in patients who progressed after AHCT. Hence, patients who obtained or remained in CR by day +90 had more favorable outcomes.
The patients who appeared to benefit from AHCT were those in first remission at the time of AHCT. The 15 patients who underwent AHCT in CR1/PR1 had a 5-year PFS and OS of 51% and 87%, respectively, with a plateau in the survival curve at a median follow-up of 59 months. Table 3 lists the characteristics of this favorable subset of patients. Investigators from the City of Hope also demonstrated a superior DFS and OS in the 12 patients who were in CR1 at AHCT compared to patients who were not in CR1 (DFS: 83% versus 35%; P = .03 and OS: 83% versus 45%, P = .006, respectively) in their retrospective series of 57 patients with a median follow-up of 22 months [11]. A large South Korean multicenter retrospective series of 139 patients also reported improved survival in patients who underwent AHCT in CR1/PR1 (n = 58) compared to those with relapsed or REF with a median follow-up of 29 months [14]. The Spanish GEL-TAMO (Spanish Group for Lymphoma and Autologous Transplantation) group published a large retrospective series of 75 patients who underwent AHCT as consolidation in first remission for PTCL with a median follow-up of 67 months from diagnosis [13]. The 5-year PFS and OS were an encouraging 63% and 68%, respectively, for all patients. However, the outcome of patients with ALCL was significantly superior compared to other histologies, and ALK status was not available in most cases. Hence, inclusion of patients with ALK+ ALCL likely biased the results in this series.
Table 3.
Characteristics of Patients Transplanted in First Response
| Age | Stage | Histology | Prior Regimens (#) | Status at AHCT | Outcome | *Duration (months) |
|---|---|---|---|---|---|---|
| 51 | IV | AITL | CHOP, hyperCVAD (2) | PR1 | REL | 15 |
| 53 | IV | AITL | CHOP (1) | CR1 | REL | 7 |
| 53 | IV | AITL | CHOP, ESHAP (2) | CR1 | REL | 8 |
| 63 | IV | AITL | CHOP (1) | CR1 | CR | 12 |
| 45 | IV | ALCL, Alk+ | CHOP, ESHAP, Bleomycin/paclitaxel (3) | PR1 | CR | 86 |
| 51 | III | ALCL, Alk− | CHOP, hyper CVAD (2) | PR1 | EXP | 1 |
| 38 | IV | Hepatosplenic | CHOP (1) | CR1 | CR | 61 |
| 43 | IV | Hepatosplenic | CHOP (1) | CR1 | CR | 81 |
| 18 | IV | nNK/T | Multiagent (1) | CR1 | EXP | 1 |
| 40 | 1 | nNK/T | CHOP + MTX + XRT (1) | CR1 | CR | 60 |
| 43 | IV | nNK/T | CHOP, hyperCVAD (2) | CR1 | CR | 22 |
| 47 | 1 | nNK/T | CHOP (1) | CR1 | EXP | 23 |
| 49 | 1 | nNK/T | CHOP, DHAP + XRT (2) | PR1 | CR | 72 |
| 54 | III | PTCL-U | CHOP (1) | CR1 | REL | 5 |
| 54 | IV | PTCL-U | CHOP (1) | CR1 | CR | 162 |
PR indicates partial response; CR, complete response; REL, relapse; EXP, expired (all deaths [EXP] were because of relapse; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; Hyper CVAD, fractionated cyclophosphamide, doxorubicin, vincristine, prednisone, methotrexate, cytarabine; DHAP, dexamethasone, cytarabine, cisplatin; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; Multi-Agent: see [27]; XRT, external radiation. See Table 1 for other abbreviations.
Notes:
Duration refers to time to progression for relapsed/expired patients and length of follow-up for patients in continuous CR.
Thus, given the poor outcomes with conventional chemotherapy and the encouraging retrospective results, the role of AHCT as consolidation therapy in first remission has been explored prospectively. A German study incorporated AHCT as frontline consolidation for PTCL-U and AITL after 4–6 cycles of CHOP and reported a PFS of 76% among the 21 patients who actually proceeded to AHCT [15]. However, median follow-up was only 15 months. The Spanish GEL-TAMO group also conducted a prospective trial of AHCT as frontline consolidation for PTCL, excluding ALK+ ALCL, and reported an encouraging OS and PFS of 84% and 56% at 2 years following AHCT in 19 patients [6]. In contrast, an Italian report combined the results of 2 prospective phase 2 trials utilizing high dose sequential therapy or MACOP-B followed by AHCT in advanced stage PTCL and reported disappointing results [5]. The 12-year estimated DFS and OS were 30% and 34%, respectively, at a median follow-up of 76 months. Excluding ALK+ ALCL, the estimated 12 year EFS was only 18%. This was an intent-to-treat analysis, and 16 patients (26%) who did not undergo AHCT were included in the survival data. In the above-mentioned German and Spanish studies, nearly 1/3 of patients did not proceed to AHCT because of inadequate responses to induction chemotherapy and were not included in the survival analysis, likely explaining the discrepancy with the Italian results.
The survival outcomes for our patients with relapsed or REF were lower than other reported series (Table 4) [2,4,11,12,14,16–18]. Duration of follow-up was relatively short in most published reports, and only the Spanish GEL-TAMO and Memorial Sloan Kettering retrospective reports have long follow-up comparable to our series. Of note, 36% of patients in the GEL-TAMO series were in PR1, although they were classified in analysis as relapsed/REF status, and 1/3 of patients in the Memorial Sloan Kettering study were considered relapsed/refractory based on response to first line therapy but were actually in PR1 prior to AHCT after a second induction regimen [2,4]. In our series, those patients would have been classified as CR1/PR1, and many of our CR1/PR1 patients required multiple induction regimens. Hence, the worse survival in our series is likely because of more stringent classification of relapsed and REF and perhaps better reflects the true poor outcome of such patients with conventional salvage chemotherapy and AHCT.
Table 4.
Autologous Hematopoietic Cell Transplantation for Relapsed and Refractory PTCL
| Group | N | DFS/PFS | OS | Median Follow-up (years) |
|---|---|---|---|---|
| Stanford: Relapsed | 28 | 12% | 40% | 4.9 |
| Stanford: Refractory | 10 | 0 | 30% | 4.9 |
| South Korea: Relapsed [14] | 73 | 33% | 33% | 2.4 |
| South Korea: Refractory [14] | 12 | 0 | 0 | 2.4 |
| GEL-TAMO [4]* | 123 | 34% | 45% | 5.1 |
| BSBMT/ABMTRR [17] | 64 | 50% | 53% | 3.1 |
| Memorial Sloan Kettering [2] | 24 | 24% | 33% | 6 |
| City of Hope [11] | 45 | 45% | 35% | 1.8 |
| Vanderbilt [12] | 28 | 50% | 69% | 3 |
| Princess Margaret Hospital [18] | 36 | 37% | 48% | 3.5 |
| Sweden [16] | 40 | 48% | 58% | 2.1 |
DFS/PFS indicates disease/progression free survival; OS, overall survival; GEL-TAMO, Spanish Group for Lymphoma and Autologous Transplantation; BSBMT/ABMTRR, British Society for Blood and Marrow Transplantation/Australasian Bone Marrow Transplant Recipient Registry.
Notes:
Included patients in first partial remission.
Although chemosensitivity prior to AHCT has been identified as prognostic for survival [2,19], all of the relapsed patients in our series were chemosensitive to salvage therapy but still had poor outcomes. Over 80% of our patients received CHOP first line, and nearly 90% received a platinum, cytarabine, or methotrexate-based regimen as salvage. Novel therapies, such as monoclonal antibodies, histone deacetylase inhibitors, and new antimetabolites, have shown promise in nontransplant trials [20–23]. Because conventional lymphoma regimens are not adequate for PTCL, inclusion of novel therapies into pretransplant salvage and conditioning regimens should be explored, particularly for relapsed and REF. Prognostic scoring systems, such as the IPI and modified PIT [24,25], can identify high-risk patients at diagnosis who might benefit from novel therapies in combination with front-line consolidation AHCT.
Because of the limitations of AHCT, allogeneic HCT has also been explored in PTCL. Myeloablative allogeneic HCT has been associated with high NRM, but reduced-intensity conditioning (RIC) has shown promising results [17,26]. We have offered a reduced-intensity allogeneic HCT to 5 patients with relapsed PTCL after prior AHCT, and 3 patients remain in remission at a median follow-up of 27 months. We are also exploring tandem AHCT followed by reduced intensity allogeneic HCT for patients with relapsed/REF.
In conclusion, our results demonstrate that patients with relapsed or REF derive minimal long-term benefit from conventional salvage therapy and AHCT. Hence, novel agents and allogeneic HCT deserve further study and should be incorporated early in the treatment course of this challenging patient population. Our findings support the use of AHCT as consolidation therapy in PTCL, but prospective randomized trials are needed to confirm benefit in patients responding to front-line therapy. Given the rarity and diversity of PTCL, the relative roles of AHCT, allogeneic HCT, and novel agents will likely require collaborative efforts for further progress.
REFERENCES
- 1.Vose JM. International Peripheral T-Cell Lymphoma (PTCL) Clinical and Pathologic Review Project: poor outcome by prognostic indices and lack of efficacy with anthracyclines. Blood. 2005;106:a811. [Google Scholar]
- 2.Kewalramani T, Zelenetz AD, Teruya-Feldstein J, et al. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br J Haematol. 2006;134:202–207. doi: 10.1111/j.1365-2141.2006.06164.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith SD, Bolwell BJ, Rybicki LA, et al. Autologous hematopoietic stem cell transplantation in peripheral T-cell lymphoma using a uniform high-dose regimen. Bone Marrow Transplant. 2007;40:239–243. doi: 10.1038/sj.bmt.1705712. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez J, Conde E, Gutierrez A, et al. The adjusted International Prognostic Index and beta-2-microglobulin predict the outcome after autologous stem cell transplantation in relapsing/refractory peripheral T-cell lymphoma. Haematologica. 2007;92:1067–1074. doi: 10.3324/haematol.11173. [DOI] [PubMed] [Google Scholar]
- 5.Corradini P, Tarella C, Zallio F, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–1538. doi: 10.1038/sj.leu.2404306. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez J, Conde E, Gutierrez A, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from The Gel-Tamo Study Group. Eur J Haematol. 2007;79:32–38. doi: 10.1111/j.1600-0609.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 8.Negrin RS, Kusnierz-Glaz CR, Still BJ, et al. Transplantation of enriched and purged peripheral blood progenitor cells from a single apheresis product in patients with non-Hodgkin's lymphoma. Blood. 1995;85:3334–3341. [PubMed] [Google Scholar]
- 9.Dreger P, Viehmann K, von Neuhoff N, et al. Autografting of highly purified peripheral blood progenitor cells following myeloablative therapy in patients with lymphoma: a prospective study of the long-term effects on tumor eradication, reconstitution of hematopoiesis and immune recovery. Bone Marrow Transplant. 1999;24:153–161. doi: 10.1038/sj.bmt.1701862. [DOI] [PubMed] [Google Scholar]
- 10.Law LY, Horning SJ, Wong RM, et al. High-dose carmustine, etoposide, and cyclophosphamide followed by allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2006;12:703–711. doi: 10.1016/j.bbmt.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Nademanee AP, Zain JM, Palmer J, et al. The impact of disease status on the outcome of high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) for peripheral T-cell lymphoma (PTCL) Blood. 2006;108:a3060. [Google Scholar]
- 12.Jagasia M, Morgan D, Goodman S, et al. Histology impacts the outcome of peripheral T-cell lymphomas after high dose chemotherapy and stem cell transplant. Leuk Lymphoma. 2004;45:2261–2267. doi: 10.1080/10428190412331272749. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez J, Conde E, Gutierrez A, et al. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: the Spanish Lymphoma and Autologous Transplantation Group experience. Ann Oncol. 2007;18:652–657. doi: 10.1093/annonc/mdl466. [DOI] [PubMed] [Google Scholar]
- 14.Yang D-H, Kim WS, Kim SJ, et al. The clinical outcomes of autologous stem cell transplantation in peripheral T cell lymphoma: from Retrospective Multicenter Study in Korea. Blood. 2007;110:a1892. [Google Scholar]
- 15.Reimer P, Schertlin T, Rudiger T, et al. Myeloablative radiochemotherapy followed by autologous peripheral blood stem cell transplantation as first-line therapy in peripheral T-cell lymphomas: first results of a prospective multicenter study. Hematol J. 2004;5:304–311. doi: 10.1038/sj.thj.6200359. [DOI] [PubMed] [Google Scholar]
- 16.Blystad AK, Enblad G, Kvaloy S, et al. High-dose therapy with autologous stem cell transplantation in patients with peripheral T cell lymphomas. Bone Marrow Transplant. 2001;27:711–716. doi: 10.1038/sj.bmt.1702867. [DOI] [PubMed] [Google Scholar]
- 17.Feyler S, Prince HM, Pearce R, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant. 2007;40:443–450. doi: 10.1038/sj.bmt.1705752. [DOI] [PubMed] [Google Scholar]
- 18.Song KW, Mollee P, Keating A, Crump M. Autologous stem cell transplant for relapsed and refractory peripheral T-cell lymphoma: variable outcome according to pathological subtype. Br J Haematol. 2003;120:978–985. doi: 10.1046/j.1365-2141.2003.04203.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim MK, Kim S, Lee SS, et al. High-dose chemotherapy and autologous stem cell transplantation for peripheral T-cell lymphoma: complete response at transplant predicts survival. Ann Hematol. 2007;86:435–442. doi: 10.1007/s00277-007-0254-1. [DOI] [PubMed] [Google Scholar]
- 20.Advani R, Hymes K, Pohlman B, et al. Belinostat (PXD101) in patients with recurrent or refractory peripheral or cutaneous T-cell lymphoma: results of a phase II study. Blood. 2007;110:a3453. [Google Scholar]
- 21.Dang NH, Pro B, Hagemeister FB, et al. Phase II trial of denileukin diftitox for relapsed/refractory T-cell non-Hodgkin lymphoma. Br J Haematol. 2007;136:439–447. doi: 10.1111/j.1365-2141.2006.06457.x. [DOI] [PubMed] [Google Scholar]
- 22.Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor OA, Hamlin PA, Gerecitano J, et al. Pralatrexate (PDX) produces durable complete remissions in patients with chemotherapy resistant precursor and peripheral T-cell lymphomas: results of the MSKCC Phase I/II experience. Blood. 2006;108:a400. [Google Scholar]
- 24.Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes de l'Adulte (GELA) Blood. 1998;92:76–82. [PubMed] [Google Scholar]
- 25.Went P, Agostinelli C, Gallamini A, et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24:2472–2479. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]
- 26.Corradini P, Dodero A, Zallio F, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22:2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 27.Coleman CN, Picozzi VJ, Jr., Cox RS, et al. Treatment of lymphoblastic lymphoma in adults. J Clin Oncol. 1986;4:1628–1637. doi: 10.1200/JCO.1986.4.11.1628. [DOI] [PubMed] [Google Scholar]