Summary
To develop a more effective vaccination method against H5N1 virus, we investigated the immunogenicity and protective efficacy after skin vaccination using microneedles coated with influenza virus-like particles containing hemagglutinin derived from A/Vietnam/1203/04 H5N1 virus (H5 VLPs). A single microneedle vaccination of mice with H5 VLPs induced increased levels of antibodies and provided complete protection against lethal challenge without apparent disease symptoms. In contrast, intramuscular injection with the same vaccine dose showed low levels of antibodies and provided only partial protection accompanied by severe body weight loss. Post-challenge analysis suggested that improved protection was associated with lower lung viral titers and enhanced generation of recall antibody secreting cells by microneedle vaccination. Thus, this study provides evidence that skin delivery of H5 VLP vaccines using microneedles designed for self-administration induces improved protection compared to conventional intramuscular immunization.
Keywords: H5N1, pandemic vaccine, single dose, skin vaccination, microneedles
Avian H5N1 influenza viruses cause sporadic zoonotic infections to humans with high fatality rates of 60% (Sims et al., 2005; Webster et al., 2005). Furthermore, the pandemic potential of these viruses poses a serious threat to public health. The influenza pandemic caused by the 2009 H1N1 virus provided an opportunity to examine the performance of current vaccination. The available evidence indicates that the second wave of infection spread through the US population in the early Fall of 2009, before the vaccine became available to the majority of targeted high-risk population groups (Litchfield, 2009; Loeb et al., 2010). This experience indicated that development of new and faster methods of vaccine manufacturing and immunization should be a priority.
The skin has been suggested as an attractive site for immunization due to the presence of potent antigen-presenting cells such as Langerhans and dermal dendritic cells (Glenn and Kenney, 2006; Hammond et al., 2001). To improve protective efficacy while reducing the antigen mass by targeting influenza antigens to the skin, intradermal (ID) immunization has been evaluated in clinical trials (Auewarakul et al., 2007; Belshe et al., 2004; Kenney et al., 2004; Khanlou et al., 2006; Van Damme et al., 2009). However, the conventional ID injection procedure requires highly trained medical personnel and is not well tolerated by vaccinees due to pain and discomfort at the site of injection (Auewarakul et al., 2007; Belshe et al., 2004; Kenney et al., 2004). Recent studies have demonstrated a promising alternative method that delivers inactivated whole-virion vaccines to the skin using microneedles, penetrating the outer layer of the skin (Kim et al., 2010; Kim et al., 2009; Quan et al., 2009; Zhu et al., 2009). This simple design could permit self-administration of vaccine by patients, possibly enabling vaccination campaigns to rapidly reach large populations (Prausnitz et al., 2009).
Conventional inactivated vaccines are produced from virus propagation in eggs. A new vaccine platform, virus-like particles (VLPs) produced in cell culture, has been shown to confer protection against highly pathogenic avian-origin influenza viruses in animal models, and can be manufactured without handling pathogenic live viruses (Bright et al., 2008; Haynes et al., 2009; Kang et al., 2009). In the present study, we investigated the immunogenicity and protective efficacy after a single vaccination using microneedles coated with dried H5 VLPs, in comparison with conventional intramuscular injection.
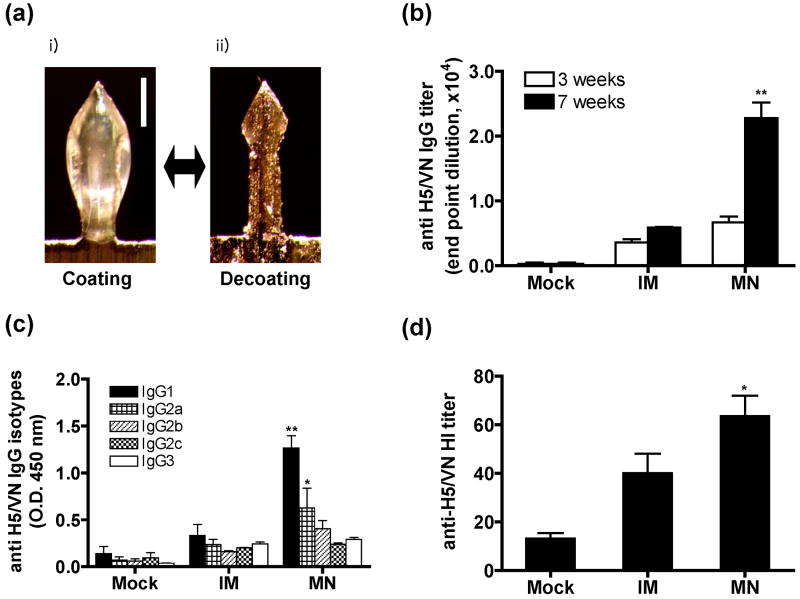
H5 VLPs derived from influenza A/Vietnam/1203/04 (A/VN/04) virus were produced in insect cells using recombinant baculovirus expression as previously described (Kang et al., 2009). Stainless steel microneedles were fabricated as arrays of 5 needles (Kim et al., 2010). The 700 μm length of microneedles used in this study is suitable for effective delivery of vaccine into mouse skin with a thickness of 500-600 μm (Azzi et al., 2005), because the whole microneedle is not fully inserted into the skin due to skin deformation during insertion. For vaccination in the skin, microneedles were coated on their surfaces with H5 VLPs in coating solution (1% carboxymethylcellulose (CMC) sodium salt as viscosity enhancer, 0.5% (w/v) Lutrol F-68 NF as surfactant, and 15% trehalose as stabilizer) and then air dried (Kim et al., 2010). A change in thickness of the microneedle was observed by bright field microscopy after coating with H5 VLPs and dissolution of coated H5 VLPs into PBS buffer (Fig. 1a). The amount of H5 VLPs coated onto each 5-microneedle array was 2.0±0.15 μg total proteins (approximately 0.2 μg HA) as determined after elution into PBS using a protein assay kit (Quan et al., 2009). Groups of mice (BALB/c, 6-8 weeks old, n=11 per group) were immunized using either i) microneedles without antigen (mock), ii) microneedles coated with 2 μg of H5 VLPs (MN), or iii) 2 μg of H5 VLPs in PBS buffer solution dissolved from coated microneedles given by intramuscular injection (IM). At weeks 3 and 7 after a single dose vaccination, antibody responses in sera were determined by quantitative ELISA using recombinant H5 HA protein as a coating antigen (Fig. 1b). Interestingly, at week 7 after a single immunization, 5-fold higher levels of H5 HA specific antibodies were observed in the microneedle vaccination group compared to the intramuscular control, which is significantly higher than those at week 3 (Fig. 1b).
Fig. 1. Immunogenicity of influenza H5 VLP vaccination in the skin using microneedles.
(a) A microneedle coated with H5 VLPs before and after dissolution in PBS. A microneedle is shown as observed by bright field microscopy after coating with influenza H5 VLPs for skin vaccination (i) and after dissolution of the coating from the microneedle in PBS (ii). Bar=250 μm. (b) H5 HA-specific IgG titers at weeks 3 and 7 after a single vaccination. Titers of antibodies specific to inactivated H5 A/Vietnam/1203/04 virus are expressed as the highest dilution of sera having a value of optical density at 450 nm (OD450) greater than the mean plus 2 standard deviations of similarly diluted naïve sera as described previously (Quan et al., 2007). (c) H5 HA-specific isotype antibodies as presented in OD450 values of 100× diluted sera at week 7. BALB/c mice (n=11 per group) were immunized once with H5 VLPs using microneedles (MN), intramuscular injection (IM), or uncoated placebo microneedles (Mock). Bars indicate means ± S.E.M. (d) Hemagglutination inhibition (HI) titers determined at week 7 after vaccination. Asterisk indicates significance between MN and IM groups (**; p<0.01, *; p<0.05, Student's 2-tailed t-test).
The pattern of antibody isotypes may provide informative insight into the T helper type 1 or 2 immune responses (Hocart et al., 1989). Therefore, we determined antibody isotypes after a single microneedle or intramuscular vaccination. IgG1 isotype antibody was induced at significantly higher levels than IgG2a antibody after microneedle delivery (p=0.031, Fig. 1c). This IgG1 isotype-dominant pattern following H5 VLP microneedle immunization is similar to that observed with microneedle vaccination using whole inactivated A/Aichi/68 virus (H3N2) (Koutsonanos et al., 2009), but different from results obtained from 0.4 μg low vaccine dose of the A/PR/8/34 (H1N1) virus or VLPs (Quan et al., 2010; Quan et al., 2009), which predominantly induced IgG2a isotype antibody after microneedle vaccination of BALB/c mice. Interestingly, the IgG1 isotype antibody was previously shown to have higher neutralizing and similar HI titers, respectively, compared to the IgG2a isotype in immune sera of BALB/c mice (Hocart et al., 1989). Although it is not clear what factor(s) influences IgG1/IgG2a ratios, there are several potential parameters affecting the antibody isotype pattern, which includes mouse strains and types of vaccines (Hocart et al., 1989), stability and integrity of vaccine antigens (Quan et al., 2010; Quan et al., 2009), routes and doses of vaccines (Bright et al., 2008), and intrinsic immunogenicity properties of vaccines such as H5 VLPs of A/Vietnam/1203/04 observed in this study.
Next, we determined hemagglutination inhibition (HI) antibody responses using 1% horse red blood erythrocyte (Fig. 1d). HI titers over 60 were detected in the microneedle vaccination group, which is 4-fold higher than the titers in the mock control and 1.5 fold higher than the IM group (p=0.03). As high doses of H5 vaccine were reported to be required in humans for moderate immunogenicity, this observation suggests that a MN H5 vaccine could bring significant improvement compared to other non-adjuvanted vaccines tested thus far in clinical trials. The low antibody response reported in a study by Patel et al. is likely related to the small doses of antigen injected intradermally (Patel et al., 2010). In other clinical trials indicating low immunogenicity of H5 vaccines, prime-boost immunizations with 90 μg HA doses of baculovirus-expressed or 30 μg of inactivated subunit vaccines produced in eggs were needed to induce antibody responses that were expected to be protective in 54 to 58% of individuals vaccinated (Nicholson et al., 2001; Treanor et al., 2006; Treanor et al., 2001).
For challenge studies to determine protective efficacy against lethal infection at 20 weeks after a single vaccination, mice (n=5 out of 11 vaccinated mice) were inoculated with 20×LD50 of wild type A/Vietnam/1203/04 (H5N1) by intranasal instillation. All mice in the mock control group showed severe losses in weight and reached the euthanasia endpoint between 6 and 7 days after challenge (Fig. 2a). Mice in the IM group also experienced severe weight losses and showed a significant delay in recovering body weight after lethal challenge infection (Fig. 2b). In contrast, the H5 VLP microneedle group (MN) did not lose body weight and showed 100% protection compared to partial protection (60%) by IM immunization. Therefore, microneedle vaccination in the skin with H5 VLPs induced enhanced protective efficacy compared to intramuscular injection.
Fig. 2. Protection by a single-dose microneedle vaccination with H5 VLPs.
Groups of BALB/c mice that had been vaccinated (Mock, IM, MN) were intranasally inoculated with a lethal dose (20×LD50) of wild type A/Vietnam/1203/04 (H5N1) virus at week 20 post vaccination. (a) Daily mean body weight (n=5 out of 11 vaccinated mice). Significant morbidity indicated by greater than 15% weight loss as well as ruffling fur and inactivity between days 6-10 post challenge was observed in the IM group. In contrast, the MN group had no weight loss. (b) Daily proportion of mice alive (n=5 out of 11 vaccinated mice).
To better characterize the protection afforded by microneedle vaccination with H5 VLPs, lungs were harvested at week 20 post vaccination from additional groups of mice (n=6 out of 11 vaccinated mice) euthanized at day 4 post lethal challenge with reassortant A/Vietnam/1203/04 (H5N1) virus (20×LD50), homogenized and titrated by plaque assay (Fig. 3a) as described previously (Quan et al., 2007). The reassortant A/Vietnam/1203/04 virus was generated to contain HA and NA genes from A/Vietnam/1203/04 (H5N1) and internal genes from A/PR/8/34 as described previously (Bright et al., 2008; Hoffmann et al., 2002). The H5 VLP microneedle vaccination group showed lung viral titers that were 20-fold lower than the mock control and 6-fold lower than the intramuscular immunization group. Thus, these results suggest that the improved protection by microneedle vaccination with H5 VLPs was partially due to more effective early control of viral replication in the lungs.
Fig. 3. Lung viral titers and virus-specific antibody-secreting cell responses.
Six out of eleven vaccinated mice (Mock, IM, MN) were sacrificed for analysis of lung viral titers and antibody secreting cell responses at day 4 post challenge as described (Quan et al., 2007). (a) Lung viral titers were determined by a plaque assay with lung homogenate obtained from 6 individual mice. (b) Recall antibody secreting cell responses of spleen cells. Antibody levels were presented as concentrations in culture supernatants (expressed in ng/ml). Values are means ± S.E.M. of individual mice (n=6) after in vitro culture. Asterisk indicates significance between MN and IM groups (*; p<0.05, **; p<0.01, Student's 2-tailed t-test).
One of the main goals of vaccination is to induce long-lasting immune memory that mediates protection upon exposure to infectious agents. We tested whether a single microneedle vaccination in the skin with H5 VLPs could induce increased levels of memory B cells that are rapidly differentiating into antibody secreting plasma cells upon viral infection (Fig. 3b). Spleen cells were collected from the same groups of mice at day 4 after challenge as described for assay of lung viral titers. Single cell suspensions from spleens were subjected to in vitro culture with inactivated H5N1 A/Vietnam/1203/04 viral antigen coated on the plates. Importantly, with antigenic stimulation by coated viral antigen on the plates, spleen cells collected from the group of mice that were immunized in the skin using microneedles showed significantly higher levels of virus-specific antibodies secreted into culture supernatants compared to those by intramuscular immunization after 6 days but not after 2 days, of culture (Fig. 3b). Therefore, these results suggest that a single microneedle vaccination with H5 VLPs is more effective in generating memory B cells that can differentiate into antibody-secreting cells compared to conventional intramuscular immunization. Regarding the cytokine-secreting splenocyte responses, intramuscular immunization with H5 VLPs showed higher IL-4 and similar IFN-γ levels of secreting cells, respectively, compared to those obtained after microneedle vaccination (data not shown), indicating that IL-4 and IFN-γ secreting cellular responses have less correlation with improved protection by microneedle vaccination in the skin with H5 VLPs.
In conclusion, this study demonstrates that a single vaccination in the skin using microneedles coated with dried H5 VLP vaccine induces improved protective immunity against highly pathogenic H5N1 virus as compared to intramuscular injection, and induces IgG1 isotype predominant antibody responses that inhibit hemagglutination. The results from this study provide evidence that microneedle vaccination with H5 VLPs in the skin is a promising vaccine delivery method. Thus, microneedle skin vaccination with H5 VLPs enables improved protection against influenza virus with pandemic potential and offers the potential for self-administration, which could dramatically expedite vaccination delivery.
Acknowledgments
This work was supported in part by NIH/NIBIB grant EB006369 (M.R.P.), NIH/NIAID grant AI0680003 and AI074579 (R.W.C.), the Georgia Research Alliance (S.M.K) and the Korea Research Foundation Grant KRF-2007-357-C00088 (J.M.S). The authors thank Dr. Robert G. Webster for providing the eight-plasmid system for generating reassortant virus. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
Footnotes
Potential conflict of interest: M.R.P. is a consultant and inventor on patents licensed to companies with an interest in microneedles. The associated conflict of interest is being managed by Georgia Tech and Emory University. R.W.C., S. M.K., and Emory University have equity in Zetra Biologicals which is developing VLP technology under license from Emory University. The VLP system reported here is different from VLP vaccine products under development and the information in this manuscript is not directly related to those products.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25:659–663. doi: 10.1016/j.vaccine.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Azzi L, El-Alfy M, Martel C, Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 2005;124:22–27. doi: 10.1111/j.0022-202X.2004.23545.x. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, Kumar NM, Pushko P, Smith G, Tumpey TM, Ross TM. Cross-Clade Protective Immune Responses to Influenza Viruses with H5N1 HA and NA Elicited by an Influenza Virus-Like Particle. PLoS ONE. 2008;3:e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–268. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- Hammond SA, Guebre-Xabier M, Yu J, Glenn GM. Transcutaneous immunization: an emerging route of immunization and potent immunostimulation strategy. Crit Rev Ther Drug Carrier Syst. 2001;18:503–526. [PubMed] [Google Scholar]
- Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, Harmsen AG, Richardson C. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2009;27:530–541. doi: 10.1016/j.vaccine.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Hocart MJ, Mackenzie JS, Stewart GA. The immunoglobulin G subclass responses of mice to influenza A virus: the effect of mouse strain, and the neutralizing abilities of individual protein A-purified subclass antibodies. J Gen Virol. 1989;70(Pt 9):2439–2448. doi: 10.1099/0022-1317-70-9-2439. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- Kang SM, Yoo DG, Lipatov AS, Song JM, Davis CT, Quan FS, Chen LM, Donis RO, Compans RW. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS ONE. 2009;4:e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- Khanlou H, Sanchez S, Babaie M, Chien C, Hamwi G, Ricaurte JC, Stein T, Bhatti L, Denouden P, Farthing C. The safety and efficacy of dose-sparing intradermal administration of influenza vaccine in human immunodeficiency virus-positive patients. Arch Intern Med. 2006;166:1417. doi: 10.1001/archinte.166.13.1417. [DOI] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27:6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield SM. Summary recommendations by the Advisory Committee on Immunization Practices (ACIP) for the use of H1N1 influenza vaccine for the 2009-2010 vaccination season. AAOHN J. 2009;57:354. doi: 10.3928/08910162-20090826-02. [DOI] [PubMed] [Google Scholar]
- Loeb M, Earn DJ, Smieja M, Webby R. Pandemic (H1N1) 2009 risk for nurses after trivalent vaccination. Emerg Infect Dis. 2010;16:719–720. doi: 10.3201/eid1604.091588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- Patel SM, Atmar RL, El Sahly HM, Cate TR, Keitel WA. A phase I evaluation of inactivated influenza A/H5N1 vaccine administered by the intradermal or the intramuscular route. Vaccine. 2010;28:3025–3029. doi: 10.1016/j.vaccine.2009.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84:7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims LD, Domenech J, Benigno C, Kahn S, Kamata A, Lubroth J, Martin V, Roeder P. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet Rec. 2005;157:159–164. doi: 10.1136/vr.157.6.159. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O'Brien D, Wolff M, Rabinovich G, Blackwelder W, Katz JM. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Webster RG, Guan Y, Poon L, Krauss S, Webby R, Govorkovai E, Peiris M. The spread of the H5N1 bird flu epidemic in Asia in 2004. Arch Virol Suppl. 2005:117–129. doi: 10.1007/3-211-29981-5_10. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang C, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]