Abstract
Objective
The association between hypertension in pregnancy and future cardiovascular disease (CVD) increasingly is recognized. We aimed to assess the role of hypertension in pregnancy as an independent risk factor for hypertension, coronary heart disease (CHD), and stroke later in life.
Methods
Women who participated in the Phase 2 (2000–2004) Family Blood Pressure Program study (n = 4782) were categorized into women with no history of pregnancy lasting more than 6 months (n = 718), women with no history of hypertension in pregnancy (n = 3421), and women with a history of hypertension in at least one pregnancy (n = 643). We used Kaplan–Meier and Cox proportional hazard models to estimate and contrast the risks of subsequent diagnoses of hypertension, CHD, and stroke among the groups.
Results
Women with a history of hypertension in pregnancy, compared with those without such a history, were at increased risks for the subsequent diagnoses of hypertension (50% hypertensive at the age 53 vs. 60, P < 0.001), CHD (14% estimated event rate vs. 11%, P = 0.009), and stroke (12% estimated event rate vs. 5%, P < 0.001). The increased risk for subsequent hypertension remained significant after controlling for race, family history of CVD, smoking, dyslipidemia, and diabetes mellitus, with an adjusted hazard ratio of 1.88 [95% confidence interval (CI) 1.49–2.39, P < 0.001]. After controlling for traditional risk factors, including subsequent hypertension, the increased risk for stroke remained statistically significant (hazard ratio 2.10, 95% CI 1.19–3.71, P = 0.01), but not for CHD.
Conclusion
Hypertension in pregnancy may be an independent risk factor for subsequent diagnoses of hypertension and stroke. J Hypertens 28:826–833
Keywords: cardiovascular disease, hypertension, pregnancy, risk factors, stroke
Introduction
Hypertension, defined by a blood pressure (BP) of 140/90 mmHg or more, affects up to 8% of pregnancies and includes a spectrum of conditions [1], namely, preeclampsia–eclampsia, preeclampsia superimposed on chronic hypertension, chronic hypertension, and gestational hypertension. Unlike other hypertensive disorders in pregnancy, preeclampsia is a multisystem disease, its distinctive feature being the sudden onset of proteinuria (≥300 mg/24-h urine). Eclampsia is a convulsive form of preeclampsia that affects 0.1% of all pregnancies. Chronic hypertension is diagnosed with BP readings equal to or greater than 140/90 mmHg prior to pregnancy or before the 20th week of gestation. It represents a major risk factor for preeclampsia, which affects 25% of these women, in contrast to 5% of women without preexisting hypertension and otherwise normal pregnancies. Gestational hypertension refers to hypertension occurring for the first time during the second half of pregnancy in the absence of proteinuria. It includes women with preeclampsia who have not yet developed proteinuria, those with hypertension only, and a subset of patients in whom BP remains elevated after delivery, leading to the diagnosis of chronic hypertension. Although the first two forms of gestational hypertension typically abate with the termination of pregnancy, the third form may lead to chronic hypertension, which is diagnosed when gestational hypertension persists beyond 12 weeks postpartum.
Hypertension in pregnancy has long been recognized as a risk factor for cerebrovascular, cardiac, and renal complications occurring during pregnancy and immediately postpartum. However, studies in the 1970s and 1980s have argued that hypertension that resolves after delivery, as in preeclampsia, does not herald hypertension later in life [2]. Subsequent studies [3], both prospective [4] and retrospective [5], have reported associations of hypertension in pregnancy with hypertension later in life, as well as with increased risks for future cardiac [6,7] and cerebrovascular events [7,8]. The results of published studies were recently summarized both in a systematic review [9] and a meta-analysis [10]. These studies, for the most part, did not control for traditional cardiovascular risk factors, were registry-based, reported a limited number of outcomes (such as cardiovascular deaths), and did not assess the impact of hypertension in pregnancy on age of onset of the events, all of which may be clinically useful when individualizing risk profiles and intervention strategies. Our principal aim was to study the associations of hypertension in pregnancy with the subsequent diagnoses of hypertension unrelated to pregnancy, coronary heart disease (CHD), and stroke in a large, multiracial cohort of women who participated in the Family Blood Pressure Program (FBPP) study. To test the hypothesis that hypertension in pregnancy is an independent risk factor for stroke and CHD later in life, we controlled for traditional risk factors including smoking, family history, dyslipidemia, diabetes mellitus, and hypertension diagnosed later in life.
Methods
Participants
This study included 4782 women from 2443 sibships participating in the FBPP study. FBPP was established in 1995 to investigate the genetics of hypertension in non-Hispanic blacks, Hispanic whites, Asians, and non-Hispanic whites [11]. The FBPP consists of four different research networks, all ascertaining families having individuals with elevated BPs or a genetic predisposition to hypertension: GenNet, Genetic Epidemiology Network of Arteriopathy (GENOA), Hypertension Genetic Epidemiology Network (HyperGEN), and Stanford Asian Pacific Program in Hypertension and Insulin Resistance (SAPPHIRe). The specific recruitment strategy for each network has been described previously [11].
For the Phase 1 (1996–2000) study examination, a questionnaire was developed to obtain participants’ personal and family medical histories, including their use of prescription medications; histories of menopause and hormone replacement were obtained from female participants. Questions regarding pregnancy and hypertension in pregnancy (as described below) were only added to the questionnaires administered during the Phase 2 study visits (2000–2004). Therefore, only the Phase 2 questionnaire data were used in the analyses.
Study visits
All individuals who participated in the FBPP gave informed consent; the Institutional Review Board at each clinic site approved all protocols. Questionnaires were administered in personal interviews by trained examiners. Although each network used slightly different definitions for recruitment, the pooled data set used the standardized definitions of hypertension. In the Phase 2 study visit, the diagnosis of hypertension was confirmed if a prior diagnosis of hypertension and use of prescription antihypertensive medication had been reported or if the average systolic or diastolic BPs were at least 140 or at least 90 mmHg, respectively. The diagnosis of CHD was established by self-reports of a previous history of myocardial infarction, coronary bypass surgery, coronary angioplasty, balloon dilatation or stent placement, whereas the diagnosis of cerebrovascular disease was based on self-reports of stroke and/or cerebral hemorrhage. Diabetes mellitus was self-reported, whereas ‘ever’ smoking was defined as having smoked more than 100 cigarettes in the past. Use of prescription medications in the previous month was recorded.
All participants underwent standardized physical examination and blood tests. The diagnosis of dyslipidemia was confirmed if one or more of the following criteria were met: use of lipid-lowering drugs or laboratory measurements at the Phase 2 examination (methods described below) revealing a total cholesterol 200 mg/dl or more, triglycerides 150 mg/dl or more, or high-density lipoprotein (HDL) 40 mg/dl or less.
Systolic and diastolic BPs were measured using an automated oscillometric BP-measurement device with a consistent protocol across networks. Height was measured while participants were standing without shoes, with heels together, against a vertically mounted ruler, and weight was measured on a balance. BMI was calculated as weight (kg)/height (m)2.
Pregnancy questionnaire
The standard questionnaire, which was previously validated [12], was used for all networks. Female participants were asked ‘Have you had at least one pregnancy that lasted more than 6 months?’ Women who responded affirmatively were asked to report the number of pregnancies and to answer whether or not they had developed hypertension during any of the pregnancies that have lasted more than 6 months. If they confirmed a history of hypertension in pregnancy, they were asked whether it occurred only in the first pregnancy; in the first pregnancy and at least one subsequent pregnancy; or only in subsequent pregnancies. Preeclampsia was defined either by self-report of this condition or by self-report of protein in the urine during the pregnancy with hypertension.
Laboratory methods
Blood was drawn by venipuncture after an overnight fast of at least 8 h. Serum glucose, total cholesterol, HDL-cholesterol, and triglyceride concentrations were measured by standard methods on a Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Indianapolis, Indiana, USA). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation when triglycerides were less than 400 mg/dl [13].
Statistical analysis
Because participants were selected for being either hypertensive or members of hypertensive sibships, logistic regression for presence or absence of hypertension later in life was deemed inappropriate. Instead, survival analysis methods using age at onset of each outcome as the time variables were used. Kaplan–Meier curves were constructed to compare the unadjusted probabilities for being free of hypertension diagnosed after age 40 years, CHD, or stroke among: nulliparous women, that is, those who had no pregnancy lasting more than 6 months; women with no history of hypertension during any pregnancy; women with a history of hypertension in pregnancy; and women with a history of preeclampsia. Age of 40 years for hypertension was chosen for the following reason. We aimed to correlate hypertension in pregnancy with the risk for hypertension later in life. Therefore, we wanted to exclude women with chronic hypertension that had occurred before pregnancy and then persisted after delivery. To accomplish this, the hazard ratio for diagnosis of hypertension was assessed only for hypertension that was diagnosed after the age of 40, as most pregnancies are likely to occur by that age. The participant’s reported age at diagnosis, or current age (in the case of hypertension diagnosed at the study visit), was used as the event time. Participants not meeting the diagnostic criteria for hypertension were considered censored free of hypertension as of their age at the study visit. Similarly, participants reporting CHD and stroke were considered as having had these events at the age they reported, or were censored, that is, considered to be free of these events, as of their current age.
Cox proportional hazard models were used to estimate the adjusted risks and hazard ratios with respective 95% confidence intervals (CIs) for age at diagnosis of hypertension, CHD, and stroke. The models were fit using custom software in order to account for the potential correlation of outcomes between women within sibships, that is, sisters [14]. We introduced two indicator variables calculated for all participants: one for the presence of at least one pregnancy lasting 6 months and a second for the presence of hypertension in at least one such pregnancy. When both indicator variables are included, the resulting coefficients can be interpreted as the contrast between ‘no pregnancy’ and ‘normotensive pregnancy’ and between ‘normotensive pregnancy’ and ‘hypertensive pregnancy’, respectively. Factors certain or likely to be present throughout adulthood (e.g., race, education) were considered as adjustment variables in all of the following models. Model A, used to model the diagnosis of hypertension after age 40 years, included race, network, family history of cardiovascular disease (CVD), education, diabetes (time-dependent), smoking status, and BMI as adjustment variables. Model B, used to model the diagnosis of CHD and stroke, included Model A variables and the diagnosis of hypertension (time-dependent). Neither Model A nor B, both of which were fit in the pooled FBPP sample, included dyslipidemia as an adjustment variable, because use of lipid-lowering drugs was not available for analyses in the pooled FBPP data set. Hence, two additional models (Model C and Model D) were fit only in a subset of GENOA participants for whom use of lipid-lowering drugs was available for analyses. They constituted 37% of the pooled FBPP sample, that is, 1754 of 4782 participants. Model C, used to model the diagnosis of hypertension after age 40 years, adjusted for dyslipidemia, in addition to race, family history, smoking, and diabetes mellitus (time-dependent). Model D, used to model the diagnosis of CHD and stroke, adjusted for Model C variables plus the diagnosis of hypertension (time-dependent), thus controlling for all traditional risk factors when comparing cardiovascular event rates between pregnancy groups.
Results
Sample description and characteristics
A total of 4782 women from 2443 sibships participated in the second FBPP study visit between 2000 and 2004. Demographic characteristics measured at the second FBPP visit are shown by network in Table 1. At least one pregnancy that lasted more than 6 months was reported by 4064 women (85%), overall. Of these women, 643 (13%) reported hypertension in at least one of their pregnancies. Among those, 209 (4.4% of the whole cohort) reported a history of preeclampsia. The percentages of women reporting hypertension in pregnancy did not differ significantly among non-Hispanic blacks (232, 18.95%), non-Hispanic whites (201, 17.14%), and Hispanic whites (162, 15.10%); however, the percentage was significantly lower in Asians (48, 8.08%; P <0.001). Education had no significant effect on the percentage of women reporting hypertension in pregnancy, that is, the percentage did not differ significantly between those who did or did not complete high school. As major differences were present across the networks, all subsequent analyses were adjusted for age, network, education, and race.
Table 1.
Family Blood Pressure Program demographics by network (n = 4782)
| Variablea | GenNet (N = 1073) | GENOA (N = 2501) | HyperGEN (N = 449) | SAPPHIRe (N = 759) |
|---|---|---|---|---|
| Age at clinic visit [median (Q1–Q3)] | 40.0 (28.0–50.0) | 61.0 (53.0–67.0) | 38.0 (31.0–43.0) | 57.0 (51.0–61.0) |
| Race [n (%)] | ||||
| Non-Hispanic white | 412 (38) | 704 (28) | 280 (62) | 0 (0) |
| Hispanic | 521 (49) | 747 (30) | 0 (0) | 0 (0) |
| Non-Hispanic black | 140 (13) | 1050 (42) | 169 (38) | 0 (0) |
| Japanese | 0 (0) | 0 (0) | 0 (0) | 759 (100) |
| High school education or higher [n (%)] | 662b (62) | 1607 (64) | 430 (96) | 751 (99) |
| Pregnancy status [n (%)] | ||||
| Nulliparous [718 (15)] | 235 (22) | 220 (9) | 98 (22) | 165 (22) |
| No history of hypertension in pregnancy [3421 (72)] | 715 (67) | 1870 (75) | 290 (65) | 546 (72) |
| History of hypertension in pregnancy [643 (13)] | 123 (11) | 411 (16) | 61 (14) | 48 (6) |
GENOA, Genetic Epidemiology Network of Arteriopathy; HyperGEN, Hypertension Genetic Epidemiology Network; SAPPHIRe, Stanford Asian Pacific Program in Hypertension and Insulin Resistance.
All variables are expressed as percentages, with the exception of age, which was expressed as median age in years (interquartile range).
Three patients from GenNet were missing education information.
We also compared traditional risk factors measured at the time of the Phase 2 FBPP examination among the pregnancy groups, after controlling for differences in age, network, and race (Table 2). Compared with women with histories of normotensive pregnancies, nulliparous women had significantly lower BMIs, a higher prevalence of a current diagnosis of diabetes mellitus and hypertension, and a lower prevalence of positive family histories of hypertension and CHD.
Table 2.
Family Blood Pressure Program risk factor data by pregnancy group
| Variablea | Nulliparous (N = 718) | Normotensive pregnancy (N = 3421) | Hypertensive pregnancy (N = 643) | Normotensive vs. nulliparous P value | Hypertensive vs. normotensive P value |
|---|---|---|---|---|---|
| History of smokingb | 24.7 | 31.5 | 29.6 | <0.001 | 0.336 |
| BMIc | 27.94 | 28.50 | 31.19 | – | – |
| Log BMId | 3.33 ± 0.25 | 3.35± 0.20 | 3.44± 0.21 | 0.028 | <0.001 |
| Diabetese | 21.1 | 18.0 | 25.5 | 0.037 | <0.001 |
| Dyslipidemiaf | 81.9 | 77.0 | 72.0 | 0.193 | 0.069 |
| Family history of stroke | 23.0 | 24.7 | 29.6 | 0.281 | 0.004 |
| Family history of CHD | 30.2 | 33.7 | 38.6 | 0.041 | 0.004 |
| Current HTNg | 49.3 | 39.8 | 57.6 | <0.001 | <0.001 |
| Family history of HTN | 67.8 | 72.3 | 79.8 | 0.018 | <0.001 |
CHD, coronary heart disease; GENOA, Genetic Epidemiology Network of Arteriopathy; HTN, hypertension.
All variables were adjusted for age, network, and race and expressed as percentages, with the exception of BMI, which was expressed as a mean. P values are calculated from a generalized linear regression model using generalized estimating equations to account for sibling relationships.
Two normotensive patients missing smoking history.
Two nulliparous, 11 normotensive, and one hypertensive patient missing BMI data.
Due to skewness of distribution, log BMI (mean ± SD) was used for comparison between the groups.
Two nulliparous and five normotensive patients missing diabetes data.
GENOA participants only, N = 149, 1303, and 293, respectively.
Two normotensive and two hypertensive patients missing current hypertension status.
Compared with women with histories of normotensive pregnancies, those with histories of hypertension in pregnancy had higher BMIs at the FBPP study visit and a higher prevalence of current diabetes mellitus, hypertension, and family histories of stroke, CHD, and hypertension.
Cardiovascular events
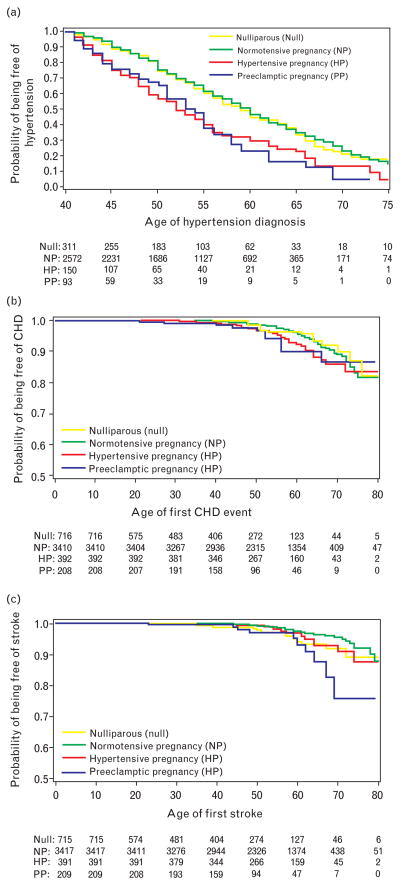
No significant differences in hypertension after the age of 40, CHD, and stroke were observed between nulliparous women and women with a history of normotensive pregnancies (P values of 0.95, 0.86, and 0.14 respectively) (Fig. 1). Women reporting hypertension in pregnancy compared with those with no history of hypertension in pregnancy had increased unadjusted risks for hypertension diagnosed after the age of 40 (50% hypertensive at the age 52 vs. 60, P <0.001), CHD (14% estimated event rate vs. 11% at 70 years, P = 0.049), and stroke (8% estimated event rate vs. 5% at 70 years, P = 0.009) (Fig. 1). Similarly, women reporting preeclampsia in pregnancy, compared with those with no history of hypertension in pregnancy, had increased unadjusted risks for hypertension diagnosed after the age of 40 (50% hypertensive at the age 54 vs. 60, P <0.001), CHD (14% estimated event rate vs. 11% at 70 years, P = 0.045), and stroke (24% estimated event rate vs. 5% at 70 years, P <0.001). No significant differences in hypertension after the age of 40, CHD, and stroke were observed between women with a history of hypertension and those with preeclampsia (P values of 0.82, 0.76, and 022 respectively) (Fig. 1). When we analyzed stroke and CHD as a combined endpoint, there was a trend for an elevated risk for women with a history of preeclampsia, although this was not statistically significant (P = 0.1). Women with a history of hypertension in pregnancy and those with a history of preeclampsia, when grouped together and compared with women with a history of normotensive pregnancies, were at increased risks for the subsequent diagnoses of hypertension (50% hypertensive at the age 53 vs. 60, P <0.001), CHD (14% estimated event rate vs. 11%, P = 0.009), and stroke (12% estimated event rate vs. 5%, P <0.001).
Fig. 1.
Kaplan–Meier plots of the cumulative probability of being free of hypertension (a), coronary heart disease (b), and stroke (c) as a function of age among nulliparous women, those with a history of normotensive pregnancies, and women with a history of either hypertensive or preeclamptic pregnancies. Numbers along the x-axis show the number of women at risk in each age group over time.
We used Cox proportional hazard models to model the age at diagnosis of hypertension (after age 40 years) and the occurrences of CHD and stroke in each pregnancy group. As the Kaplan–Meier curves showed no significant difference in cardiovascular outcomes between women with a history of hypertension in pregnancy and those with a history of preeclampsia (Fig. 1), these two subsets of women were grouped together under ‘hypertensive pregnancy’ for these analyses. The hazard ratios for each event type were contrasted in nulliparous women relative to women with normotensive pregnancies, and in women with a history of normotensive pregnancies relative to those with hypertensive pregnancies (Table 3), after adjusting for those potentially confounding variables that differed among pregnancy groups (Table 2).
Table 3.
Adjusted hazard ratios for hypertension after age 40, coronary heart disease, and stroke between pregnancy groupsa
| FBPP participants |
GENOA sub-sample |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension (after age 40) (n = 3093)b |
CHD (n = 4720)b |
Stroke (n = 4726)b |
Hypertension (after age 40) (n = 1288)b |
CHD (n = 1741)b |
Stroke (n = 1744)b |
|||||||||||||
| HRc | 95% CI | P | HRd | 95% CI | P | HRd | 95% CI | P | HRe | 95% CI | P | HRf | 95% CI | P | HRb | 95% CI | P | |
| Normotensive vs. nulliparous | 0.88 | 0.73–1.08 | 0.22 | 1.02 | 0.60–1.75 | 0.94 | 0.55 | 0.31–1.00 | 0.050 | 0.78 | 0.59–1.04 | 0.095 | 0.84 | 0.39–1.82 | 0.67 | 0.61 | 0.27–1.40 | 0.24 |
| Hypertensive vs. normotensive | 1.53 | 1.25–1.87 | <0.001 | 1.14 | 0.78–1.68 | 0.50 | 1.86 | 1.16–2.98 | 0.010 | 1.88 | 1.49–2.39 | <0.001 | 0.65 | 0.32–1.30 | 0.22 | 2.10 | 1.19–3.71 | 0.010 |
CHD, coronary heart disease; CVD, cardiovascular disease.
Expressed as hazard ratios (HRs) with 95% confidence intervals (CIs) from Cox proportional hazards models fit using custom software to account for potential correlation among women within sibships.
Due to missing data, sample sizes are smaller than the total number of 4782 and 1754 women in the Family Blood Pressure Program (FBPP) and Genetic Epidemiology Network of Arteriopathy (GENOA) cohorts, respectively.
Model A adjusted for network, race, family history of CVD, smoking, BMI, education, and diabetes (time-dependent).
Model B adjusted for network, race, family history of CVD, smoking, BMI, education, diabetes mellitus (time-dependent), and hypertension (time-dependent).
Model C adjusted for race, family history of CVD, smoking, dyslipidemia, and diabetes (time-dependent).
Model D adjusted for race, family history of CVD, smoking, dyslipidemia, diabetes mellitus (time-dependent), and hypertension (time-dependent).
Among FBPP participants (Table 3), the hazard ratio for hypertension after age 40 years (Model A) in nulliparous women relative to those with a history of normotensive pregnancies did not differ significantly from 1.0. The hazard ratios for CHD and stroke (Model B) were also not significantly different from 1.0, although the estimated hazard ratio for stroke was 0.55 (95% CI 0.31–1.00, P = 0.05), suggesting a lower hazard of stroke in those with a history of normotensive pregnancy compared with nulliparous women.
Compared with women with a history of normotensive pregnancies, those with a history of hypertensive pregnancies had an increased hazard ratio of 1.53 (95% CI 1.25–1.87, P <0.001) for the diagnosis of hypertension (Model A). After controlling for diagnosis of hypertension after age 40 (Model B), the hazard for stroke remained significantly increased in women with a history of hypertensive pregnancies relative to those with a history of normotensive pregnancies (hazard ratio 1.86, 95% CI 1.16–2.98, P = 0.01). The hazard for CHD was no longer significantly increased (hazard ratio 1.14, 95% CI 0.78–1.68, P = 0.50).
In the GENOA subset of FBPP participants (Table 3), in which we were able to also control for dyslipidemia (Model C), the hazard ratio for diagnosis of hypertension after age 40 years was also significantly increased in women with a history of hypertension in pregnancy relative to those with normotension in pregnancy (hazard ratio 1.88, 95% CI 1.49–2.39, P <0.001). In Model D, which included hypertension and dyslipidemia, and thus controlled for all traditional risk factors, the hazard ratio for stroke (hazard ratio 2.10, 95% CI 1.19–3.71, P = 0.010), but not for CHD (hazard ratio 0.65, 95% CI 0.32–1.30, P = 0.22), was significantly increased in women with a history of hypertension in pregnancy relative to those with normotension in pregnancy.
Discussion
Our results indicate that women with a history of hypertension in pregnancy, including preeclampsia, compared with those who were normotensive while pregnant, have an increased risk for developing hypertension after age 40, and they develop it earlier in life. They were also more likely to have CHD, but adjusted risks were not significantly different, suggesting that the association between hypertension in pregnancy and CHD may be partially mediated by traditional risks factors, including a greater risk of hypertension. In contrast, the increased risk for stroke in women who reported hypertension in pregnancy remained significantly elevated, even after controlling for traditional risk factors, including the greater risk of hypertension.
Previous studies have indicated that women who remain normotensive in pregnancy may experience a lower risk for CVD than nulliparous women [15] and a lower incidence of hypertension later in life, that is, unrelated to pregnancy, compared with either nulliparous women [15] or controls from the general population that were age-matched and race-matched [16]. Our observations do not lend support to this hypothesis overall, inasmuch as the risks for subsequent hypertension and CHD did not differ significantly between the pregnancy groups and there was only a trend toward a lower risk for stroke in women with a history of normotensive pregnancy compared with nulliparous women.
Our study has several limitations. The prevalence of hypertension in pregnancy among our study participants appears to be higher than commonly reported, as high as 18% for GENOA participants compared with 8% in the general population [1]. A possible reason may relate to our sampling scheme that preferentially recruited persons who either had hypertension or were at greater risk to become hypertensive (by virtue of higher BP levels or positive family history of hypertension). Furthermore, our analyses were based on self-reported, physician-diagnosed hypertension in pregnancy and CVD events, and thus were subject to recall bias. Of note, a potential for recall bias exists whenever self-reports of preceding medical conditions are requested from study participants, particularly in case–control or cross-sectional studies with retrospective components, such as ours. Differential recall between cases and controls may serve as a source of bias, leading to inaccurate (frequently inflated) risk estimates [17]. We tried to minimize the recall bias by using standardized protocols and by collecting data from cases and controls in the same way and at similar time points. Another potential source of recall bias in our study relates to distinction between preeclampsia and other, non-proteinuric hypertensive pregnancy disorders, which was also based on self-reports. However, recent studies have indicated adequate recalls of pregnancy-related complications [18]. Specifically, a history of preeclampsia was reported by women with sensitivity of 72–80% and specificity of 96–99% in two studies [12,19]. With such a high specificity, it is unlikely that women without a history of preeclampsia would self-report positive history. On the contrary, 20–30% of women with such a history may not report it, which, if anything, may lead to an underestimation of its association with future CVD. Further limitations relate to our inability to differentiate early from term deliveries and to study the effect of hypertension in multiple pregnancies, both of which have been previously shown to confer particularly high risks for future CVD [20,21].
Despite these limitations, our results not only extend previous reports by confirming this association in a large, multiracial cohort but also provide new evidence for a positive association between hypertension in pregnancy and CVD later in life, after controlling for essentially all traditional risk factors. Thereby, our results suggest that hypertension in pregnancy predicts stroke, but not CHD, independently of traditional risk factors. However, the CHD risk increases with age and tends to develop in women 10–20 years later than in men. Given the age of the FBPP cohort, the impact of this association might have been underestimated. Our future studies will focus on testing whether or not the association between CHD and hypertension in pregnancy become independent of traditional risk factors with aging. A longer follow-up may also facilitate further distinctions among different hypertensive pregnancy disorders with respect to CVD outcomes, and contrast the long-term cardiovascular effects of preeclampsia to other, nonproteinuric hypertensive pregnancy disorders.
One possible mechanism underlying the association between hypertension in pregnancy and future CVD is that these two conditions share several common risk factors, including obesity, diabetes mellitus, and renal disease, which may lead to hypertension and hypertensive sequelae during the childbearing years, only when pregnancy is superimposed. Alternatively, hypertension in pregnancy may induce metabolic and vascular changes that may not resolve after pregnancy, thus increasing the risk for CVD later in life. Results from the study of neuroimaging abnormalities in women with eclamptic seizure may support this hypothesis [22]. One-fourth of the women studied (five of 27), although clinically asymptomatic, had persistent imaging abnormalities 6 weeks postpartum, presumably caused by gliosis in response to infarction. In addition, women who have had eclampsia may experience impaired cognitive functioning, potentially due to permanent neurological damage [23]. The answer to the question whether or not hypertensive pregnancy disorders, and particularly preeclampsia, may cause CVD is crucially dependent on longitudinal studies of risk factors and CVD events before, during, and after hypertensive pregnancies. These may have significant influences, not only on the screening and primary prevention strategies in women but also on the treatment of hypertensive pregnancy disorders as well. Pending results of such studies, we suggest that women who develop hypertension during pregnancy be informed regarding their increased risks for hypertension and other CVD sequelae later in life and that questions regarding not only pregnancy but also hypertension in pregnancy become a routine part of their medical history. Those women should have their BP checked regularly and be treated for modifiable risk factors.
Acknowledgments
The study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K08HD051714), and the National Heart, Lung, and Blood Institute, National Institutes of Health (U01HL054481, U01HL054471, U01HL054512, and U01HL054498).
Abbreviations
- BP
blood pressure
- CHD
coronary heart disease
- CI
confidence intervals
- CVD
cardiovascular disease
- FBPP
Family Blood Pressure Program
- GENOA
Genetic Epidemiology Network of Arteriopathy
- HDL
high-density lipoprotein
- HyperGEN
Hypertension Genetic Epidemiology Network
- LDL
low-density lipoprotein
- SAPPHIRe
Stanford Asian Pacific Program in Hypertension and Insulin Resistance
Footnotes
This study has been presented in an abstract form at the American Society of Nephrology, American Heart Association, International Society for Study of Hypertension in Pregnancy in 2008, and 19th Scientific Meeting of the European Society of Hypertension in 2009.
There are no conflicts of interest.
The following investigators are associated with the Family Blood Pressure Program:
GenNet Network: Alan B. Weder (Network Director), Lillian Gleiberman (Network Coordinator), Anne E. Kwitek, Aravinda Chakravarti, Richard S. Cooper, Carolina Delgado, Howard J. Jacob, and Nicholas J. Schork.
GENOA Network: Eric Boerwinkle (Network Director), Tom Mosley, Alanna Morrison, Kathy Klos, Craig Hanis, Sharon Kardia, and Stephen Turner.
HyperGEN Network: Steven C. Hunt (Network Director), Janet Hood, Donna Arnett, John H. Eckfeldt, R. Curtis Ellison, Chi Gu, Gerardo Heiss, Paul Hopkins, Aldi T. Kraja, Jean-Marc Lalouel, Mark Leppert, Albert Oberman, Michael A. Province, D.C. Rao, Treva Rice, and Robert Weiss.
SAPPHIRe Network: David Curb (Network Director), David Cox, Timothy Donlon, Victor Dzau, John Grove, Kamal Masaki, Richard Myers, Richard Olshen, Richard Pratt, Tom Quertermous, Neil Risch, and Beatriz Rodriguez.
National Heart, Lung, and Blood Institute: Dina Paltoo and Cashell E. Jaquish.
Web Site: http://www.biostat.wustl.edu/fbpp/FBPP.shtml.
References
- 1.Anonymous. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy [comment] Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 2.Chesley SC, Annitto JE, Cosgrove RA. The remote prognosis of eclamptic women. Sixth periodic report. Am J Obstet Gynecol. 1976;124:446–459. doi: 10.1016/0002-9378(76)90168-x. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis [see comment] BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindeberg S, Axelsson O, Jorner U, Malmberg L, Sandstrom B. A prospective controlled five-year follow-up study of primiparas with gestational hypertension. Acta Obstet Gynecol Scand. 1988;67:605–609. doi: 10.3109/00016348809004272. [DOI] [PubMed] [Google Scholar]
- 5.Selvaggi L, Loverro G, Schena FP, Manno C, Cagnazzo G. Long term follow-up of women with hypertension in pregnancy. Int J Gynaecol Obstet. 1988;27:45–49. doi: 10.1016/0020-7292(88)90086-0. [DOI] [PubMed] [Google Scholar]
- 6.Mann JI, Doll R, Thorogood M, Vessey MP, Waters WE. Risk factors for myocardial infarction in young women. Br J Prev Soc Med. 1976;30:94–100. doi: 10.1136/jech.30.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS, Olafsson O. Cardiovascular death in women who had hypertension in pregnancy: a case–control study. BJOG. 2005;112:286–292. doi: 10.1111/j.1471-0528.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WCS. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis. 2008;2:249–259. doi: 10.1177/1753944708094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Multi-Center Genetic Study of Hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39:3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 12.Diehl CL, Brost BC, Hogan MC, Elesber AA, Offord KP, Turner ST, Garovic VD. Preeclampsia as a risk factor for cardiovascular disease later in life: validation of a preeclampsia questionnaire. Am J Obstet Gynecol. 2008;198:e11–e13. doi: 10.1016/j.ajog.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Therneau TM, Grambsch PM, Pankratz VS. Penalized survival models and frailty. J Comput Graph Stat. 2003;12:156–175. [Google Scholar]
- 15.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77:154–158. doi: 10.1136/hrt.77.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia–eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011–1016. doi: 10.1016/0002-9378(86)90336-4. [DOI] [PubMed] [Google Scholar]
- 17.Raphael K. Differential recall as a source of bias. J Clin Epidemiol. 1991;44:1281–1282. doi: 10.1016/0895-4356(91)90163-4. [DOI] [PubMed] [Google Scholar]
- 18.Sou SC, Chen WJ, Hsieh WS, Jeng SF. Severe obstetric complications and birth characteristics in preterm or term delivery were accurately recalled by mothers. J Clin Epidemiol. 2006;59:429–435. doi: 10.1016/j.jclinepi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women [see comment] Am J Epidemiol. 2007;166:117–124. doi: 10.1093/aje/kwm139. [DOI] [PubMed] [Google Scholar]
- 20.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after preeclampsia: population based cohort study. BMJ. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikstrom A-K, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005(112):1486–1491. doi: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 22.Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190:714–720. doi: 10.1016/j.ajog.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol. 2007;197:365.e1–365.e6. doi: 10.1016/j.ajog.2007.06.044. [DOI] [PubMed] [Google Scholar]