Abstract
Background & Aims
Alcohol use and cigarette smoking associate with various pancreatic diseases, but it is not known whether they associate with post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP). We performed a retrospective case-control study to determine if these activities increase the risk of PEP.
Methods
We identified 7,638 patients that had undergone ERCP in the University of Michigan Health System and applied exclusion criteria to identify 123 with PEP. We randomly selected 308 age- and gender-stratified controls (2.5-fold case sample); after applying exclusion criteria 248 remained. In a masked fashion, we collected data for alcohol use, cigarette smoking, and 5 internal control variables: suspected sphincter of Oddi dysfunction (SOD), pancreatic sphincterotomy, moderate/difficult cannulation, ≥ 2 pancreatic injections and pancreatic stent placement.
Results
The univariate model showed an increased frequency of PEP in current drinkers (P<0.001), former drinkers (P<0.001) and former smokers (P<0.001), as well as patients who were suspected of having SOD (P<0.001), had undergone pancreatic sphincterotomy (P<0.001), had a moderate/difficult cannulation (P=0.001), and/or had ≥2 pancreatic injections (P=0.007). The frequency of PEP was reduced in current smokers (P<0.001). The multivariate model showed that the only independent significant predictors of PEP were current drinking (OR=4.70, 95% CI 2.60–8.50, P<0.0001), former cigarette smoking (OR=3.29, 95% CI 1.28–8.44, P<0.013), suspected SOD (OR= 3.69, 95% CI 1.94–7.02, P<0.001), and pancreatic sphincterotomy (OR=5.91, 95% CI 2.04–17.14, P=0.001).
Conclusions
Current alcohol use and potentially former cigarette smoking are new risk factors for PEP. It is important to consider these variables in designing PEP prevention trials.
INTRODUCTION
Pancreatitis is a potential serious complication of endoscopic retrograde cholangiopancreatography (ERCP)1,2–4. Commonly reported risk factors include suspected sphincter of Oddi dysfunction (SOD)5–10, pancreatic sphincterotomy6, moderate/difficult cannulation6, 7, 11, 12, ≥ 2 pancreatic duct contrast injections6–8, 13, 14 and a prior history of post-ERCP pancreatitis (PEP)5, 6. Risk stratification of patients allows endoscopists to identify candidates who might benefit from the placement of a prophylactic pancreatic duct stent, the only intervention proven to reduce the incidence of PEP in high-risk patients15, 16. Unfortunately, this decision making is not always straight forward17, 18 and evidence is inconclusive whether chemopreventive agents reduce PEP17, 19, 20. A potential consideration for all post-ERCP studies is that data may be confounded by the effects of unknown risk factors.
Alcohol use and cigarette smoking are known risk factors for various pancreatic diseases but they have escaped attention in PEP studies21. Alcohol use is a major risk factor for acute and chronic pancreatitis22, 23 and alcohol exposure increases the risk of pancreatic necrosis in acute pancreatitis, independent of etiology24. Although there appears to be a dose-response relationship between alcohol use and the incidence of acute-25 and chronic pancreatitis26, there is no clear alcohol toxicity threshold on the human pancreas26.
Cigarette smoking alters pancreatic secretion27 and with few exceptions28, 29 it increases the risk of developing acute25, 30, 31 and chronic30–35 alcoholic pancreatitis, idiopathic chronic pancreatitis (ICP)31, pancreatic calcifications in alcoholic chronic pancreatitis (ACP)36 and late-onset ICP37, and pancreatic cancer35, 38, 39. Cigarette smoking also associates with an earlier onset of ACP33 and an accelerated disease progression of both ACP32 and late-onset ICP40. Finally, the dose-response relationship is strong between cigarette smoking and the incidence of acute alcoholic pancreatitis25, 31, 41, but less so for idiopathic25, 31 and “other or unknown25, 41” forms of acute pancreatitis, and absent for gallstone pancreatitis25, 31, 34, with one exception41.
To test the hypothesis that alcohol use and cigarette smoking are risk factors for developing PEP, we performed a retrospective case-control study of 7,638 patients who had ERCP between 1/1/1998 – 6/30/2007 at the University of Michigan Health System. We collected and analyzed data to address two a priori aims: 1) to determine whether alcohol use and/or cigarette smoking in current and/or former drinkers/smokers influences the risk of PEP and 2) to determine whether a dose-response relationship exists between alcohol use and/or cigarette smoking and the risk of PEP.
MATERIALS AND METHODS
Ascertainment of Case and Control Samples
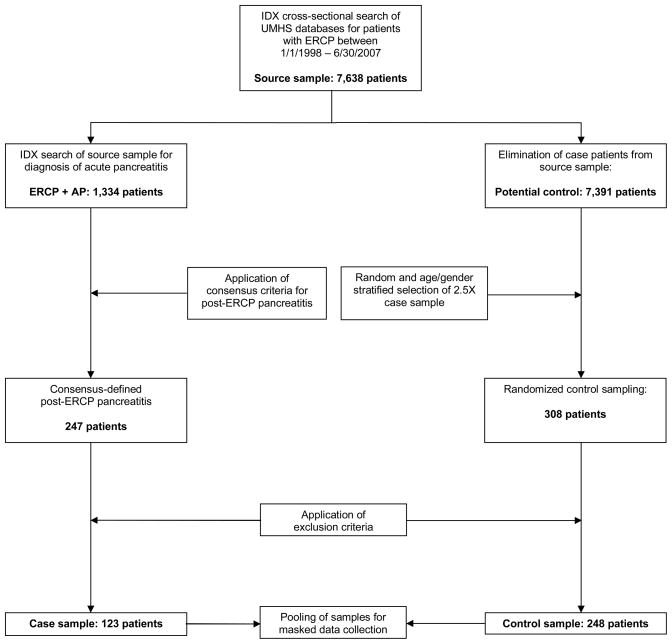
Permission to review patient records for a retrospective case-control study was granted by the University of Michigan Institutional Review Board. We performed an IDX cross-sectional search of our health system’s databases and identified 7,638 patients who had ERCP performed between 1/1/1998 – 6/30/2007. Within this source sample we identified 1,334 patients who had an ICD-9 code (577.0) diagnosis of acute pancreatitis. Based on medical chart review, 247 patients met criteria by Cotton et al.3 for PEP and 123 formed our case sample after applying the following exclusion criteria: age < 18, pregnancy, planned biliary stent removal or exchange without planned pancreatogram and factors that might confound the diagnosis of PEP, including 1) active pancreatitis pre-ERCP, 2) known chronic pancreatitis based on relevant symptoms, imaging modalities, and assessment of pancreatic function42, 3) known pancreatic cancer, or 4) prior pancreatic surgery. We utilized a random-number generator program to select 308 age- and gender stratified controls from the remaining source sample, equivalent to 2.5-fold the number of cases. After applying these exclusion criteria, 248 remained to form our control sample (Figure 1). Age (either < 60 or ≥60) and gender were selected as stratification variables rather than other control variables because age and gender data were accessible in the demographic sections of the medical records and did not require opening and exposing the entire medical record, which could introduce bias associated with the collection of the variables of interest.
Figure 1.
Methodological Summary
Variable Selection and Definitions
We collected data for a set of five internal control variables and both alcohol use and cigarette smoking. The specific internal control variables were selected because they were consistent risk factors for PEP based on reported odds ratios in two multicenter, prospective trials5, 6. We included 4 of 5 identifiable risk factors (suspected SOD, pancreatic sphincterotomy, moderate/difficult cannulation, ≥ 2 pancreatic duct contrast injections) and one known prophylactic measure (pancreatic stent placement)15, 16. A history of prior PEP was not included because selection bias would have occurred in the process of choosing retrospectively one ERCP among all ERCPs performed for an individual patient. To this end, in those with multiple episodes of PEP, we selected the ERCP associated with the first episode of PEP.
We defined cigarette smoking and alcohol use as current, former, or never drinker/smoker. We defined cigarette smoking exposure as number of packs-per-day, years smoking, and pack-years. Quantitative information for alcohol use was commonly absent and inadequate for analysis. Suspected SOD was defined according to Cheng et al5 as a clinically documented pre-ERCP suspicion of SOD independent of manometric findings. Cannulation difficulty was judged as moderate/difficult if the endoscopist performed ≥ 6 cannulation attempts on either the common bile duct or the pancreatic duct or if the endoscopist’s report described a “difficult” or “tough” cannulation on either duct. We used common definitions for pancreatic sphincterotomy, pancreatic injections and pancreatic stent placement (see Supporting Documents).
Data Collection and Management
To limit bias, we pooled and masked the case and control electronic medical records. We utilized a computer program known as “EMERSE43” to electronically, automatically and reproducibly search the entire electronic medical record at our institution for our data of interest (see Supporting Documents).
During the timeframe of the study, ERCP reports were generated from one of two computer software driven template programs: EndoPRO (Pentax writer) from 1/1/1998 – 6/30/2006 and ProVation (ProVation Medical®, Inc) from 7/1/06–6/30/07 (see Supporting Documents).
Variations in the data recorded in the electronic medical record were reconciled by extracting the data most consistently reported in the medical record or if necessary the data from the most current medical document. Microsoft Excel spreadsheets were utilized for data management.
Statistical Analyses
The primary outcome analyzed was PEP. Descriptive statistics were compiled on all variables to evaluate variable distribution prior to modeling. Univariate analyses were conducted prior to multivariate logistic regression analyses, which contained variables with P values < 0.2 from the univariate analysis. Categorical and dichotomous variables were assessed by χ2 tests and continuous variables were assessed using either two sample t-tests for normally distributed variables or the Kolmogorov-Smirnov two-sample tests for non-normal variables. Broad frequency age and gender stratification was used in the selection of controls with all results adjusted for age < 60 and gender. The multivariate analysis examined the risk of PEP associated with substance status (current and former drinkers/smokers), cumulative cigarette exposure (represented as continuous pack-years), suspected SOD, pancreatic sphincterotomy, moderate/difficult cannulation, ≥ 2 pancreatic injections, and pancreatic stent placement. The model was used to estimate odds ratios and associated 95% confidence intervals. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
Univariate Analysis
The case and control samples had similar mean ages (52.4 years vs. 52.2 years, P=0.928), persons < 60 years old (74.0% vs. 75.4%, P=0.767), and women (74.0% vs. 73.8%, P=0.968), indicating successful age and gender stratification (Table 1). Similar to prior studies, the frequency of PEP was greater for the internal control variables known to be associated with PEP – suspected SOD (39.8% vs. 16.1%, P<0.001), pancreatic sphincterotomy (20.3% vs. 4.4%, P<0.001), moderate/difficult cannulation (39.8% vs. 23.4%, P=0.001), and ≥ 2 pancreatic injections (11.4% vs. 4.0%, P=0.007).
Table 1.
Univariate Analysis
| Variable | Case (%) | Control (%) | P-value |
|---|---|---|---|
| Mean Age (std dev) | 52.4 (15.4) | 52.2 (16.1) | 0.928* |
| Age < 60 years old | 91/123 (74.0%) | 187/248 (75.4%) | 0.767 |
| Female | 91/123 (74.0%) | 183/248 (73.8%) | 0.968 |
| Suspected SOD | 49/123 (39.8%) | 40/248 (16.1%) | <0.001 |
| Pancreatic Sphincterotomy | 25/123 (20.3%) | 11/248 (4.4%) | <0.001 |
| Mod./Diff. Cannulation | 49/123 (39.8%) | 58/248 (23.4%) | 0.001 |
| ≥ 2 Pancreatic Injections | 14/123 (11.4%) | 10/248 (4.0%) | 0.007 |
| Pancreatic Stent Placement | 32/123 (26.0%) | 31/248 (12.5%) | 0.001 |
| Alcohol Use Status | |||
| Current drinker | 67/120 (55.8) | 56/222 (25.2) | <0.001 |
| Former drinker | 14/120 (11.7) | 17/222 (7.7) | <0.001 |
| Cigarette Smoking Status | |||
| Current smoker | 16/120 (13.3) | 43/222 (19.4) | <0.001 |
| Former smoker | 33/120 (27.5) | 23/222 (10.3) | <0.001 |
| Mean pack–years for smokers (std dev) | 28.1 (31.4) | 19.2 (19.3) | 0.171† |
Two Sample T-Test
Kolmogorov-Smirnov Two-Sample Test
The frequency of PEP was greater in both current (55.8% vs. 25.2%, P<0.001) and former (11.7% vs. 7.7%, P<0.001) drinkers. Former smokers had a greater frequency of PEP (27.6% vs. 10.3%, P<0.001) and current smokers had a lesser frequency of PEP (13.3% vs. 19.4%, P<0.001).
Multivariate Analysis
According to the multivariate logistic model, the only independent significant predictors of PEP were current drinking (Table 2, OR=4.70, 95% CI 2.60–8.50, P<0.0001), former cigarette smoking (OR=3.29, 95% CI 1.28–8.44, P<0.013), suspected SOD (OR= 3.69, 95% CI 1.94–7.02, P<0.001), and pancreatic sphincterotomy (OR=5.91, 95% CI 2.04–17.14, P=0.001). We could not examine an alcohol dose-response relationship because there was insufficient quantitative data for alcohol use. There was no dose response relationship between continuous pack-years of cigarette smoking with PEP in either current or former smokers.
Table 2.
Multivariate Analysis
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Suspected SOD | 3.69 | 1.94–7.02 | <0.001 |
| Pancreatic Sphincterotomy | 5.91 | 2.04–17.14 | 0.001 |
| Mod./Diff. Cannulation | 1.70 | 0.92–3.14 | 0.091 |
| ≥ 2 Pancreatic Injections | 1.52 | 0.53–4.34 | 0.439 |
| Pancreatic Stent Placement | 0.70 | 0.29–1.70 | 0.430 |
| Alcohol Use Status | |||
| Current drinker | 4.70 | 2.60–8.50 | <0.0001 |
| Former drinker | 2.33 | 0.87–6.22 | 0.091 |
| Cigarette Smoking Status | |||
| Current smoker | 0.30 | 0.09–1.03 | 0.055 |
| Continuous pack-years | 1.04 | 0.99–1.09 | 0.098 |
| Former Smoker | 3.29 | 1.28–8.44 | 0.013 |
| Continuous pack-years | 1.00 | 0.98–1.02 | 0.978 |
All results adjusted for stratified variables of age < 60 and gender.
Interaction Analysis
We were not able to analyze interactions between alcohol use and cigarette smoking, which frequently co-associate44, 45, because subgroups of drinkers and smokers had few persons, which prevented a meaningful interaction analysis. For example, the combined group of former drinkers and current smokers had three persons in the control sample and two persons in the case sample. Further, the combined group of current drinkers and former smokers had only 18 persons in the case sample and five persons in the control sample.
DISCUSSION
The multivariate analysis of our data reveals that current drinking, former cigarette smoking, suspected SOD and pancreatic sphincterotomy are risk factors for PEP. Of these the most important new finding is the association of current alcohol use with PEP. Perhaps surprising and open to other interpretations are the association of former cigarette smoking with PEP and the lack of association of former drinkers and current smoking with PEP (and potential protection from PEP).
Current alcohol use may enhance the vulnerability of the pancreas to injury via disruption of normal pancreatic neurohormonal control mechanisms46, dysregulation of the immune system47, reduction in pancreatic microperfusion23, and/or triggering multiple pathological changes in pancreatic acinar cells recently reviewed by Pandol et al.23, such as increased intracellular calcium concentrations, mitochondrial damage, increased activation of the inflammatory transcription factor NF-kB and trypsinogen activation. In addition, drinking alcohol may predispose to pancreatic diseases by uncoupling NO production from the enzyme endothelial NO synthase (eNOS)21, 48. eNOS helps maintain normal endothelial function and has protective effects on acute pancreatitis49, possibly by maintaining pancreatic exocrine secretion48, augmenting pancreatic blood flow49 or indirectly inhibiting intracellular trypsinogen activation49.
Why former drinking does not and former smoking does increase the risk of PEP is uncertain. Possibly former drinkers developed subclinical pancreatic damage that reduced the risk for PEP, analogous to patients with established chronic pancreatitis, who appear to have a lower risk of PEP6. It is unknown whether pancreatic damage persists after cessation of smoking, although cigarette smoke in experimental studies increases pancreatic inflammation and reduces pancreatic blood flow50, 51. A possible explanation for increased risk of PEP in former smokers is that they may be predisposed to PEP on the basis of ischemia as they were older than the cohorts and current smokers (60.3 vs. 52 vs. 45.7 years old, see Supporting Documents) and had a greater frequency of coronary heart disease compared to current and never smokers (27% vs. 19% vs. 6%, see Supporting Documents), which is associated with increased pancreatic microvascular atheroma52, 53. The association of age, independent of smoking, is unclear. One multicenter prospective trial5 showed that younger age associated with greater PEP but another trial6 made no such association; neither trial considered smoking (or drinking) in statistical analyses.
Whether current cigarette smokers had less PEP is uncertain. Only by univariate analysis we found that current smokers had less PEP (Table 1, OR 13.3% vs. 19.4%, P=0.001) but by multivariate analysis this was a nonsignificant trend (Table 2, P=0.055). If however, current smoking reduces PEP, a possible explanation may be that nicotine activates the nicotinic anti-inflammatory pathway, which reduces pancreatic inflammation and ameliorates experimental pancreatitis54.
We performed an observational retrospective case control study, which has inherent disadvantages such as confounding (unrecognized differences in the drinkers and/or smokers vs. the control populations might explain the associations with PEP rather than the risk exposure) and incorrect reporting of the risk factors, particularly if the factors are deemed socially undesirable (alcohol use, cigarette smoking).
To address incorrect reporting of risk factors, we point out that our data reporting was interview-based, which is more reliable than self-reporting55. Secondly we obtained a statistically acceptable rate of missing data (7.8%)56, showed that the site of data collection (inpatient versus outpatient) had a nonsignificant effect on study outcome and in subgroup analyses that former and current smokers compared to never smokers had a greater frequency of Chronic Obstructive Pulmonary Disease (COPD) and a greater odds of Coronary Artery Disease (CAD), giving support for the accuracy of the smoking data. Thus, although data reporting bias is possible, such an effect was likely minimal in our study, as suggested by the analyses of data reliability in this paragraph (please see Supporting Documents for additional results and discussion).
In addition we provide supporting evidence that our data is reliable by confirming that recognized risk factors increased the odds of PEP. Specifically, we showed that suspected SOD5–10 and pancreatic sphincterotomy6 are independent risk factors for PEP. Also, we showed that as a prophylactic intervention15, 16, pancreatic stent placement occurred more frequently in the case sample (26% vs. 12.5%), and, as expected, was not a risk factor for PEP. Moderate/difficult cannulation associated with increased risk of PEP in several studies6, 7, 11, 12 but not in our study. As a possible explanation, we expanded our definition of the difficulty of the cannulation to include the endoscopists’ subjective interpretation of the cannulation in addition to a preset number of cannulation attempts (≥ 6 attempts), because the number of attempts was lacking in the majority of ERCP reports. Similarly, we speculate that non-standardized reporting by the endoscopists may explain why ≥ 2 pancreatic duct contrast injections was not a significant risk factor for PEP in our study as it is in other studies6–8, 13, 14.
An additional limitation of our study is failure to include all variables possibly associated with PEP. Of statistical necessity we had to limit the number of variables because the number of variables a statistical model can accommodate is directly proportional to the sample size. Hence, we excluded variables that had a doubtful or unclear association to PEP such as miscellaneous indications for ERCP, an endoscopist’s experience, trainee involvement, a patient’s body-mass index, or the presence of co-morbidities like diabetes, anemia, and hemodialysis5–8, 13, 14, 57–60
In summary, this study is the first to attempt to examine the relationship between alcohol use, cigarette smoking, and other established risk factors in PEP. We report that current alcohol use and potentially former cigarette smoking are new risk factors for PEP. Because of inherent limitations of the retrospective study design, including the reliability of former smoking and drinking data, these findings require validation, preferably by a large, prospective multi-center study of detailed drinking and smoking habits, which could also address interactions between alcohol use and cigarette smoking and further elucidate dose-response relationships. Nevertheless, our findings are potentially important because if confirmed by prospective studies, drinking and smoking status may aid assessing the risk of PEP prior to ERCP and guide implementation of risk lowering strategies for PEP such as prophylactic pancreatic stent or chemoprevention.
Acknowledgments
Grant Support: Research support provided by NIH grants DK073298 (M.J.D.).
We thank Dawn Chien (administrative assistant, University of Michigan) for assistance in performing our IDX cross-sectional searches of the University of Michigan databases.
Abbreviations
- ACP
alcoholic chronic pancreatitis
- ERCP
endoscopic retrograde cholangiopancreatography
- ICP
idiopathic chronic pancreatitis
- post-ERCP pancreatitis
PEP
- SOD
Sphincter of Oddi
Footnotes
Financial Disclosures: The authors declare no competing interests.
Writing Assistance: None.
Transcript Profiling: Not applicable.
References
- 1.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–8. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 2.Freeman ML. Adverse outcomes of endoscopic retrograde cholangiopancreatography: avoidance and management. Gastrointest Endosc Clin N Am. 2003;13:775–98. xi. doi: 10.1016/s1052-5157(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 3.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–93. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 4.Huibregtse K. Complications of endoscopic sphincterotomy and their prevention. N Engl J Med. 1996;335:961–3. doi: 10.1056/NEJM199609263351309. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139–47. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 6.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–34. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 7.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–18. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SN, Pavone E, Barkun JS, et al. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy. 1998;30:457–63. doi: 10.1055/s-2007-1001308. [DOI] [PubMed] [Google Scholar]
- 9.Masci E, Mariani A, Curioni S, et al. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta-analysis. Endoscopy. 2003;35:830–4. doi: 10.1055/s-2003-42614. [DOI] [PubMed] [Google Scholar]
- 10.Aronson N, Flamm CR, Bohn RL, et al. Evidence-based assessment: patient, procedure, or operator factors associated with ERCP complications. Gastrointest Endosc. 2002;56:S294–302. doi: 10.1067/mge.2002.129021. [DOI] [PubMed] [Google Scholar]
- 11.Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652–6. doi: 10.1067/mge.2002.129086. [DOI] [PubMed] [Google Scholar]
- 12.Friedland S, Soetikno RM, Vandervoort J, et al. Bedside scoring system to predict the risk of developing pancreatitis following ERCP. Endoscopy. 2002;34:483–8. doi: 10.1055/s-2002-32004. [DOI] [PubMed] [Google Scholar]
- 13.Andriulli A, Clemente R, Solmi L, et al. Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc. 2002;56:488–95. doi: 10.1067/mge.2002.128130. [DOI] [PubMed] [Google Scholar]
- 14.Loperfido S, Angelini G, Benedetti G, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 15.Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544–50. doi: 10.1016/s0016-5107(04)02013-9. [DOI] [PubMed] [Google Scholar]
- 16.Das A, Singh P, Sivak MV, et al. Pancreatic-stent placement for prevention of post-ERCP pancreatitis: a cost-effectiveness analysis. Gastrointest Endosc. 2007;65:960–8. doi: 10.1016/j.gie.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–44. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 18.Elta GH. Temporary prophylactic pancreatic stents: which patients need them? Gastrointest Endosc. 2008;67:262–4. doi: 10.1016/j.gie.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Lankisch PG, Lerch MM. Pharmacological prevention and treatment of acute pancreatitis: where are we now? Dig Dis. 2006;24:148–59. doi: 10.1159/000090318. [DOI] [PubMed] [Google Scholar]
- 20.DiMagno MJ, DiMagno EP. New advances in acute pancreatitis. Curr Opin Gastroenterol. 2007;23:494–501. doi: 10.1097/MOG.0b013e3282ba566d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiMagno MJ. Nitric-oxide pathways and evidence based perturbations in acute pancreatitis. Pancreatology. 2007;7:403–408. doi: 10.1159/000108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 23.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–51. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 24.Papachristou GI, Papachristou DJ, Morinville VD, et al. Chronic alcohol consumption is a major risk factor for pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2006;101:2605–10. doi: 10.1111/j.1572-0241.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindkvist B, Appelros S, Manjer J, et al. A Prospective Cohort Study of Smoking in Acute Pancreatitis. Pancreatology. 2008;8:63–70. doi: 10.1159/000114868. [DOI] [PubMed] [Google Scholar]
- 26.Durbec JP, Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion. 1978;18:337–50. doi: 10.1159/000198221. [DOI] [PubMed] [Google Scholar]
- 27.Murthy SN, Dinoso VP, Jr, Clearfield HR, et al. Simultaneous measurement of basal pancreatic, gastric acid secretion, plasma gastrin, and secretin during smoking. Gastroenterology. 1977;73:758–61. [PubMed] [Google Scholar]
- 28.Haber PS, Wilson JS, Pirola RC. Smoking and alcoholic pancreatitis. Pancreas. 1993;8:568–72. doi: 10.1097/00006676-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Levy P, Mathurin P, Roqueplo A, et al. A multidimensional case-control study of dietary, alcohol, and tobacco habits in alcoholic men with chronic pancreatitis. Pancreas. 1995;10:231–8. doi: 10.1097/00006676-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Talamini G, Bassi C, Falconi M, et al. Cigarette smoking: an independent risk factor in alcoholic pancreatitis. Pancreas. 1996;12:131–7. [PubMed] [Google Scholar]
- 31.Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. Am J Gastroenterol. 2004;99:731–8. doi: 10.1111/j.1572-0241.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- 32.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510–4. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourliere M, Barthet M, Berthezene P, et al. Is tobacco a risk factor for chronic pancreatitis and alcoholic cirrhosis? Gut. 1991;32:1392–5. doi: 10.1136/gut.32.11.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowenfels AB, Zwemer FL, Jhangiani S, et al. Pancreatitis in a native American Indian population. Pancreas. 1987;2:694–7. doi: 10.1097/00006676-198711000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999;44:1303–11. doi: 10.1023/a:1026670911955. [DOI] [PubMed] [Google Scholar]
- 36.Cavallini G, Talamini G, Vaona B, et al. Effect of alcohol and smoking on pancreatic lithogenesis in the course of chronic pancreatitis. Pancreas. 1994;9:42–6. doi: 10.1097/00006676-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Imoto M, DiMagno EP. Cigarette smoking increases the risk of pancreatic calcification in late-onset but not early-onset idiopathic chronic pancreatitis. Pancreas. 2000;21:115–9. doi: 10.1097/00006676-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–70. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 39.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27:87–93. doi: 10.1016/s0361-090x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 40.Mullhaupt B, Truninger K, Ammann R. Impact of etiology on the painful early stage of chronic pancreatitis: a long-term prospective study. Z Gastroenterol. 2005;43:1293–301. doi: 10.1055/s-2005-858733. [DOI] [PubMed] [Google Scholar]
- 41.Blomgren KB, Sundstrom A, Steineck G, et al. A Swedish case-control network for studies of drug-induced morbidity--acute pancreatitis. Eur J Clin Pharmacol. 2002;58:275–83. doi: 10.1007/s00228-002-0471-4. [DOI] [PubMed] [Google Scholar]
- 42.Witt H, Apte MV, Keim V, et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Hanauer DA. EMERSE: The Electronic Medical Record Search Engine. AMIA Annu Symp Proc. 2006:941. [PMC free article] [PubMed] [Google Scholar]
- 44.Klatsky AL, Friedman GD, Siegelaub AB, et al. Alcohol consumption among white, black, or oriental men and women: Kaiser-Permanente multiphasic health examination data. Am J Epidemiol. 1977;105:311–23. doi: 10.1093/oxfordjournals.aje.a112388. [DOI] [PubMed] [Google Scholar]
- 45.Sirtori CR, Avogaro P, Tremoli E. Metabolic Effects of Alcohol: Proceedings of the International Symposium on Metabolic Effects of Alcohol Held in Milan; June 18–21, 1979; Elsevier Science Ltd; 1979. [Google Scholar]
- 46.Deng X, Wood PG, Eagon PK, et al. Chronic alcohol-induced alterations in the pancreatic secretory control mechanisms. Dig Dis Sci. 2004;49:805–19. doi: 10.1023/b:ddas.0000030093.25897.61. [DOI] [PubMed] [Google Scholar]
- 47.Kovacs EJ, Jerrells TR. Alcohol and immunology: introduction to and summary of the 2003 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2004;33:171–4. doi: 10.1016/j.alcohol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Reddy RC, Hao Y, Lee SH, et al. Pioglitazone reverses insulin resistance and impaired CCK-stimulated pancreatic secretion in eNOS(−/−) mice: therapy for exocrine pancreatic disorders? Am J Physiol Gastrointest Liver Physiol. 2007;293:G112–20. doi: 10.1152/ajpgi.00442.2006. [DOI] [PubMed] [Google Scholar]
- 49.DiMagno MJ, Williams JA, Hao Y, et al. Endothelial nitric oxide synthase is protective in the initiation of caerulein-induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2004;287:G80–7. doi: 10.1152/ajpgi.00525.2003. [DOI] [PubMed] [Google Scholar]
- 50.Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21:272–8. doi: 10.1097/00006676-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Wittel UA, Pandey KK, Andrianifahanana M, et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101:148–59. doi: 10.1111/j.1572-0241.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 52.Stumpf HH. Microatheromas of very small arteries: unusual lesions involving primarily the pancreas. Hum Pathol. 1983;14:1039–43. doi: 10.1016/s0046-8177(83)80259-7. [DOI] [PubMed] [Google Scholar]
- 53.Pollak OJ. Human pancreatic atherosclerosis. Ann N Y Acad Sci. 1968;149:928–39. doi: 10.1111/j.1749-6632.1968.tb53847.x. [DOI] [PubMed] [Google Scholar]
- 54.van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–30. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84:1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Little RJA, Rubin DB. Statistical Analysis With Missing Data. John Wiley & Sons, Inc; 2002. [Google Scholar]
- 57.Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417–23. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]
- 58.Rabenstein T, Schneider HT, Bulling D, et al. Analysis of the risk factors associated with endoscopic sphincterotomy techniques: preliminary results of a prospective study, with emphasis on the reduced risk of acute pancreatitis with low-dose anticoagulation treatment. Endoscopy. 2000;32:10–9. doi: 10.1055/s-2000-138. [DOI] [PubMed] [Google Scholar]
- 59.Maldonado ME, Brady PG, Mamel JJ, et al. Incidence of pancreatitis in patients undergoing sphincter of Oddi manometry (SOM) Am J Gastroenterol. 1999;94:387–90. doi: 10.1111/j.1572-0241.1999.00864.x. [DOI] [PubMed] [Google Scholar]
- 60.Rabenstein T, Hahn EG. Post-ERCP pancreatitis: is the endoscopist’s experience the major risk factor? JOP. 2002;3:177–87. [PubMed] [Google Scholar]