Abstract
Background: Bevacizumab has demonstrated antitumor activity in multiple diseases. This phase II study was undertaken to determine the effects of adding bevacizumab to a regimen of docetaxel and oxaliplatin in patients with advanced adenocarcinoma of the stomach or gastroesophageal junction.
Patients and methods: Previously untreated patients with locally advanced or metastatic disease and a performance status (PS) of 0–1 were eligible for this study. Patients received bevacizumab at 7.5 mg/kg, docetaxel at 70 mg/m2, and oxaliplatin at 75 mg/m2 administered on day 1 of a 21-day cycle. The primary end point of the study was progression-free survival (PFS).
Results: A total of 38 eligible patients (median age 57 years, 45% gastric, 55% PS 0) were enrolled on to the study. Median PFS was 6.6 months [95% confidence interval (CI) 4.4–10.5] and median survival 11.1 months (95% CI 8.2–15.3). Complete responses were documented in 2 (5%) patients, partial responses in 14 (37%), and stable disease in 14 (37%). No treatment-related deaths were observed. The most commonly reported grade 3–4 toxicity was neutropenia (34%), and gastrointestinal perforation occurred in three patients (8%).
Conclusion: The combination of bevacizumab, docetaxel, and oxaliplatin has promising activity for further evaluation in randomized trials.
Keywords: bevacizumab, docetaxel, gastric cancer, oxaliplatin
introduction
Gastric cancer is the second leading cause of cancer death worldwide [1–2]. In the USA, the incidence of gastroesophageal junction (GEJ) cancer has increased threefold, making it among the cancers with the fastest rising incidence [3]. Therapeutic research in gastric and GEJ cancers has focused on screening cytotoxic agents. Despite decades of research, the impact of conventional chemotherapy combinations on advanced gastric and GEJ cancers has reached a plateau. Combinations of two or three cytotoxic drugs demonstrate objective response rates and median progression free-survival (PFS) estimates of 21%–47% and 3.4–7.4 months, respectively[4–6]. Median survival generally remains <1 year.
Cytotoxic agents commonly used in this disease include platinum compounds, fluoropyrimidines, taxanes, irinotecan, and anthracyclines. The addition of docetaxel to cisplatin and 5-fluorouracil (5-FU) resulted in a significantly higher response rate (39% versus 23%), a longer time to progression (5.2 versus 3.7 months), and an improved median overall survival (OS; 10.2 versus 8.5 months) [4]. Cunningham et al. [5] evaluated the impact of substituting oxaliplatin for cisplatin and capecitabine for 5-FU in the epirubicin, cisplatin, and 5-FU (ECF) regimen. Oxaliplatin as compared with cisplatin demonstrated comparable efficacy, with a lower incidence of myelosuppression, thromboembolic complications, and nephrotoxicity. The combination of docetaxel and oxaliplatin has been evaluated in gastric cancer in four phase II trials [7–10]. The observed response rates, median PFS, and median OS were 34%–55%, 4.3–8.9 months, and 8.5–12.7 months, respectively. These results indicated that the oxaliplatin–docetaxel combination is an active and a safe platform for incorporation of targeted agents.
Angiogenesis is an essential step for tumor growth and metastasis [11]. The vascular endothelial growth factor (VEGF) promotes the proliferation and migration of endothelial cells [12]. In gastric cancer, overexpression of the VEGF is associated with increasing microvascular density, advancing stage, and reduced survival [13–15]. Inhibition of VEGF in preclinical models of gastric cancer resulted in increased apoptosis and reduction in metastases [16]. Furthermore, inhibition of VEGF by bevacizumab is thought to improve the efficacy of cytotoxic therapy in colorectal [17], breast [18], and non-small-cell lung cancers [19]. Two phase II trials in patients with advanced gastric and GEJ cancers reported increased median PFS (8.3 and 12 months) and OS (12.3 and 16.2 months) with the addition of bevacizumab to cisplatin-based chemotherapy [20, 21] The rationale of this multi-institutional study was to evaluate the efficacy and tolerability of bevacizumab in combination with oxaliplatin and docetaxel in patients with advanced gastric and GEJ cancers.
materials and methods
patient eligibility
Patients were required to have a histological or cytological diagnosis of locally advanced unresectable or metastatic adenocarcinoma of stomach or GEJ, Southwest Oncology Group performance status of 0–1, measurable disease, and adequate hematological, renal, and hepatic function defined by the following criteria: neutrophil count ≥1500/mm3, platelet count ≥100 000/mm3, serum creatinine ≤1.5 mg/dl, total serum bilirubin less than or equal to the upper limit of the institutional normal range (ULN), and serum alkaline phosphatase and aspartate transaminase ≤2.5 times ULN. Patients were excluded if they had other active malignancy within the preceding year except for adequately treated basal cell cancer, squamous cell skin cancer, or in situ cervical cancer. Additional exclusion criteria included uncontrolled hypertension, brain metastases, history of deep venous thrombosis (DVT) requiring anticoagulation, or arterial thrombotic events including angina, myocardial infarction, or cerebrovascular accident within 1 year. Patients were also ineligible if they had a major surgery within 4 weeks, incompletely healed surgical wounds, or an active peptic ulcer disease. Previous chemotherapy for gastric or GEJ cancer was not allowed except for patients relapsing >6 months after the completion of adjuvant chemotherapy that did not include a taxane or platinum compound. All patients provided written informed consent in accordance with the institutional Human Investigation Committee guidelines before enrolment on the study.
study design and treatment plan
Oxaliplatin (Eloxatin; Sanofi-Aventis, Bridgewater, NJ) 75 mg/m2 i.v. and docetaxel (Taxotere; Sanofi-Aventis) 70 mg/m2 i.v. were administered on day 1 of a 21-day treatment cycle. Bevacizumab (Avastin; Genentech Inc., San Francisco, CA) was administered at a dose of 15 mg/kg i.v. on day 1 of the treatment cycle. After the occurrence of two gastrointestinal (GI) perforations in the first five patients, the dose of bevacizumab was reduced to 7.5 mg/kg for the remainder of the study.
A new cycle of therapy could begin only if the neutrophil count was ≥1500/mm3, platelet count was ≥100 000/mm3, and all relevant non-hematological toxic effects were grade 1 or lower. Dose reductions were based on the toxicity in the preceding cycle. The docetaxel and oxaliplatin doses were reduced by 20% for any grade 3 or 4 hematological toxicity, except anemia. A 20% dose reduction of oxaliplatin was carried out for grade 3 neuropathy lasting >7 days but resolving before the next treatment cycle. Dose reduction of 20% for oxaliplatin and docetaxel was carried out for grade 2 neuropathy present at day 1 of a treatment cycle. No dose reduction was carried out for grade 3 or lower neuropathy lasting <7 days. Oxaliplatin and docetaxel were discontinued for grade 3 neuropathy present at day 1 of a treatment cycle or for grade 4 neuropathy regardless of duration. Treatment was held for grade 3 or 4 non-hematological toxic effects (excluding nausea or vomiting), until resolution to grade 1 or lower, and resumed at a 20% reduction of docetaxel and oxaliplatin doses. No dose adjustments for bevacizumab were planned. Bevacizumab was discontinued for grade 4 hypertension, grade 3 or 4 hemorrhage, grade 4 venous thromboembolic event (VTE), grade 4 proteinuria, or any grade of GI perforation, wound dehiscence, or arterial thromboembolic event. Patients requiring a delay in therapy of longer than 2 weeks because of toxicity or requiring more than two dose reductions were removed from the study. In addition, patients were removed from study for disease progression, unacceptable toxicity, or withdrawal of consent.
on-study evaluation
Efficacy end points of objective response, PFS, and OS were assessed. Imaging studies were carried out at baseline and repeated after every two cycles of therapy or whenever there was any clinical suspicion of disease progression. Objective tumor responses were determined and categorized by the RECIST criteria [22] as complete response, partial response, disease progression, or stable disease. Objective responses required at least one additional confirmatory follow-up scan to be carried out at ≥3 weeks after the first documentation of response. OS was measured from study registration to the date of death or last follow-up. PFS was measured from study registration to the date of first documented progressive disease or death. Time to treatment failure (TTF) was measured from study registration to the date of first documented progressive disease or date off treatment due to toxicity, patient refusal, or death, whichever occurred first. Response duration (RD) was measured from the start of objective response to the date of first evidence of relapse or censored at the last tumor assessment for those patients still responding. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [23].
statistical methods
The primary end point was PFS. A single-stage, one-sample survival-type study design was planned. The primary goal was to detect evidence (with 90% power and one-sided type I error of 0.15) that patients on this new regimen had a median PFS of at least 6 months, in contrast to an assumed population reference value of 4 months. Due to the lack of reasonably effective treatments for this disease, we purposely relaxed the type I error probability to ≤0.15 but limited the type II error probability to ≤0.10. Under the assumptions of exponential PFS times and uniform accrual rate, the required sample size was N = 38 patients via the method of Lawless.
Baseline patient characteristics, response, and toxic effects were described using summary statistics. Wilson's method was used to compute a 95% confidence interval (CI) for a proportion. Standard Kaplan‑Meier (K–M) estimates of the censored RD, TTF, PFS, and OS distributions were computed. PFS was the primary end point of the study. Due to modest sample sizes (or numbers of events), time-to-event (TTE) statistics (e.g. median, 6-month rate) were estimated more conservatively using linear interpolation between successive event times on the K‑M curves [24]. All point estimates of TTE statistics were accompanied by a 95% CI. Censored PFS was compared by disease site using the log-rank test.
results
patient characteristics
Study patients were enrolled from 25 July 2005 until 14 July 2009. Patient characteristics are shown in Table 1. Thirty-nine patients were enrolled on to the study. One patient was ineligible. Twenty-one patients had cancer of GEJ and 17 patients had primary gastric cancer. One patient had prior surgery for localized disease, and none had received adjuvant chemotherapy. Of the 36 patients with metastatic disease, 22 had more than one site of metastasis including liver (19 patients), lung (14), lymph nodes (23), peritoneum (4), and bone (3).
Table 1.
Characteristics of the 38 eligible patients with advanced gastroesophageal junction (GEJ) or gastric cancer treated with bevacizumab, docetaxel, and oxaliplatin
| Characteristic | n | % | Years |
| Age: median (range) | 57 (22–73) | ||
| Sex | |||
| Male | 29 | 76 | |
| Female | 9 | 24 | |
| Race | |||
| African American | 4 | 11 | |
| Caucasian | 32 | 84 | |
| Other | 2 | 5 | |
| Prior surgery | |||
| No | 37 | 97 | |
| Yes | 1 | 3 | |
| Performance status | |||
| 0 | 21 | 55 | |
| 1 | 17 | 45 | |
| Site of primary tumor | |||
| Stomach | 17 | 45 | |
| GEJ | 21 | 55 | |
| Stage | |||
| Locally advanced | 2 | 5 | |
| Metastatic disease | 36 | 95 | |
| Liver | 19 | 50 | |
| Lung | 14 | 37 | |
| Peritoneum | 4 | 11 | |
| Other (bone, lymph nodes) | 27 | 71 |
efficacy
A total of 208 cycles were administered, with a median of 6 cycles given per patient (range 1–12). Thirteen patients (34%) are still receiving therapy. Reasons for removal from the study included disease progression (12 patients), neuropathy (5), GI perforation (3), tracheoesophageal (TE) fistula (1), DVT (1), worsening dysphagia (1), and patient preference (2). Eleven patients subsequently went on to receive second-line therapy.
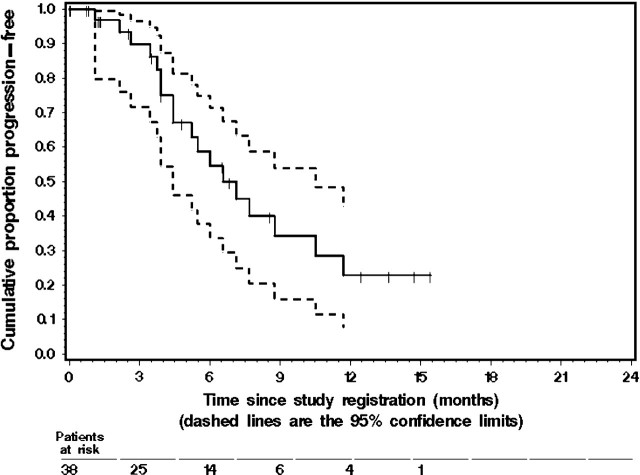
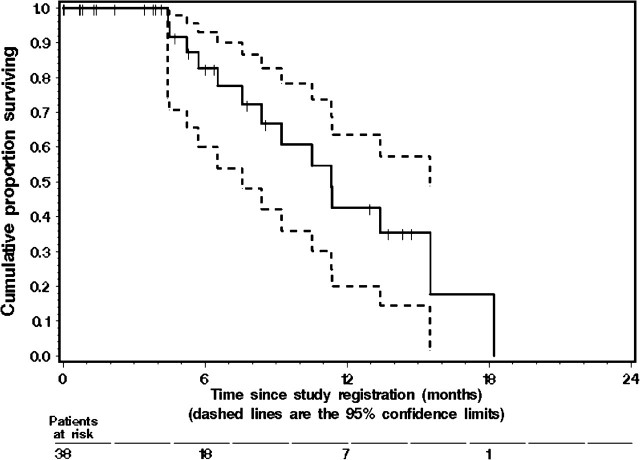
The median PFS for all 38 eligible patients was 6.6 months (95% CI 4.4–10.5 months). Twenty patients were still without progression, and their median follow-up for progression was 3.0 months. The K–M estimate of the probability of PFS is given in Figure 1. No significant difference in PFS was observed between patients with gastric and GEJ tumors (P = 0.435). The median TTF was 4.5 months (95% CI 3.6–6.3 months). The median OS was 11.1 months (95% CI 8.2–15.3 months). Twenty-four patients were still alive, and their median follow-up for survival was 3.9 months. The K–M estimate of the probability of OS is shown in Figure 2.
Figure 1.
The Kaplan–Meier estimate of progression-free survival (PFS) in the 38 eligible patients with metastatic gastroesophageal junction cancer or gastric cancer who were treated with docetaxel, oxaliplatin, and bevacizumab. The dashed lines represent the 95% confidence interval (CI) about each successive estimate of the progression-free rate. The median PFS was 6.6 months (95% CI 4.4–10.5 months). The 6-month PFS rate was 55% (95% CI 35% to 74%).
Figure 2.
The Kaplan–Meier estimate of overall survival (OS) in the 38 eligible patients with metastatic gastroesophageal junction cancer or gastric cancer enrolled in the study. The dashed lines represent the 95% confidence interval (CI) about each successive estimate of the survival rate. The median OS was 11.1 months (95% CI 8.2–15.3 months). The 12-month OS rate was 40% (95% CI 17% to 63%).
Objective tumor responses were seen in 16 patients (42%, 95% CI 28% to 58%) including two complete responses. Stable disease as the best response was seen in an additional 14 patients. The median RD was 4.0 months (95% CI 1.3–10.8 months). Seven patients were still in remission, and their median follow-up for relapse was 1.9 months.
toxicity
The treatment was generally well tolerated in the outpatient setting. No treatment-related deaths were reported. Table 2 summarizes the grade 3 and 4 toxic effects observed in the study. The most common grade 3–4 toxic effects were hematological. Thirteen patients had grade 3–4 neutropenia. Two patients had a grade 3 febrile neutropenia. Five patients had grade 2 or 3 neuropathy. The median number of cycles before development of neuropathy was 8 (range 6–10 cycles).
Table 2.
The frequency of treatment-related toxic effects in 38 treated patients with gastric or gastroesophageal junction cancer expressed as the worst toxicity per patient
| Toxicity type | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
| Bevacizumab related | ||||
| GI perforation | 0 (0) | 0 (0) | 1 (3) | 2 (5) |
| Tracheoesophageal fistula | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| Hypertension | 4 (11) | 1 (3) | 2 (5) | 0 (0) |
| Venous thromboembolism | 1 (3) | 0 (0) | 1 (3) | 0 (0) |
| Proteinuria | 1 (3) | 1 (3) | 0 (0) | 0 (0) |
| Hematological | ||||
| Neutropenia | 0 (0) | 1 (3) | 5 (13) | 8 (21) |
| Anemia | 1 (3) | 2 (5) | 0 (0) | 0 (0) |
| Leukopenia | 2 (5) | 0 (0) | 1 (3) | 0 (0) |
| Neuropathy | ||||
| Acute | 3 (8) | 6 (16) | 2 (5) | 0 (0) |
| Chronic | 5 (13) | 0 (0) | 3 (8) | 0 (0) |
| GI-related toxic effects | ||||
| Stomatitis | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 7 (18) | 3 (8) | 4 (11) | 0 (0) |
| Vomiting | 3 (8) | 1 (3) | 2 (5) | 0 (0) |
| Diarrhea | 7 (18) | 5 (13) | 3 (8) | 0 (0) |
| Hepatotoxicity | ||||
| Aspartate transaminase | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Alanine transferase | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Alkaline phosphatase | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
| Other | ||||
| Dehydration | 0 (0) | 0 (0) | 4 (11) | 0 (0) |
| Fever | 1 (3) | 0 (0) | 2 (5) | 0 (0) |
| Infection | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
| Fatigue | 7 (18) | 7 (18) | 2 (5) | 0 (0) |
| Anorexia | 2 (5) | 1 (8) | 2 (5) | 0 (0) |
| Myalgia | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Alopecia | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 scale for toxicity grading.
GI, gastrointestinal.
Bevacizumab-related toxic effects included hypertension, VTEs, GI perforation, and TE fistula. No arterial thrombotic events, wound dehiscence, hemorrhage, or grade 4 proteinuria was observed. Bevacizumab-related grade 3 hypertension occurred in two patients (5%). One patient (3%) developed a grade 3 DVT after the first cycle of therapy.
GI perforation occurred in three (8%) patients each of whom had intact primary tumors and two of whom also had peritoneal metastases. GI perforation developed following the second cycle of chemotherapy in each patient. In one, the perforation was at a gastric ulcer site and the patient required surgery. The site of perforation could not be ascertained in the second patient who was managed medically. The third patient had the perforation in the sigmoid colon and underwent successful surgical repair. One additional patient had a TE fistula develop after an 11th cycle of therapy. This patient had previously received chemoradiotherapy to the mediastinum and had surgical resection of the primary tumor. All four patients went on to receive additional alternate systemic therapy. Two perforations occurred in the first five patients enrolled in the study raising safety concerns and leading to a reduction in the bevacizumab dose from 15 to 7.5 mg/kg in the remaining cohort of patients.
In total, 20 patients required hospitalization for listed reasons during study therapy [neutropenia (6), fever (2), dehydration (4), GI perforation (3), infection (2), pain (2), TE fistula, DVT].
discussion
The impact of conventional cytotoxic therapy on gastric and GEJ adenocarcinoma remains modest at best. An increase in efficacy gained by the use of a triplet cytotoxic combination (e.g. with docetaxel, cisplatin, and 5-FU or ECF) has been offset by increased toxic effects associated with these regimens. The inclusion of a targeted agent such as bevacizumab into a cytotoxic regimen might improve antitumor activity with less of an increase in toxicity. In this multi-institutional study, we evaluated the impact of adding bevacizumab to oxaliplatin and docetaxel in patients with advanced gastric and GEJ cancers. The regimen resulted in an objective response rate, median PFS, and median OS of 42%, 6.6 months, and 11.1 months, respectively. Three other phase II trials have evaluated bevacizumab in combination with cytotoxic chemotherapy in untreated gastric and GEJ cancers [20, 21, 25]. These trials differed in the patient selection and the chemotherapy regimens used and are summarized in Table 3 with our trial. In total, the reported response rates, PFS, and OS in these trials indicate that bevacizumab is active in gastric and GEJ tumors. The definition and magnitude of that benefit with bevacizumab in this disease will require randomized trials.
Table 3.
Phase II trials of bevacizumab + chemotherapy in gastric and GEJ tumors
| Regimen | Disease site | n | ORR (%) | Median PFS (months) | Median OS (months) | Reference |
| Cisplatin/irinotecan | GEJ/gastric | 47 | 47 | 8.3 | 12.3 | Shah et al. [20] |
| Docetaxel/cisplatin/5-FU | GEJ/gastric/esophagus | 44 | 67 | 12.0 | 16.2 | Kelsen et al. [21] |
| Docetaxel/cisplatin/irinotecan | GEJ/gastric/esophagus | 26 | 68 | N/A | N/A | Enzinger et al [25] |
| Oxaliplatin/docetaxel | GEJ/gastric | 38 | 42 | 6.6 | 11.1 | Current trial |
GEJ, gastroesophageal junction; ORR, overall response rate; PFS, progression-free survival; OS, overall survival; 5-FU, 5-fluorouracil; N/A, not available.
The bevacizumab, oxaliplatin, and docetaxel combination in general was well tolerated. The addition of bevacizumab did not significantly change the side-effect profile associated with oxaliplatin and docetaxel. However, bevacizumab-related toxicity in this trial, including GI perforations observed in three patients (8%), warrants comment. The perforations appear to occur early in the course of therapy, which is similar to the pattern reported in advanced colorectal cancer (CRC) treated with bevacizumab. Although the perforations resulted in significant morbidity, there were no deaths due to perforations in our study. The incidence rates of GI perforation observed in two previous trials of bevacizumab in gastric and GEJ cancers were 2.5% and 6% [20, 21]. The reported rates of GI perforation in patients with gastric cancer receiving systemic chemotherapy without bevacizumab and in patients with CRC treated with bevacizumab were 1.1% and 1.8%, respectively [26, 27]. These results clearly indicate that there is a higher likelihood of GI perforation in patients with gastric and GEJ tumors treated with bevacizumab. This may be attributed to a higher proportion of patients with an unresected primary tumor or peritoneal metastases. A recent meta-analysis of GI perforations in randomized trials of bevacizumab revealed an increased risk for perforation in patients receiving the 5 mg/kg per week dose as compared with those receiving the 2.5 mg/kg per week dose [28]. Strategies to address the higher risk for GI perforations in future clinical trials of bevacizumab in gastric or GEJ tumor could include consideration of a lower dose of bevacizumab [28] or exclusion of patients with clinical risk feature for perforation such as diffuse peritoneal metastasis or unresected primary tumor. One patient who had previously received radiation to the chest developed a TE fistula. The safety of administering bevacizumab after thoracic radiation therapy in patients with GEJ cancers requires further evaluation.
The incidence of other bevacizumab-related side-effects appeared to be lower in our study as compared with previously reported trials in patients with gastric and GEJ cancers. Grade 3 hypertension was observed in only 5% of the patients and was lower than the 28% rate previously reported in gastric and GEJ cancer trials [20]. VTEs were observed in one patient (3%) in the current study. The incidence of grade 3–4 VTE observed with bevacizumab in other gastric and GEJ cancer trials was 25%–31% [20, 21]. This difference in the toxicity profile between the current study and previously reported data may be related to the use of a lower dose of bevacizumab and/or the concurrent chemotherapy regimen. In CRC trials, the incidence of bevacizumab-related side-effects was similar in trials that used 2.5 and 5 mg/kg per week dose of bevacizumab [17, 29], and therefore, the reduction in the dose of bevacizumab in our study is unlikely to explain the difference in the observed rate of grade 3 hypertension and VTE. Instead, the lower incidence of these toxic effects observed in our study may be related to the substitution of oxaliplatin for cisplatin. This is supported by the results of the REAL-2 (Randomized ECF for Advanced and Locally advanced Esophagogastric cancer) trial where the incidence rates of VTE with cisplatin and oxaliplatin arms were 13.3%–16.9% and 7.5%–7.7%, respectively [5]. Similarly, the impact of cisplatin on renal function may explain the increased incidence of hypertension.
In conclusion, the results of this trial support the development of and need for further evaluation of bevacizumab in gastric and GEJ adenocarcinoma. In addition and perhaps more importantly, the identification and validation of predictive biomarkers for response or resistance to anti-VEGF therapies in gastric or GEJ cancer is vital to help select those patients most likely to benefit from this type of therapy.
funding
National Cancer Institute (Cancer Center Support grant CA-22453); Sanofi-Aventis and Genentech.
disclosure
The following authors have received support from Genentech or Sanofi-Aventis: PA Philip, BF El-Rayes, T Bekai-Saab, and U Vaishampayan.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–1289. [PubMed] [Google Scholar]
- 4.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 6.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15(1):261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Park YS, Chang MH, et al. A phase I/II trial of docetaxel and oxaliplatin in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2009;64(2):347–353. doi: 10.1007/s00280-008-0878-4. [DOI] [PubMed] [Google Scholar]
- 8.Hejna M, Raderer M, Zacherl J, et al. Phase II study of docetaxel in combination with oxaliplatin in patients with metastatic or locally advanced esophagogastric cancer previously untreated with chemotherapy for advanced disease: results of the Central European Cooperative Oncology Group Study ESGAS.1.2.001. Anticancer Drugs. 2008;19(5):535–539. doi: 10.1097/CAD.0b013e3282fb178a. [DOI] [PubMed] [Google Scholar]
- 9.Kim JG, Sohn SK, Chae YS, et al. Multicenter phase II study of docetaxel plus oxaliplatin combination chemotherapy in patients with advanced gastric cancer: Daegu Gyeongbuk Oncology Group. Br J Cancer. 2008;98(3):542–546. doi: 10.1038/sj.bjc.6604188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards D, McCollum D, Wilfong L, et al. Phase II trial of docetaxel and oxaliplatin in patients with advanced gastric cancer and/or adenocarcinoma of the gastroesophageal junction. Ann Oncol. 2008;19(1):104–108. doi: 10.1093/annonc/mdm449. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Endothelial cells and angiogenic growth factors in cancer growth and metastasis. Introduction. Cancer Metastasis Rev. 1990;9(3):171–174. doi: 10.1007/BF00046358. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto S, Yasui W, Kitadai Y, et al. Expression of vascular endothelial growth factor in human gastric carcinomas. Pathol Int. 1998;48(7):499–506. doi: 10.1111/j.1440-1827.1998.tb03940.x. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi Y, Cleary KR, Mai M, et al. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996;2(10):1679–1684. [PubMed] [Google Scholar]
- 15.Ikeguchi M, Oka S, Saito H, et al. The expression of vascular endothelial growth factor and proliferative activity of cancer cells in gastric cancer. Langenbecks Arch Surg. 1999;384(3):264–270. doi: 10.1007/s004230050202. [DOI] [PubMed] [Google Scholar]
- 16.Kanai T, Konno H, Tanaka T, et al. Anti-tumor and anti-metastatic effects of human-vascular-endothelial-growth-factor-neutralizing antibody on human colon and gastric carcinoma xenotransplanted orthotopically into nude mice. Int J Cancer. 1998;77(6):933–936. doi: 10.1002/(sici)1097-0215(19980911)77:6<933::aid-ijc23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24(33):5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 21.Kelsen DP, Jhawer D, Ilson DH, et al. Analysis of survival with modified docetaxel, cisplatin, fluorouracil and bevacizumab in patients with metastatic gastric and GEJ adenocarcinoma: results of a phase II trial. 2009 ASCO Annual Meeting, Orlando, FL. J Clin Oncol 2009; 27:15s (Abstr 4512) [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. National Cancer Institute Common Toxicity Criteria Version 3. http://ctep.cancer.gov/reporting/ctc.html (date last accessed, 28 February 2010) [Google Scholar]
- 24.Lee E, Wang JW. Statistical Methods for Survival Data Analysis. 3rd edition. Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 25.Enzinger PC, Ryan DP, Regan EM, et al. Phase II trial of docetaxel cisplatin irinotecan and bevacizumab in metastatic esophagogastric cancer. 2008 ASCO Annual Meeting, Chicago, IL. J Clin Oncol 2008; 26 (Suppl): (Abstr 4552) [Google Scholar]
- 26.Asmis TR, Capanu M, Kelsen DP, Shah MA. Systemic chemotherapy does not increase the risk of gastrointestinal perforation. Ann Oncol. 2007;18(12):2006–2008. doi: 10.1093/annonc/mdm357. [DOI] [PubMed] [Google Scholar]
- 27.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26(33):5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 28.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10(6):559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 29.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]