Abstract
CD8+ Tumor infiltrating lymphocyte (TIL) in human melanomas express high levels of PD-1 and are functionally impaired. However, adoptive cell therapy using in vitro-expanded TIL can be a highly effective therapy for patients with advanced melanoma. This discrepancy led us to further analyze the CD8+PD-1+ TILs. We found that the percentage of PD-1 expressing CD8+ T-cells was higher in the tumor digests that generate tumor-reactive TILs after in vitro culture in IL-2 (P=0.0007). Also sorted and expanded CD8+PD-1+ T-cells in tumor digests showed much higher tumor specific IFN-γ production compared with CD8+PD-1- T-cells. These results suggested that tumor-specific CD8+ T-cells in melanoma tumor digests are largely PD-1+, and this population can recover function after culturing in IL-2. PD-1 has been reported as an inhibitory receptor on T-cells. We found that the in vitro functional suppression of cultured-TILs from native levels of PD-L1 expression on melanomas was minimal, and moreover expression level of PD-1 on CD8+ tumor-specific TILs decreased during the culture. As a consequence, the PD-1 receptor can be a useful biomarker for enriching tumor specific T-cells from fresh melanomas.
Keywords: PD-1, PD-L1, TIL, tumor immunity, melanoma, tumor digest, tumor microenvironment
Introduction
Programmed cell Death 1 (PD-1, PDCD1, CD279) has been characterized as an inhibitory receptor on T-cells. Its physiological effects have been well delineated in animal models of chronic viral infection where chronically antigen exposed virus-reactive T-cells can enter a state of “exhaustion” mediated to some extent by PD-1 (1). Also in humans, a correlation between the disease progression of some viral infections and PD-1 expression levels in viral specific CD8+ T-cells in patient peripheral blood has been reported (2) (3). There are additional reports suggesting a role for PD-1 in the immune response to tumors. Our previous report showed that a majority of CD8+ T-cells in enzymatically-dispersed fresh melanomas (particularly the tumor reactive HLAA2/MART-1: 27-35 tetramer positive population) express PD-1, in contrast to T cells in normal tissues and peripheral blood lymphocytes (PBL) (4). This suggested that encountering tumor antigen in the tumor microenvironment may induce PD-1 expression on T-cells that recognize tumor. This report also showed that PD-1 expressing CD8+ T-cells in tumor digests displayed a globally impaired capacity to produce IFN-γ even when stimulated with PMA-ionomycin which bypasses TCR signaling. (4). These data suggested that the majority of tumor-specific T cells infiltrating melanomas are rendered non-reactive to tumors by the tumor microenvironment and this could be an explanation for continued tumor growth despite their presence.
Programmed cell death ligand 1 (PD-L1, B7-H1, CD274) is a ligand for PD-1, that can mediate inhibition of cytokine production and cell proliferation in PD-1 expressing T cells (5). PD-L1 is expressed by various tissues, including several human tumor types (6). A correlation of PD-L1 expression levels on tumor and an unfavorable prognosis for certain tumors has been reported (7) (8) (9) (10). Extending our previous report that PD-1 can be a marker of “exhausted” T-cells in tumor digests, these data point to PD-1 engaging PD-L1 on tumor as a potential mediator of the anergic state of these tumor resident, tumor-reactive T-cells.
Yet the paradox remains, that adoptive transfer of in vitro expanded tumor-infiltrating lymphocyte (cultured-TIL) can be clinically effective in patients with advanced melanoma. Using a regimen of preparative host lymphodepletion with cyclophosphamide and fludarabine followed by infusion of cultured-TIL, given with supportive systemic IL-2, objective responsive rates of 49 –72% have been seen with some patients maintaining complete responses beyond 5 years (11) (12) (13). The majority of responding patients had previously progressed after treatment with IL-2 without transfer of cultured-TIL. Therefore we undertook to further examine the state of PD-1 expressing TIL in fresh melanomas and follow their status during culture in IL-2. We also sought to determine if separation based on PD-1 expression in fresh TIL prior to culture could be used to enrich for tumor reactivity and if other markers could serve a similar purpose. Such information could be of utility in devising better approaches to selecting and growing TIL populations and perhaps predicting which patients may respond in future adoptive cell therapy trials.
Materials and methods
Tumor samples
Tumor specimens from A2+ (HLA-A*0201+; to facilitate detection of melanoma-associated antigen reactivity with available reagents) patients were processed as described (14). Briefly, surgically resected tumors were enzymatically digested with 0.1% collagenase type IV, 0.01% hyaluronidase type V, and 30 U/mL deoxyribonuclease I type IV (Sigma Chemical Co., St. Louis, MO, USA) in RPMI 1640 (Life Technologies, Gaithersburg, MD, USA) at room temperature overnight. After filtration and separation by density gradient, digested tumors were cryopreserved until use. On thawing, these tumor preparations showed total cell viabilities which ranged from 50-90%.
To establish tumor cell lines, 1 × 107 digested tumor cells were cultured in T75-cm2 flasks in RPMI 1640, including 10% fetal bovine serum (FBS; Life Technologies), 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL gentamicin, and 0.25 μg/mL amphotricin B (Fungizone; Biofluids, Camarillo, CA, USA). Tumors were passaged at approximately 80% – 90% confluence and used when free of fibroblasts and proliferating beyond the tenth passage.
Antibodies and reagents
The following monoclonal antibodies (mAb) specific for human antigens were purchased from BD Bioscience (San Jose, CA): APC, PE, or PE-Cy7-conjugated anti-CD3 (SK7), FITC-conjugated anti-CD8 (SK1), PE-conjugated anti-CD25 (2A3), PE-conjugated anti-CD27 (M-T271), PE-conjugated anti-CD28 (CD28.2), FITC-conjugated anti-CD57 (HNK-1), PE-conjugated anti-CD62L (Dreg-56), PE-conjugated anti-CD80, PE-conjugated anti-4-1BB (4B4-1), PE-conjugated anti-CTLA-4 (BNI3), PE-conjugated anti-HLA-DR (L243), APC-conjugated anti-human PD-1 (MIH4), PE-conjugated anti-human PD-L1 (MIH1), PE-conjugated anti-human IFN-γ (B27), and isotype controls conjugated to FITC, PE or APC. FITC-conjugated anti-human LAG-3 (17B4) was purchased from Alexis Biochemicals (San Diego, CA), and PE-conjugated anti-Tim3 (clone 344823) was purchased from R & D systems (Minneapolis, MN). HLAA2/MART-1: 27-35 tetramer was purchased from Beckman Coulter Immunotech. (Fullerton, CA). Gating was always freshly determined on concurrently stained isotype controls as background staining varied with time in culture.
In vitro expansion of TIL
Cryopreserved tumor digests were thawed into complete medium consisting of RPMI-1640 supplemented with 10% heat-inactivated human AB serum (Gemini Bioproducts, Calabasa, CA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotricin B. After washing, tumor digests were initiated in 2ml wells of complete medium and rhIL-2 (recombinant human interleukin 2, 6000 IU/ml, (Novartis, East Hanover, NJ) in a humidified 37 °C incubator with 5% CO2. Five days after initiation, one-half of the media was aspirated from the wells and replaced with fresh complete medium and IL-2, and this was repeated every 2-3 days thereafter as needed.
Flow Cytometry Analysis
Tumor digests or cultured TILs were re-suspended in staining buffer (phosphate-buffered saline [PBS] containing 3% FBS) and stained with fluorochrome-conjugated antibodies specific for human antigens (BD Bioscience). Stained cells were subsequently washed in staining buffer twice and briefly stained with propidium iodide (PI) to exclude nonviable cells prior to analyzing in a FACS Caliber (Becton Dickinson, San Jose, CA).
Intracellular staining of cultured-TIL to detect IFN-γ production was performed by using Cytofix/Cytoperm Kit (BD Bioscience) according to the product instruction. Before fixation and extra-cellular staining, cultured-TILs were co-cultured with melanoma cells (1 × 106 cells per well) in the presence of blefeldin A (Golgiplug; BD Bioscience) at 37°C for 6h, in 2 ml complete medium consisting of RPMI-1640 supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.5 μg/mL amphotricin B.
Tumor recognition by cultured-TIL
Cultured tumor lines (5-10 × 104 cells per well) were used as target cells. Cultured-TILs at 105 cells per well were added to tumor in a final volume of 200 μL and incubated for 20h. In some experiments, anti-PD-L1 mAb (eBioscience, San Diego, CA) or isotype control (P3: eBioscience) were added at 10μg/mL. Supernatant was assayed by ELISA for IFN-γ (Endogen, Woburn, MA). A cultured-TIL was defined as possessing specific reactivity if IFN-γ release was twice background (co-cultures of cultured-TIL with HLA-mismatched tumors) and at least 200 pg/ml.
Establishment of PD-L1 over-expressing tumor cell lines
To introduce PD-L1 into melanoma tumor lines, the PD-L1 gene was cloned by RT-PCR from a melanoma cell line (526mel) treated with IFN-γ (1000U/ml, 48h). The gene was sequenced and subcloned into the retroviral vector pRx-IRES-Bsr. Vesicular stomatitis virus G protein – pseudotyped retrovirus was prepared by transiently transfecting the 293 GP cell line as described (15). Melanoma tumor cell lines were infected with filtered cell-free viral supernatant in the presence of 8μg/mL polybrene and subsequently maintained in selection with 10 μg /mL Blasticidin S (EMD chemicals, Gibbstown, NJ).
PD-1 enrichment and T-cell expansion
For FACS sorting, cryopreserved tumor digests were thawed and re-suspended in complete medium consisting of RPMI-1640 supplemented with 10% heat-inactivated human AB serum, and incubated with FITC-conjugated anti-CD8, PE-conjugated anti-CD3, and APC conjugated anti-human PD-1 at 4°C for 1h. After wash, cells were briefly stained with propidium iodide for exclusion of nonviable cells. Cells were sorted into lymphocyte gated, PI-, CD3+, CD8+ and PD-1+ population, and lymphocyte gated, PI-, CD3+, CD8+ and PD-1- population by FACSAria (Becton Dickinson, San Jose, CA). If TIL were separated with magnetic beads, the tumor digest was washed and stained for 20 minutes on ice with APC-conjugated anti-PD-1 antibody (BD). After washing, the cells were incubated with microbeads-conjugated anti-APC (Miltenyi Biotec, Auburn, CA) and selected using LS columns (Miltenyi Biotec). These sorted or separated populations and unseparated tumor digest were expanded in T25 flasks with 2.5× 107 irradiated PBMCs pooled from 3 allogeneic donors in 25ml of RPMI + 10% human serum + anti-CD3 (30ng/ml OKT-3, Ortho-McNeil Phameceuticals, Rarian, NJ) (Rapid Expansion Protocol: REP). IL-2 (300 IU/ml) was added the next day. Fresh RPMI1640 + 10% human serum + IL-2 (300 IU/ml) were replenished on day 5. Reactivity of the expanded cells was tested on day 21 – day 28 by IFN-γ production.
Results
Our previous report showed that CD8+ T-cells in melanoma tumor digests contain a significantly higher percentage of PD-1+ cells compared with CD8+ T-cells in normal tissues and peripheral blood. Also, the majority of the tumor specific CD8+ population in tumor digests expressed PD-1 (4). This may imply that encountering tumor antigen in the tumor microenvironment induces PD-1 expression on tumor specific T-cells. Therefore, there is the possibility that tumor digests with a higher percentage of PD-1+ CD8+ T-cells would contain a higher proportion of tumor specific, albeit in situ anergized, T-cells. To examine this hypothesis, we analyzed the relationship between PD-1 expression in CD8+ T-cells in multiple tumor digests and tumor specific reactivity during long term in vitro culture (Figure 1). We found a clear correlation between the percentage of PD-1+ cells in the CD8+ T-cell population in fresh melanoma digests as well as on day 5 of culture and the reactivity of the TILs after 14-28 days in culture with IL-2. On the other hand, other T-cell markers we have examined (CD27, 4-1BB, and LAG-3 in Figure 1 and CD25, CD28, CD40L, CD80, CTLA-4, HLA-DR, and Tim-3; data not shown) did not show any correlation with tumor specific reactivity, except for 4-1BB which showed a correlation of phenotype with subsequent reactivity only at day 5 of culture.
Figure 1. Correlation between the initial expression level of PD-1 in tumor digest and TIL reactivity after in vitro culture.
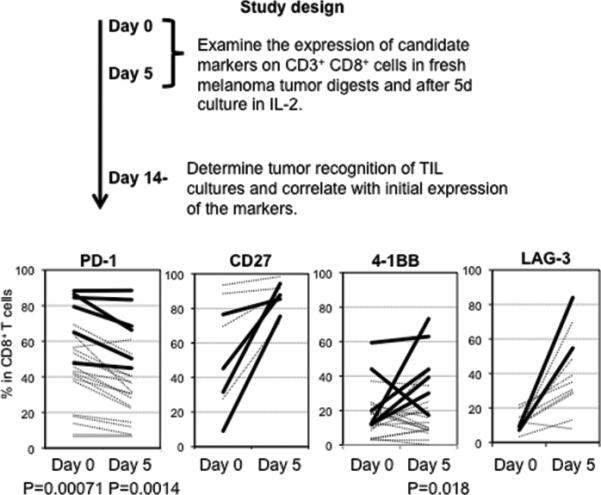
Cryopreserved tumor digests from HLA-A*0201 expressing patients with metastatic melanoma were thawed and half of them were stained with anti-CD3, anti-CD8, PI, and antibodies for the candidate molecules, immediately after thawing (day 0). Left tumor digests were cultured by standard TIL generating method (described in materials and methods), and at day 5, half of them were stained with same antibodies. The remaining TILs were grown for another 1-3 weeks, and the tumor reactivity of each was determined by IFN- γ production. A cultured TIL was defined as possessing specific reactivity if IFN-γ release was twice background (co-culture of cultured-TIL with HLA-mismatched tumors) and at least 200 pg/ml. The graph shows expression % of candidate molecules in lymphocyte, PI+, CD3+, and CD8+ gated population. Bold line shows expression level in “reactive” cultured-TILs, and broken line shows expression level in “non-reactive” cultured-TILs. Frequencies of marker expression in reactive and non-reactive TIL were analyzed using the two-tailed Student's t-test.
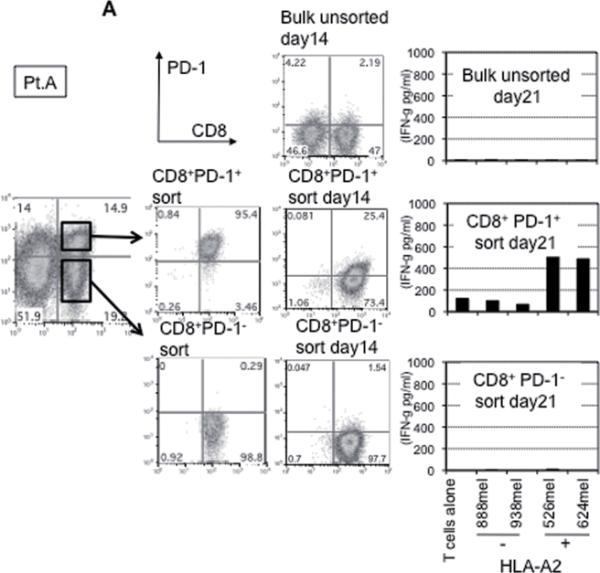
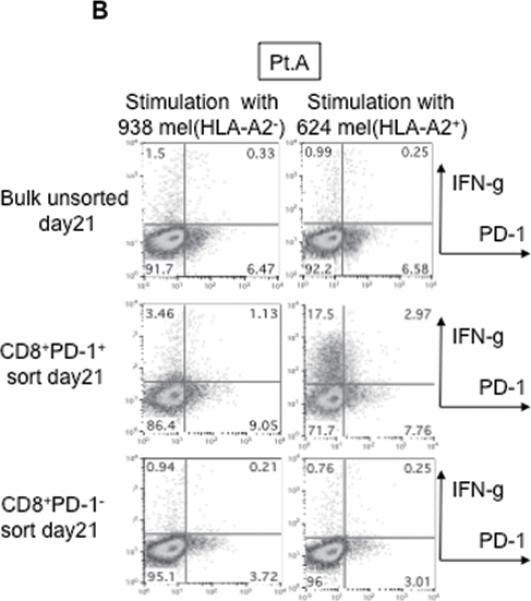
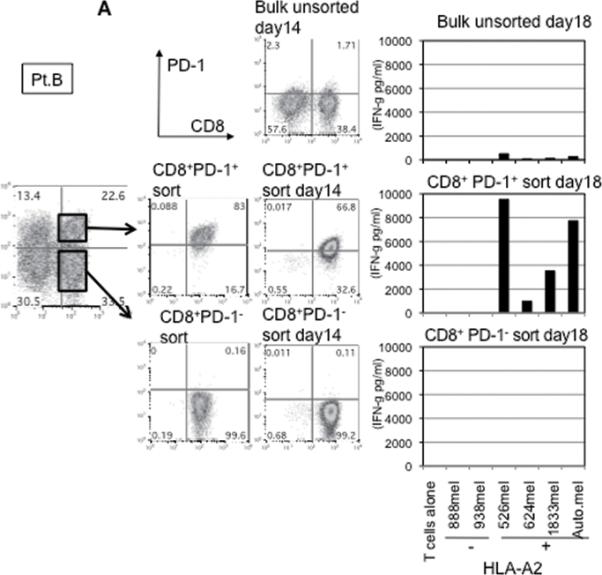
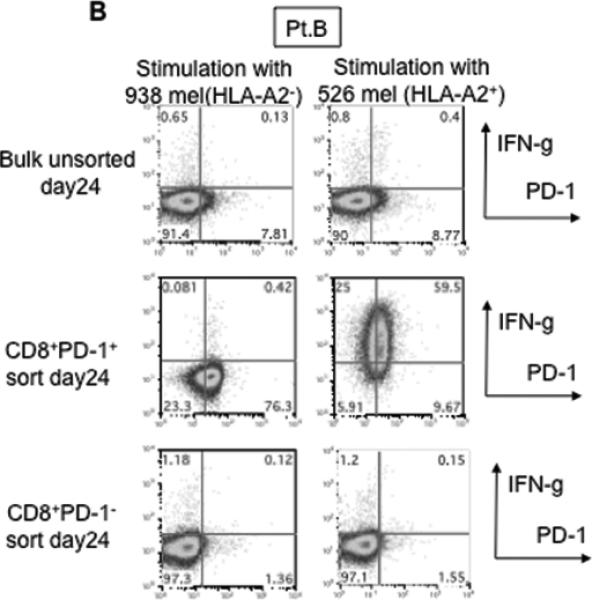
This finding raised the possibility that tumor specific CD8+ T-cells in tumor digests could be enriched by sorting of CD8+ PD-1+ T-cells prior to culture. To examine this hypothesis, we compared the reactivity of sorted CD8+ PD-1+ T cells, CD8+ PD-1- T-cells, and unsorted bulk TIL from several uncultured melanoma specimens. Each population was separated from freshly digested tumors by FACS and immediately expanded using anti-CD3 Ab, IL-2, and irradiated feeder cells to obtain sufficient cells for analysis. After 14 days of culture in IL-2, the expression levels of PD-1 were reexamined. The expression level of PD-1 on all sorted populations was down-regulated during in vitro expansion (Figure 2A and 3A). The reactivity of each population was assessed by IFN-γ release after stimulation with melanoma tumor cells (autologous tumor cells, HLA-A2 matched tumor cells, or HLA mismatched tumor cells as a control). In 3 of 5 patients tested, TILs expanded from CD8+ PD-1+ T-cells showed higher tumor specific IFN-γ release in ELISA, compared to CD8+ PD-1- sorted T-cells or unsorted TILs. Two examples are shown in Figure 2A and 3A. Also, the frequency of tumor reactive, IFN-γ producing T cells detected by IFN-γ intracellular staining was the highest in TILs expanded from PD-1+ population (Figure 2B and 3B). Among these three patients, two had sufficient tumor to replicate the entire sequence of separation, expansion and testing, one once and the other twice. All of these replicates showed intrapatient confirmation that tumor reactivity was highly enriched in the PD1+CD8+ fraction (details not shown). A sixth patient's tumor underwent positive separation of PD-1+ TIL (with no CD8 separation) to explore preparative scale methods for possible clinical application. Again the PD1+ fraction contained the highest reactivity and greatest tumor specificity. PD1- and unseparated cells showed both lower reactivity and more non-specific recognition of MHC-incompatible target cells similar to the unseparated TIL (Fig. 4). In the remaining 2 cases we examined, no specific tumor reactivity was detected in any of the separated populations (data not shown). PD-1 is reported as a marker of terminally differentiated “exhausted” T-cells in chronic viral infection. However, the PD-1+ sorted TIL population in these studies did not consistently develop an exhausted phenotype (CD27-, CD28-, CD62L-, and CD57+) after 14 to 18 days in culture in IL-2 (Figure 2A, 3A, and data not shown) (16).
Figure 2. Tumor reactive T cells in tumor digest can be enriched by sorting and subsequent in vitro expansion of CD8+ PD-1+ population in tumor digests.
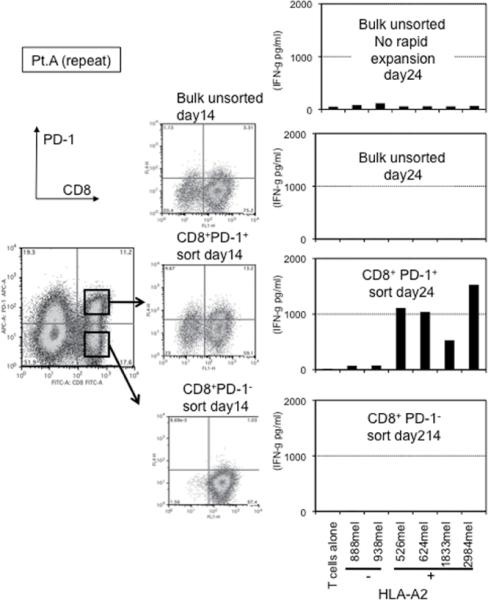
(A) CD8+PD-1+ and CD8+PD-1- populations in tumor digests were enriched by FACS sorting. Sorted cells and un-sorted bulk tumor digests were immediately stimulated and expanded by placing in a T25 flask with irradiated 2.5×107 PBMCs from 3 allogeneic donors in the presence of 30ng/ml OKT-3 Ab. IL-2 was added the next day (REP; rapid expansion). PD-1 expression level of each sorted population, immediately after sorting and after the expansion was determined by FACS analysis. Lymphocyte, PI-, and CD3+ population are shown. At day 18 – day 24, reactivity of each population was examined by IFN- γ release assay or (B) intracellular IFN- γ staining assay by tumor stimulation. Lymphocyte, PI-, and CD8+ population are shown. Representative data of 2 independent experiments for each case are shown.
Figure 3. Tumor reactive T cells in tumor digest can be enriched by sorting and subsequent in vitro expansion of CD8+ PD-1+ population in tumor digests.
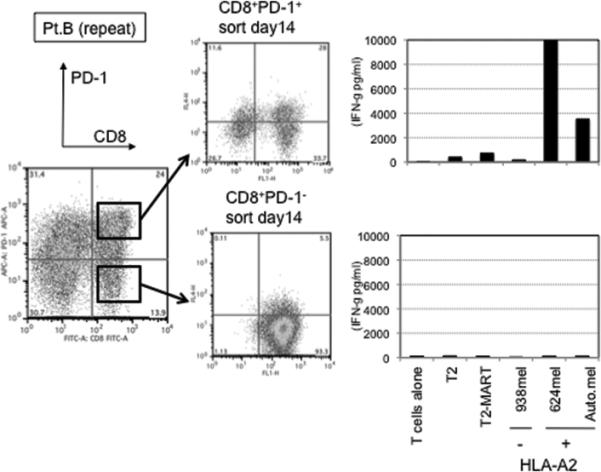
(A) CD8+PD-1+ and CD8+PD-1- populations in tumor digests were enriched by FACS sorting. Sorted cells and un-sorted bulk tumor digests were immediately stimulated and expanded by placing in a T25 flask with irradiated 2.5×107 PBMCs from 3 allogeneic donors in the presence of 30ng/ml OKT-3 Ab. IL-2 was added the next day (REP; rapid expansion). PD-1 expression level of each sorted population, immediately after sorting and after the expansion was determined by FACS analysis. Lymphocyte, PI-, and CD3+ population are shown. At day 18 – day 24, reactivity of each population was examined by IFN- γ release assay or (B) intracellular IFN- γ staining assay by tumor stimulation. Lymphocyte, PI-, and CD8+ population are shown. Representative data of 2 independent experiments for each case are shown.
Figure 4. Tumor reactive T cells in tumor digest can be enriched by bead separation of PD-1+ TIL and subsequent in vitro expansion.
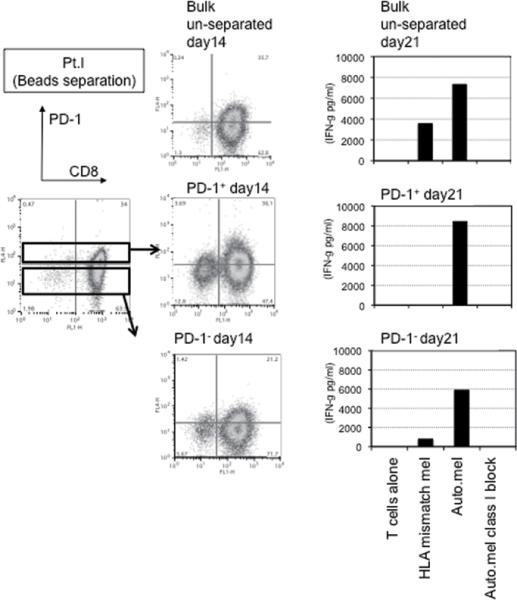
PD-1+ and PD-1- populations in fresh tumor digest were enriched by magnetic bead separation. Separated cells and un-separated bulk tumor digests were stimulated by the same method used on sorted populations. On day 21, reactivity of each population was examined by IFN- γ release assay against autologous tumor and MHC-mismatched allogeneic melanoma.
PD-1 functions as an immune-inhibitory receptor, and engagement of one of its ligands, PD-L1, inhibits TCR-mediated proliferation and cytokine production (5). Cultured TILs did not express their own PD-L1 even after PMA-ionomycin or IFN-γ stimulation (data not shown), however previous reports showed that PD-L1 is expressed on many tumor cells including melanoma in vivo (6). Therefore, we examined the kinetics of PD-1 expression on TILs during long term in vitro culture, when IL-2 is added and co-cultured tumor is lysed. Figure 5 shows that the percentage of CD8+PD-1+ cells in total CD3+ T-cells decreased during in vitro culture in IL-2. Also, the percentage of PD-1+ cells within the HLA-A2/MART-1: 27-35 tetramer positive populations decreased during culture (Figure 5). To exclude the possibility that this is due to overgrowth of CD8+ PD-1- T-cells, we confirmed that it is also observed in sorted CD8+ PD-1+ T-cells (Figure 2A and 3A).
Figure.5. Expression level of PD-1 in total CD8+ T-cells and tumor specific T-cells in tumor digests decreases during long-term in vitro culture.
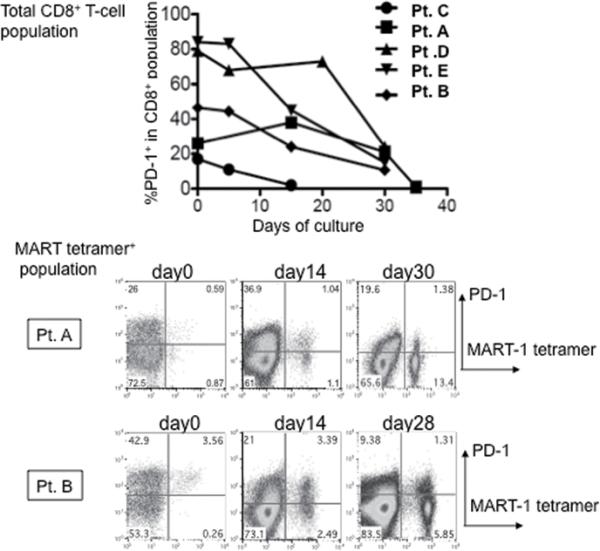
Kinetics of PD-1 expression level of total CD8+ T cells (upper panel; % PD-1+ in lymphocyte, PI-, CD3+, and CD8+ gated population) and HLA-A2/MART-1:27-35 tetramer+ population (bottom panel: dot plot of lymphocyte, PI-, and CD8+ gated population) are shown.
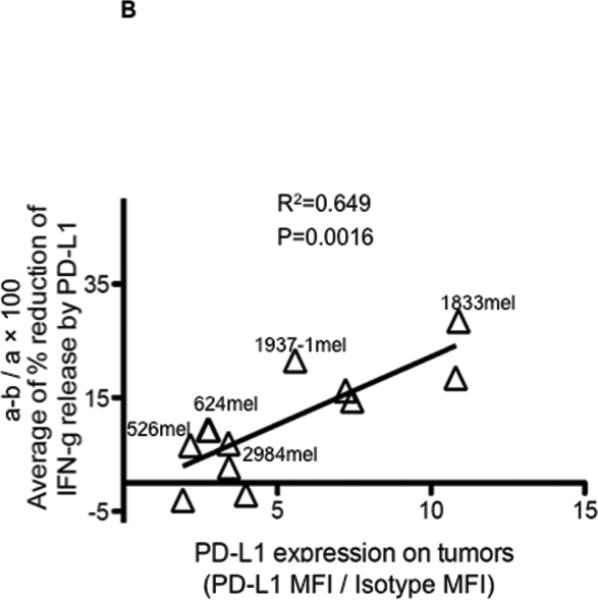
Finally, we investigated whether there was any short-term effect from PD-L1 expressed by melanoma cells, on tumor recognition by PD-1 expressing tumor-specific cultured-TILs. As shown in Figure 6A, recognition of tumors by PD-1 expressing cultured-TILs was suppressed by PD-L1 over-expressed on tumor cells, and this suppression was reversed after adding anti-PD-L1 antibody (top figure, control). The effect of anti-PD-L1 antibody blockade on the recognition of native melanomas was substantially less compared to tumors transduced with the gene encoding PD-L1, particularly with tumors where little PD-L1 could be induced with IFN-γ (bottom left). In fact, the impact of PD-L1 blockade was significantly correlated with PD-L1 expression level induced by in vitro pre-treatment with IFN-γ on tumor targets (Figure 6B).
Figure.6. Effect of PD-1 – PD-L1 signal blockade on tumor recognition by PD-1 expressing cultured-TILs are minimal.

(A) Reactivity of 3 different cultured-bulk TILs (expanded by in vitro culture in IL-2 for 20-30 days) that express PD-1 were assessed by 24 hour IFN- γ release assay by stimulation with HLA-A2 matched melanoma cell lines in the presence of anti-PD-L1 antibody or isotype control (10ug/ml). PD-1 expression on cultured-TILs used in this experiment is shown at the top of the figure. Lymphocyte, PI-, and CD8+ population are shown. Top figure is a control for the effect of anti-PD-L1 antibody, using tumor cells transduced with mock or PD-L1 gene. Histograms show PD-L1 expression level of tumor cells transduced with PD-L1. Shaded is stained for PD-L1, and the line is the isotype control. At bottom is a concurrent study using untransduced, unstimulated native melanomas.
At left are histograms are showing PD-L1 expression on tumor cells before (left) and after (right) exogenous IFN- γ stimulation (1000 U/ml, 48h). Filled is staining for PD-L1, and line is isotype control.
(B) Reduction of IFN- γ release by PD-L1 on native tumor cells is correlated with expression level of PD-L1 on native tumor cells after stimulation with exogenous IFN- γ. The expression level of PD-L1 on native tumors after IFN- γ stimulation (1000U/ml, 48h) is indicated as a ratio of MFI (MFI of PD-L1 staining and IgG1 isotype control staining). Percentage Reduction of IFN- γ release by PD-L1 is calculated by recognition of native melanoma cell lines in the presence of anti-PD-L1 antibody (a, indicated Figure 6A) and recognition to native melanoma cell lines in the presence of isotype control (b, indicated Figure 6A). Average values derived from the 3 different cultured-TILs used in Figure 6A are used for the correlation analysis.
Discussion
PD-1 was identified as a gene up-regulated in a T-cell hybridoma undergoing apoptotic cell death(17). The cytoplasmic domain of PD-1 contains two tyrosine signaling motifs, that can attenuate the TCR/CD28 signal(18). PD-1 knockout mice develop autoimmune disease in various tissues (19, 20). These findings support the concept that this molecule plays a role as an inhibitory receptor. Recently, PD-1 was reported as a marker of exhausted viral specific CD8+ T-cells and its expression level correlated with increased viremia in chronic viral infection(1-3). Our previous report also showed that PD-1+ T-cells in freshly dispersed melanomas were functionally impaired(4) when assessed by IFN-γ production, a cytokine considered essential to an effective anti-tumor immune response(21). Thus despite T-cells with anti-tumor potential being present, melanomas seem to be populated with PD-1 expressing CD8+ T-cells incompetent in their cognate secretion of IFN-γ.
In this report we have examined the character of CD8+ PD-1+ T-cells in freshly dispersed melanomas and examined the functional role of PD-1 expressed by these TILs. Although in our previous report, the CD8+ PD-1+ T-cell population in un-cultured tumor digests could not make IFN-γ by stimulation with PMA-ionomycin, this report shows that purified populations of these cells regain function during in vitro expansion in IL-2 and can make IFN-γ on stimulation with autologous or HLA matched tumor cells (Figure 2 and 3). This is in accord with clinical results where the adoptive transfer of cultured-TIL which have tumor reactivity after in vitro expansion can mediate the regression of metastatic disease (11-13). Although we saw consistent declines in the expression of PD1 on TIL during culture in IL-2, we have not shown that this is the mechanism by which these TIL lose their anergy. In fact, the possibility remains that other inhibitory receptors, cells and cytokines could also play important roles in anergizing TIL in situ.
The fact that PD-1 on CD8+ T-cells is induced by antigen-specific activation suggested that the PD-1+ T-cells in the tumor microenvironment may be exactly the population that is being chronically exposed to relevant tumor antigens. If the anergy that results is indeed reversible, then PD-1 expression may be an excellent biomarker for cells with tumor reactivity. Such a biomarker may circumvent the need for cumbersome assays and autologous tumor target cells currently needed to select the most tumor-reactive T-cell subpopulations (22). The results in this report suggest that PD-1 could serve that function and the clinical anti-tumor activity of these selected cells after administration in vivo should be evaluated. There have been reports of CD137 (4-1BB) and CD131 being biomarkers for enrichment of antigen specific populations from antigen stimulated bulk PBLs(23, 24). However the requirement for stimulating the bulk T-cell population before assessing these biomarkers and the consequent need for defined T-cell reactivities or having autologous tumor cells for stimulation limits the utility of these approaches. In contrast, PD-1 can be applied directly to freshly dispersed tumors with no stimulation required.
PD-1 is reported as a receptor mediating an inhibitory signal via interaction with PD-L1(5) and this ligand has been reported to be expressed by melanoma cells. Therefore, we determined the impact of this potential interaction on cultured-TIL in the tumor microenvironment(6). However, we showed that PD-1 expression by the total CD3+ CD8+ population and the MART-1 specific T-cell population (by tetramer staining) in tumor digests decreased during in vitro culture in IL-2 (Figure 5). Although high levels of PD-L1 on tumor after retroviral transduction could decrease T-cell effector functions in vitro, this impact was much less apparent using native melanoma lines (Figure 6). Still the complex interaction between PD-L1 induction on tumor cells by IFN-γ and the modulation of IFN-γ secretion by T-cells when exposed to tumors expressing PD-L1 remains unclear. Short term functional assays and acute exposures to IFN-γ and PD-L1 may not accurately reflect the biology of the tumor microenvironment.
Whatever the physiological role of PD-1 as an inhibitory receptor on melanoma TIL, its behavior as an activation-induced inhibitory receptor and the fact that its impact diminishes as TIL are cultured in IL-2, suggests that it might have utility as a biomarker of T-cells in melanoma chronically exposed to relevant tumor antigens. Here, purified fresh TIL expressing this marker contained the vast majority of tumor-reactive CD8+ T-cells which regained functional activity after culture in IL-2. This finding may greatly simplify the methods for selecting and expanding TIL with tumor reactivity and improve the efficacy of adoptive T-cell therapy for melanoma.
Acknowledgement
The authors are grateful to A. Mixon and S. Farid for performing flow cytometry.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 2.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 3.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009 doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 8.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 9.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang QJ, Hanada K, Perry-Lalley D, et al. Generating renal cancer-reactive T cells using dendritic cells (DCs) to present autologous tumor. J Immunother. 2005;28:551–9. doi: 10.1097/01.cji.0000175495.13476.1f. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 21.Palmer DC, Chan CC, Gattinoni L, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci U S A. 2008;105:8061–6. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–51. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfl M, Kuball J, Ho WY, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–10. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selleri S, Deola S, Pos Z, et al. GM-CSF/IL-3/IL-5 receptor common beta chain (CD131) expression as a biomarker of antigen-stimulated CD8+ T cells. J Transl Med. 2008;6:17. doi: 10.1186/1479-5876-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


