Abstract
Objectives
The designer antibacterial peptide A3-APO is efficacious in mouse models of Escherichia coli and Acinetobacter baumannii systemic infections. Here we compare the efficacy of the peptide with that of imipenem and colistin in A. baumannii wound infections after burn injury.
Methods
CD-1 mice were inflicted with burn wounds and different inocula of A. baumannii, isolated from an injured soldier, were placed into the wound sites. The antibiotics were given intramuscularly (im) one to five times. Available free peptide in the blood and the systemic toxicity of colistin and A3-APO were studied in healthy mice.
Results
While toxicity of colistin was observed at 25 mg/kg bolus drug administration, the lowest toxic dose of A3-APO was 75 mg/kg. In the A. baumannii blast injury models, 5 mg/kg A3-APO improved survival and reduced bacterial counts in the blood as well as in the wounds and improved wound appearance significantly better than any other antibiotic treatment. The free peptide concentration in the blood did not reach 1 µg/mL.
Conclusions
Peptide A3-APO, with an intramuscular therapeutic index of 15, is more efficacious and less toxic than any existing burn injury infection therapy modality against multidrug-resistant Gram-negative pathogens. A3-APO administered by the im route probably binds to a biopolymer that promotes the peptide's biodistribution.
Keywords: bacterial counts, burn injury, MDR Gram-negative bacteria, mouse models, survival rate, therapeutic index
Introduction
Infections caused by Gram-negative bacteria are the major causes of morbidity and mortality after burn injuries in both military and civilian hospitals.1,2 The most common pathogens in blast wounds are Acinetobacter baumannii strains (33%) followed by Escherichia coli and Pseudomonas spp. (14%), with Klebsiella spp., Enterobacter spp. and Proteus spp. in lesser proportion (5%–6%).3 In addition to systemic infections leading to sepsis and clinical syndromes of soft tissue and skin infections,4 osteomyelitis from multidrug-resistant (MDR) A. baumannii has emerged in >30% of combat-related injuries in Iraq and Afghanistan.5
β-Lactam-resistant A. baumannii infections are usually treated with imipenem or, if it fails, with colistin,6 although the mortality rate after burn injuries and colistin treatment at an intensive care unit remained high at 41%.7 Unfortunately, in a 2 year period starting from October 2005, resistance to imipenem in patients injured in Operation Iraqi Freedom and Operation Enduring Freedom increased from 13% to 46%, with similarly disturbing trends of an increasing resistance rate to colistin treatment.8 Approximately half of the current carbapenem-resistant A. baumannii clinical strains are also resistant to colistin.9 Colistin also suffers from a low therapeutic index, with an intramuscular (im) LD50 value of <40 mg/kg in CD-1 mice, while the efficacious im dose is 2.5–5 mg/kg given twice daily.10 Colistin is a cyclic peptide containing a number of non-natural diaminobutyric acid residues. Contemporary regimens that are used against A. baumannii involve two additional drugs that contain at least one amide bond: tigecycline and daptomycin.11,12
In our quest to find a suitable therapy for MDR Gram-negative infections, we turned to proline-rich designer antimicrobial peptides represented by their flagship analogue, designated A3-APO.13 The sequence of A3-APO [(Chex-Arg-Pro-Asp-Lys-Pro-Arg-Pro-Tyr-Leu-Pro-Arg-Pro-Arg-Pro-Pro-Arg-Pro-Val-Arg)2-Dab] is optimized for maximal in vitro efficacy against MDR Enterobacteriaceae alone or in combination with conventional antibiotics (Chex corresponds to 1-amino cyclohexyl carboxylic acid and Dab corresponds to 2,4-diaminobutyric acid).14,15 The main selection criteria were increased activity on bacterial membrane disintegration while maintaining the peptide's ability to bind the intracellular target DnaK and inhibit chaperone-assisted protein folding.16 Armed with these properties, A3-APO is one of the very few peptide antibiotics that in mouse models are able to fight systemic MDR E. coli and A. baumannii infections at levels similar to or better than imipenem administered at a significantly higher dose.17,18 Intriguingly, the in vivo efficacy parameters greatly exceed those that could have been expected purely from in vitro MIC values against the infectious strains. The optimal intraperitoneal (ip) and im doses are 10 and 5 mg/kg, respectively.17,18 A3-APO is actually a prodrug; when administered intravenously (iv) it quickly decomposes in the blood into smaller fragments, among which a single-chain derivative retains, or sometimes exceeds, the in vivo activity of the parent dimer.17–19
When given ip or iv, antimicrobial peptides frequently exhibit low therapeutic indices20 and A3-APO is no exception. However, the im therapeutic index in our preliminary studies was at least 12 and this value is superior to that of colistin.18 The favourable im toxicity profile and MDR A. baumannii systemic efficacy data prompted us to investigate the therapeutic potential of A3-APO in mouse models of soft tissue infections in burn wounds in comparison with colistin and imipenem. We also performed a brief pharmacokinetic analysis to obtain insights into the biodistribution and toxicity parameters of A3-APO following its administration im.
Materials and methods
Antibiotics and MIC determinations
Peptide A3-APO was synthesized on the solid phase and purified by reversed-phase HPLC (RP-HPLC) as described previously.14 After purification, the peptide was lyophilized twice from 2% aqueous acetic acid solution to eliminate all residual chromatography solvents and counterions. To exclude the possibility that the biological activity is due to non-peptide-related contaminants, four efficacy assays were run using three different peptide preparations. The colistin sulphate preparation (15 000 IU/mg) was from Sigma-Aldrich (Budapest, Hungary) and imipenem was from MSD (Budapest, Hungary; tienamycin formamidine monohydrate:sodium cilastatin 1:1, marketed as Tienam).
Conventional antibacterial growth inhibition assays were performed using sterile 96-well polypropylene plates in a final volume of 100 µL.16 Briefly, 50 µL of a suspension of mid-logarithmic phase bacterial cultures diluted to 5 × 105 cfu/mL in Mueller-Hinton broth was added to 50 µL of serially diluted antibiotics. The highest peptide concentration was 128 mg/L. Cultures were then incubated at 37°C for 16–20 h without shaking, and growth inhibition was measured by recording the absorbance at 600 nm using a microplate reader. MICs were identified as the lowest antimicrobial concentrations at which the 600 nm absorbance did not exceed that of the negative control (medium only) values.
Animals
Female CD-1 mice weighing 20–25 g (Charles River Laboratories, Budapest, Hungary) were used for the in vivo studies. Mice were housed in plastic type 2 cages, three to five mice per cage on softwood granules as bedding. The room was kept between 21°C and 25°C with a 12 h : 12 h light : dark cycle. The animals had free access to tap water and pelleted rodent food. The assays were initiated after a 3 week acclimatization period. Upon completion of the experiments, the mice were euthanized by diethyl ether inhalation. Animals were maintained and handled in accordance with the recommendations of the Guidelines for the Care and Use of Laboratory Animals and the experiments were approved by the Animal Care Committee of Semmelweis University (permission no. 399/003/2005).
In vivo toxicity and preliminary pharmacokinetics
For acute toxicity studies, peptide A3-APO and colistin sulphate were dissolved in water and given as bolus injections at 5, 25, 50, 75 or 100 mg/kg im in the left thigh. Mice were observed for signs of systemic toxicity (see footnotes to Table 1).
Table 1.
Gross toxicity in mice treated with im colistin or peptide A3-APO
| Dose (mg/kg) | Mice (n) receiving colistin | Mice (n) receiving A3-APO |
|---|---|---|
| 5 | 3, no effect | therapy dose |
| 25 | 1, no effect; 2, toxicity level 1 | 3, no effect |
| 50 | 3, toxicity level 2 | 3, no effect |
| 75 | NT | 2, no effect; 1, toxicity level 1 |
| 100 | NT | 3, toxicity level 2 |
NT, not tested.
In each group three CD-1 female mice received a bolus inoculation of drugs into the left thigh.
Toxicity grading: level 1, narrowing of eyes; level 2, crouching and cuddling.
Most mice recovered 3 h after treatment.
For the pharmacokinetic analysis, 100 µg of peptide A3-APO (5 mg/kg) dissolved in 200 µL of sterile PBS, pH 7.2, was injected im into healthy CD-1 mice (Charles River Laboratories) using four mice for each timepoint. About 100 µL of blood was taken from the eye at 0 (immediately after peptide administration), 5, 15, 45, 90 and 120 min. Each animal was used only at two timepoints. Cells were centrifuged at 5000 rpm and 20 µL of aqueous 15% trichloroacetic acid was added per 60 µL of plasma. After repeated centrifugation at 12 000 rpm, 0.5 µL of supernatant was loaded on to a matrix-assisted laser ionization/desorption (MALDI) time-of-flight (TOF) tandem mass spectrometer (MS) (MALDI-TOF/TOF-MS, 4700 Proteomics Analyzer; Applied Biosystems, Weiterstadt, Germany) using α-cyano-4-hydroxycinnamic acid as the matrix. Additionally, a combined volume of 210 µL of supernatant was loaded on to a C18 HPLC column that had previously been calibrated with 50 ng, 200 ng, 500 ng and 1 µg of peptide A3-APO dissolved in PBS. Absorbance was measured at 214 nm.
Efficacy assays
Mice were anaesthetized with an im injection of a mixture of 6 mg/kg ketamine and 1 mg/kg xylazine and burn injury was inflicted by pressing a 1 g hot metal weight (diameter 11 mm, temperature 90°C) to their shaved dorsal skin.21 The weight was pressed to the skin for 2 min. Three hours after burn injury, A. baumannii BAA-1805 (purchased from ATCC, and isolated from a Canadian soldier injured in Afghanistan)22 was injected (0.5 × 16 mm 25G needle) into the wound. Treatment started immediately after bacterial inoculation. For the treatment, the drugs were administered im into the right hind leg for single dosing and into the right and left hind legs and right and left front legs (in this order) for repeat treatment.
Assay 1: low-dose daily A. baumannii infection, daily A3-APO treatment
An amount of 2 × 103 cfu/wound/day of A. baumannii BAA-1805 was injected into six mice on 5 consecutive days. Three mice were treated with 5 mg/kg A3-APO on days 0, 1, 2, 3 and 4 immediately after infection. Blood was taken from the tail vein 3 h after the first inoculation, after 2 days and on day 4. On day 5 the mice were killed by diethyl ether inhalation and their wound bacterial load was counted.
Assay 2: high-dose single A. baumannii infection, single-dose A3-APO, imipenem and colistin treatment
An amount of 7.2 × 109 cfu/wound A. baumannii BAA-1805 was injected once into 28 mice. The mice were split into four groups. One group of seven mice remained untreated, one group received 5 mg/kg A3-APO, one group received 5 mg/kg colistin and one group received 40 mg/kg imipenem im immediately after infection. Blood was taken from the tail vein, and the blood bacterial counts were determined 1, 2, 3 and 4 h after treatment. Surviving mice were photographed on day 5 and killed by diethyl ether inhalation, their wounds were excised and the bacterial load in the wound was counted.
Assay 3: extremely high-dose single A. baumannii infection, daily treatment with A3-APO, imipenem and colistin
An amount of 2 × 1011 cfu/wound A. baumannii BAA-1805 was injected into 28 mice on day 0. The mice were split into four groups. One group of seven mice remained untreated, one group received 5 mg/kg A3-APO, one group received 5 mg/kg colistin and one group received 40 mg/kg imipenem im on days 0, 1, 2, 3 and 4. Surviving mice were killed on day 5 by diethyl ether inhalation, their wounds were excised and the bacterial load in the wound was counted.
Effect of A3-APO on uninfected burn wounds
Four mice did not receive any medical intervention after burn injury and four mice received 5 mg/kg A3-APO im 3 h later and on 4 consecutive days. On day 5 the mice were killed by diethyl ether inhalation, and their wounds were removed and fixed in 10% buffered formaldehyde pH, 7.4. After 24 h fixation, the specimens were dehydrated in graded ethanol and placed in methylbenzoate for 1 h then transferred to xylene and embedded in paraffin. Paraffin sections (6 μm thick) were deparaffinized and stained with haematoxylin and eosin. Photomicrographs were taken using a Zeiss light microscope equipped with a charge-coupled device camera. The final magnification of the pictures was 100-fold.
Results
Toxicity and pharmacokinetics
Earlier we reported that we did not observe any systemic toxic effect of peptide A3-APO when given im up to the highest tested dose of 60 mg/kg.18 However, the mice exhibited signs of muscle pain at the site of inoculation, which we interpreted as being potentially due to leftover solvents and trifluoroacetate counterions after peptide RP-HPLC purification and lyophilization. After all trifluoroacetates had been replaced with acetates, the A3-APO toxicity assay was repeated side-by-side with colistin. For A3-APO, the mice did not show any signs of systemic toxicity at 25 and 50 mg/kg doses (Table 1). The first toxic sign appeared at a 75 mg/kg dose when one of the three animals narrowed its eyes. At 100 mg/kg all three mice crouched, signalling more serious discomfort. Clearly, highly purified peptide A3-APO lacks low-dose toxicity when given im. In contrast, two of the three colistin-treated mice exhibited signs of minor toxic effects at a dose as low as 25 mg/kg, with universal and more serious discomfort at 50 mg/kg (Table 1). While we could not reproduce the lethal effects of colistin at 40 mg/kg, our toxicity data are in the neighbourhood of those reported by others.10
We failed to detect any free peptide or degradation product in mouse blood by mass spectrometry or chromatography in the time period 5 min to 2 h after im A3-APO administration. Identical mass spectrometry technology allowed us earlier to identify A3-APO degradation products upon iv dosing.19 Spiking the samples with 0.5 or 500 ng A3-APO provided clear and quantifiable signals in MALDI and RP-HPLC, respectively. Thus, the free A3-APO concentration in the blood or that of any single metabolite did not reach 1 µg/mL in the plasma taken from the animals. This amount would correspond to 20% bioavailable A3-APO or any single degradation product, assuming homogeneous biodistribution all over the body. We concluded that upon im administration A3-APO binds to a carrier biomolecule and the complex remains fully active in the circulation.
In vivo efficacy assay design
Acinetobacter infections among combat casualties appear to be primarily the result of nosocomial transmission and not from colonization of the casualty at the time of injury or environmental contamination.23 In general, A. baumannii wound infections occur in civilian human and veterinary hospitals during periods when antibiotics are freely available to the physicians.24,25 Accordingly, we waited 3 h after the burn injury to mimic the time until the casualties reach hospital and applied the first antibiotic treatment immediately after infecting the burn wounds. To model various scenarios of infection, we used three treatment regimens. In assay 1, a low-dose daily infection was applied with daily A3-APO treatment. As 40% of the gowns and gloves worn by healthcare workers entering the rooms of colonized MDR A. baumannii patients are contaminated with the bacterium,26 this model represents a uniform nosocomial transmission scenario. Since in this assay the untreated mice remained alive, the efficacy of A3-APO alone was studied and only on a small number of animals. In assay 2 we modelled a more serious single infection event when some of the untreated mice were expected to succumb to sepsis. Here we treated the animals only once, and the efficacy of A3-APO and the currently employed antimicrobial drugs was compared. All three drugs were applied at the clinically recommended dose, but to allow direct comparison of the efficacies all were administered im. In the third assay an extraordinarily high infectious inoculum was used and the animals were treated with the same antibiotics on 5 consecutive days, representing life-saving efforts in military and civilian hospitals.
Assay 1
Due to the low daily bacterial inocula, no bacterium was found in the blood 3 h after the first inoculation, or 2 or 4 days later. On day 5 the average wound bacterial counts of mice treated with A3-APO were 3.4 × 101 cfu/mg tissue (1.4 × 101, 4.5 × 101 and 4.2 × 101 cfu/mg), representing an almost 3 log10 unit reduction compared with untreated animals, in which the average wound bacterial counts were 1.5 × 104 cfu/mg tissue (1.5 × 103, 2 × 103 and 4.2 × 104 cfu/mg). These initial results confirmed the utility of the peptide in A. baumannii wound infections after burn injuries.
Assay 2
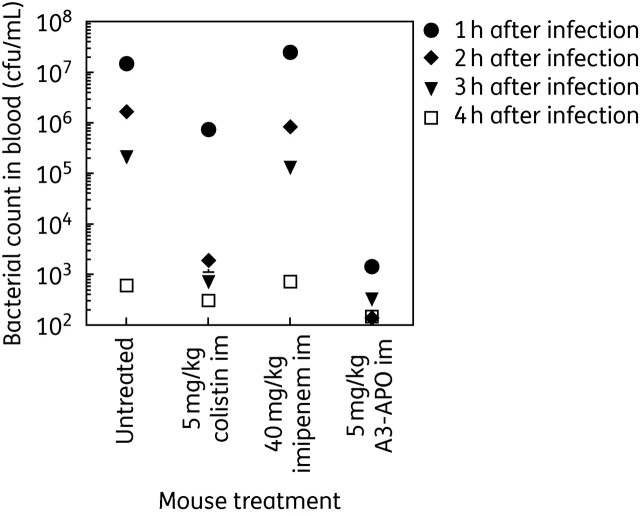
Two of the seven untreated control mice succumbed to bacterial sepsis on day 2, reflecting the increased bacterial inoculum compared with assay 1. At 1–3 h after infection/treatment, mice receiving no drug or dosed with imipenem or colistin were ill but later recovered. The A3-APO-treated animals had no visible signs of discomfort. Two animals per group were bled 1, 2, 3 and 4 h after infection/treatment. The blood bacterial counts immediately after infection mirrored the behavioural signs: 1 h after the first dose of antimicrobials, the bacterial load in the blood of untreated, colistin-treated and imipenem-treated animals exceeded 106 cfu/mL (Figure 1). In contrast, the blood bacterial counts of A3-APO-treated mice were in the low 103 cfu/mL range, representing a 4 log10 reduction compared with untreated and imipenem-treated animals and a 3 log10 reduction compared with colistin-treated animals (Figure 1). With advancing time the blood bacterial load was reduced in all four groups but was always the lowest in the peptide-treated animals. In fact, only the peptide-treated mice had close to sterile blood in the 3–4 h time period.
Figure 1.
Blood bacterial counts without or after im drug dosing in A. baumannii BAA-1805-infected CD-1 female mice. Bacteria (7.2 × 109) were inserted into burn wounds 3 h after injury. Drugs were injected into the left leg simultaneously with infection. Blood was taken from two animals after 1, 2, 3 and 4 h of infection/treatment and the number of bacteria in the blood was counted. Treatment with peptide A3-APO reduced bacterial counts to close to the detection level and significantly better than any other therapy measure.
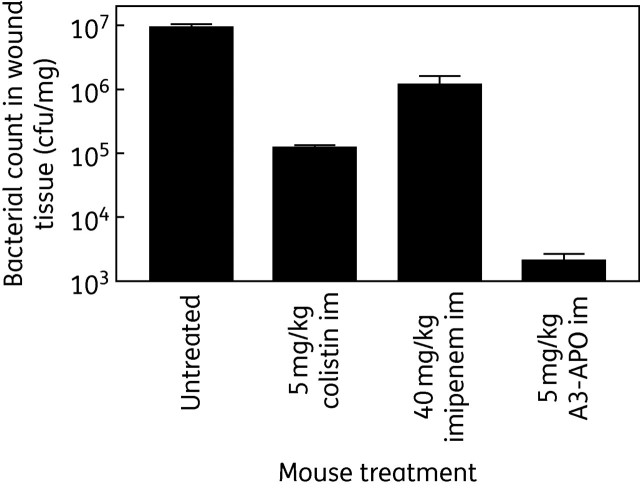
After 5 days, surviving mice still had wounds on their backs, but the wound sizes were dependent upon the treatment they received. Untreated mice carried the most extensive lesions in addition to their coat being wet and knotted. The colistin-treated and imipenem-treated animals had smaller wound sizes but the damage was not localized to the burn site. Mice receiving A3-APO treatment had small wounds with clear surroundings. After euthanasia the wound bacterial loads were counted from two animals in each group. Once again, the wounds of untreated animals contained the highest bacterial load (107 cfu/mg), followed by imipenem-treated (106 cfu/mg) and colistin-treated (105 cfu/mg) mice (Figure 2). Peptide A3-APO-treated mice had wound bacterial loads close to the lowest detection limit of 1000 cfu/mg tissue.
Figure 2.
Long-term success of single-dose antimicrobial treatment of CD-1 mice infected with A. baumannii BAA-1805 under a burn wound. Five days after infection/im treatment the mice were photographed and euthanized. Wounds were excised and the remaining bacterial loads were counted. The graph shows the average bacterial counts from two mice, determined in duplicate from each mouse. The only treatment option that managed to reduce the bacterial load to close to the detection limit was that with peptide A3-APO. In addition, peptide-treated mice looked healthier than others and had smaller wound sizes.
Assay 3
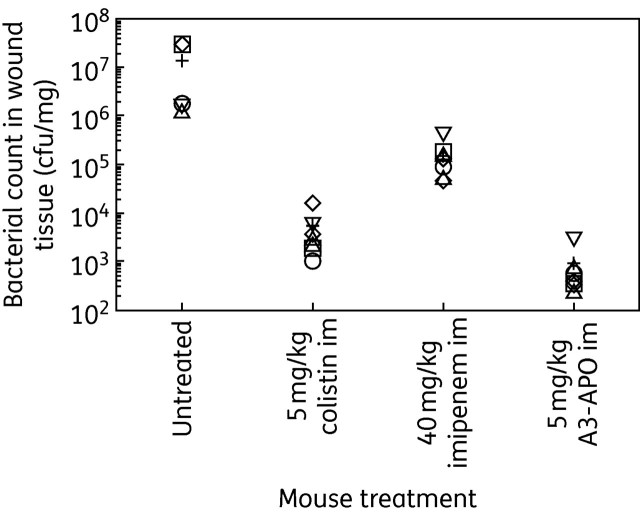
The very high infectious dose had a major effect on the gross health condition of the mice. Two of the seven animals in the control group died on day 2. The imipenem-treated mice survived but were generally very ill and the colistin-treated mice were ill after each treatment, although they recovered after 1 h. Mice receiving A3-APO treatment looked healthy throughout the experiment. The higher bacterial inocula compared with assay 2 were more than compensated for by the daily antibiotic treatment. By day 5 imipenem had reduced the wound bacterial counts 100-fold to 105 cfu/mg tissue and colistin managed to reduce it further to the mid-103 cfu/mg range (Figure 3). A3-APO-treated mice had fewer than 1000 cfu/mg A. baumannii in their wounds.
Figure 3.
Treatment of CD-1 mice infected with a very high dose of A. baumannii BAA-1805 under a burn wound. The mice received 2 × 1011 cfu of bacteria/wound. The first dose of antibiotics was administered at the time of infection and was followed by daily dosing for 4 consecutive days. Two untreated mice in the untreated group died of sepsis on day 2. On day 5 the surviving mice were euthanized, their wounds were excised and the remaining bacterial loads were counted. Open symbols represent the bacterial load in the wounds of single mice; the black cross is the average from seven (five in the untreated group) animals. A3-APO was more effective than any other antibiotic.
Promotion of wound healing without direct pathogen inoculation
Although the main thrust of this report is the treatment of A. baumannii wound infections when physical contact is made between soft tissue injury and bacteria, we also wanted to examine whether peptide A3-APO can improve wound healing when bacteria reach the injury site via environmental contamination. To this end, in one group of mice the burn injury was untouched for 5 days and in another group mice were treated five times with A3-APO after injury infliction. The wound tissues were removed after 5 days, fixed on slides and submitted to histopathology evaluation. In the tissue of untreated mice, more than half of the epithelial region was excoriated (Figure 4). Under the epithelium, a homogeneous eosinophilic and necrotic material was observed that contained polymorphonuclear pus cells and was surrounded with loose granulation tissue. In contrast, in the tissue of A3-APO treated mice there were clear signs of a regenerated epithelial layer under the necrotic surface, without pus cells and with decreased levels of inflamed granulation (Figure 4). Apparently peptide A3-APO improved the skin conditions by prophylactic elimination of invading environmental microbes.
Figure 4.
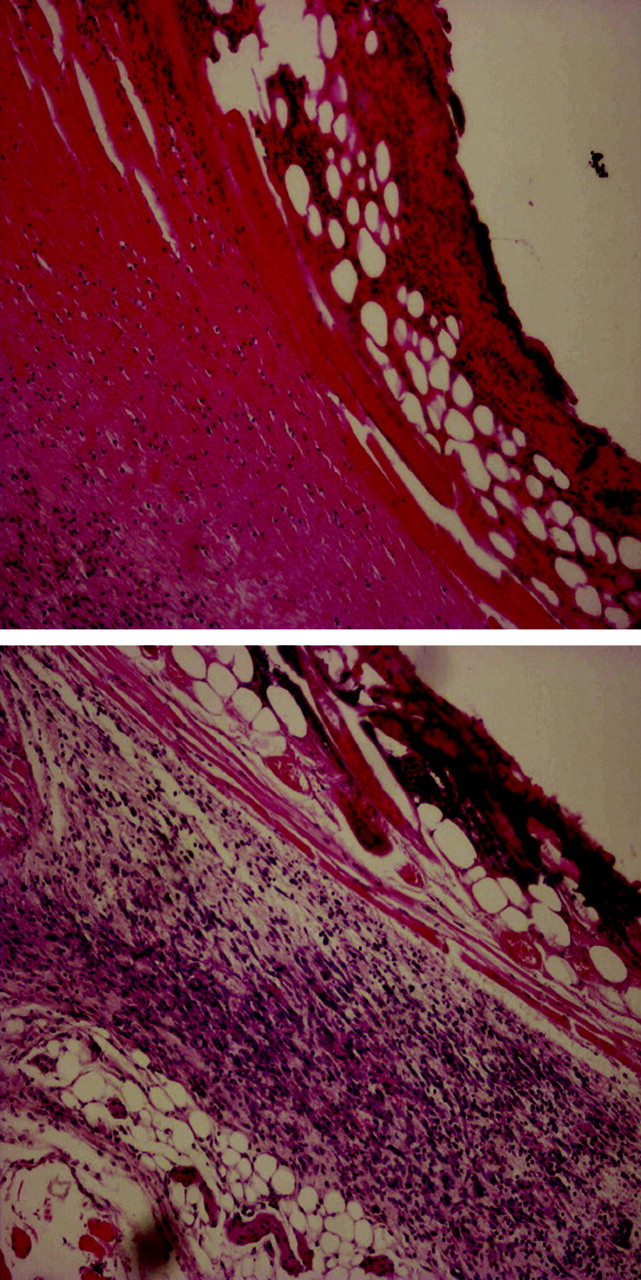
Peptide A3-APO prevents environmental microbial contamination of wounds. Sections of injury tissue from untreated mice (top) and mice treated with 5 mg/kg A3-APO (bottom). The sections were stained with haematoxylin and eosin. Mice receiving A3-APO treatment exhibited improved skin condition, probably due to prophylactic elimination of contaminating bacteria. This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
Discussion
A3-APO was efficacious against wound A. baumannii infection and environmental contamination in all four assays and was significantly more active than either imipenem or colistin in the two comparative A. baumannii wound infection models. In addition, the peptide exhibited a better safety profile than colistin and could be used in lower doses than imipenem. Thus, we conclude that peptide A3-APO is a very efficacious treatment option against experimental MDR A. baumannii wound infections after burn injuries.
This is somewhat surprising because the MIC of the peptide against this A. baumannii strain is 32–64 mg/L, a value that would suggest little utility in an in vivo setting for small-molecule antibiotics.20 However, A3-APO shows remarkable efficacy against E. coli and A. baumannii bacteraemia even in systemic infection models,17,18 suggesting that (i) in vitro bacterial killing figures will not have an ultimate predictive value for the clinical utility of peptide-based antimicrobials, (ii) the peptide is modified/derivatized to a more potent form or (iii) there are immunomodulatory interactions that enhance efficacy. Cationic antibacterial peptides generally exhibit immunostimulating properties in vivo.27 Not only are they the most versatile effectors of innate immunity in mammals and lower animals,28,29 but cationic antimicrobial peptides have also been shown to protect animals from bacterial invasion by up-regulating cellular immune responses. For example, lactoferricin analogues have been shown to protect the host via concomitant direct antimicrobial activity and immunostimulatory effects in several infection models of mice.30 The lack of distinction between microbiological and immunological effects extends to a hyaluronic acid-binding peptide that prevents experimental staphylococcal wound infections and is considered an immunological therapeutic agent rather than an antibiotic,31 although its sequence shows striking similarity to cationic antibacterial peptides with lysines, arginines and histidines interspersed with stretches of hydrophobic residues. In turn, this suggests that in vitro MIC determinations or immunostimulatory properties alone will not identify the potentially best antimicrobial peptide derivative in the clinical setting. The analogues need to be selected based on their efficacies in animal models against certain (or closely related) bacterial pathogens, body sites of infections and administration routes.
The lethal dose of the A3-APO dimer is 50 mg/kg when given ip,17 a value significantly lower than that we reported for im dosing earlier18 and examined in detail in the current report. The main manifestation of the toxic effects is haemolysis.17 Upon iv administration, peptide A3-APO rapidly decomposes to shorter fragments, among which the single-chain monomer appears to be the active metabolite.19 While this peptide fragment is more active than the parent prodrug in vitro against some strains and in vivo in a few animal models, it is also more toxic and induces resistance sooner when applied in sublethal concentrations.16 As in the current report we could not detect either the peptide dimer or any cleavage product in the blood upon im dosing under conditions identical to those in which we identified the shorter iv metabolites, we hypothesize that upon im administration A3-APO binds to a carrier biomolecule before it enters the circulation but remains fully active in the complex. Known mammalian plasma proteins that bind cationic antibacterial peptides include serum albumin,32 apolipoprotein A-I33 and heparin,34 just to name a few. We also showed that proline-rich antibacterial peptides bind the conserved substrate-binding pocket of the 70 kDa heat shock protein.35 Alternatively, the high concentration of trichloroacetic acid used for removing serum proteins may also precipitate this large peptide,36 a process that can be prevented in future by using ethanol or trifluoroacetic acid dissolved in aqueous acetonitrile.37,38
Regardless of the identity of the putative carrier biomolecule, the delivery mechanism outlined above would prevent haemolysis and improve the biodistribution of peptide A3-APO. Indeed, subcutaneously administered A3-APO is distributed in the body very poorly and lacks measurable in vivo antibacterial activity.13 Thus, im administration is the safest and most advantageous dosing modality for peptide A3-APO.
A3-APO is efficacious either when bacteria are already rapidly proliferating in the body17,18 or when they are just about to invade the host (current report). While we do not suggest that the peptide should be preferentially given prophylactically before hospital admission, the protective properties of the peptide immediately before infection takes place are worth considering during clinical development. The major drawback of the preventive, low-dose use of antimicrobials is of course the potential for resistance induction. Nevertheless, multifunctional antimicrobial peptides have multiple bacterial targets. In the case of A3-APO they are the bacterial membrane and the heat shock protein DnaK. Bacteria will find it extremely difficult, if not impossible, to concomitantly modify the composition of two independent target structures.39 Indeed, while co-culturing of Enterobacteriaceae with the single-chain metabolite, acting mainly on protein folding inhibition, rapidly induces resistance in vitro (although genetic alterations do not involve the putative DnaK D-E helix domain), such an exercise leaves the MIC of the microbiologically two-armed A3-APO dimer unchanged.40 We are confident that, if successfully developed as a drug, peptide A3-APO will be by far the preferred treatment option for MDR Gram-negative infections for many years to come. In addition, the complementary mode of action of A3-APO and small-molecule antibiotics, frequently overcoming existing antimicrobial resistance,15,16,40 makes it an exceptionally attractive candidate for combination therapies.
Funding
This study was supported by the Department of Health, Commonwealth of Pennsylvania. R. A. B. is supported by the VISN 10 GRECC, Merit Review Program of the VA and by grants from the National Institutes of Health.
Transparency declarations
None to declare.
Acknowledgements
We thank Drs Suode Zhang, Fazel Shabanpoor and Feng Lin (Howard Florey Institute) for peptide synthesis and Chief Consultant Judit Harsing, MD (Department of Dermatology, Venerology and Dermatooncology, Semmelweis University) for the slides.
References
- 1.Wurtz R, Karajovic M, Dacumos E, et al. Nosocomial infections in a burn intensive care unit. Burns. 1995;21:181–4. doi: 10.1016/0305-4179(95)80005-9. doi:10.1016/0305-4179(95)80005-9. [DOI] [PubMed] [Google Scholar]
- 2.Johnson EN, Burns TC, Hayda RA, et al. Infectious complications of open type III tibial fractures among combat injuries. Clin Infect Dis. 2007;45:409–15. doi: 10.1086/520029. doi:10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 3.Petersen K, Riddle MS, Danko JR, et al. Trauma-related infections in battlefield casualties in Iraq. Ann Surg. 2007;245:803–11. doi: 10.1097/01.sla.0000251707.32332.c1. doi:10.1097/01.sla.0000251707.32332.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CK, Wilkins K, Molter NC, et al. Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma. 2009;66:S138–44. doi: 10.1097/TA.0b013e31819d894c. doi:10.1097/TA.0b013e31819d894c. [DOI] [PubMed] [Google Scholar]
- 5.Crane DP, Gromov K, Li D, et al. Efficacy of colistin-impregnated beads to prevent multidrug-resistant A. baumannii implant-associated osteomyelitis. J Orthop Res. 2009;27:1008–5. doi: 10.1002/jor.20847. doi:10.1002/jor.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban C, Segal-Murer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003;36:1268–74. doi: 10.1086/374847. doi:10.1086/374847. [DOI] [PubMed] [Google Scholar]
- 7.Ganapathy H, Pal SK, Teare L, et al. Use of colistin in treating multi-resistant Gram-negative organisms in a specialized burns unit. Burns. 2010;36:522–7. doi: 10.1016/j.burns.2009.07.010. doi:10.1016/j.burns.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Hawley JS, Murray CK, Griffith ME. Susceptibility of Acinetobacter strains isolated from deployed U.S. military personnel. Antimicrob Agents Chemother. 2007;51:376–8. doi: 10.1128/AAC.00858-06. doi:10.1128/AAC.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez CH, De Ambrosio A, Bajuk M, et al. In vitro antimicrobials activity against endemic Acinetobacter baumannii multiresistant clones. J Infect Dev Ctries. 2010;29:164–7. doi: 10.3855/jidc.604. [DOI] [PubMed] [Google Scholar]
- 10.Lin B, Zhang C, Xiao X. Toxicity, bioavailability and pharmacokinetics of a newly formulated colistin sulfate solution. J Vet Pharmacol Ther. 2005;8:349–4. doi: 10.1111/j.1365-2885.2005.00666.x. doi:10.1111/j.1365-2885.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 11.Giamarellou H, Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options. Drugs. 2009;69:1879–901. doi: 10.2165/11315690-000000000-00000. doi:10.2165/11315690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Sauermann R, Rothenburger M, Graininger W, et al. Daptomycin: a review 4 years after first approval. Pharmacology. 2007;81:79–91. doi: 10.1159/000109868. doi:10.1159/000109868. [DOI] [PubMed] [Google Scholar]
- 13.Rozgonyi F, Szabo D, Kocsis B, et al. The antibacterial effect of a proline-rich antibacterial peptide A3-APO. Curr Med Chem. 2009;16:3996–4002. doi: 10.2174/092986709789352295. doi:10.2174/092986709789352295. [DOI] [PubMed] [Google Scholar]
- 14.Otvos L, Jr, Wade JD, Lin F, et al. Designer antibacterial peptides kill fluoroquinolone-resistant clinical isolates. J Med Chem. 2005;48:5349–9. doi: 10.1021/jm050347i. doi:10.1021/jm050347i. [DOI] [PubMed] [Google Scholar]
- 15.Otvos L, Jr, de Olivier Inacio V, Wade JD, et al. Prior antibacterial peptide-mediated inhibition of protein folding in bacteria mutes resistance enzymes. Antimicrob Agents Chemother. 2006;50:3146–9. doi: 10.1128/AAC.00205-06. doi:10.1128/AAC.00205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassone M, Vogiatzi P, La Montagna R, et al. Scope and limitations of the designer proline-rich antibacterial peptide dimer, A3-APO, alone or in synergy with conventional antibiotics. Peptides. 2008;29:1878–86. doi: 10.1016/j.peptides.2008.07.016. doi:10.1016/j.peptides.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Szabo D, Ostorhazi E, Binas A, et al. The designer proline-rich antibacterial peptide A3-APO is effective against systemic Escherichia coli infections in different mouse models. Int J Antimicrob Agents. 2010;35:357–61. doi: 10.1016/j.ijantimicag.2009.10.015. doi:10.1016/j.ijantimicag.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Ostorhazi E, Rozgonyi F, Szabo D, et al. Intramuscularly administered peptide A3-APO is effective against carbapenem-resistant Acinetobacter baumannii in mouse models of systemic infections. Biopolymers. 2010 doi: 10.1002/bip.21443. doi:10.1002/bip.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noto PB, Abbadessa G, Cassone M, et al. Alternative stabilities of a proline-rich antibacterial peptide in vitro and in vivo. Protein Sci. 2008;17:1249–55. doi: 10.1110/ps.034330.108. doi:10.1110/ps.034330.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush K, Macielag M, Weidner-Wells M. Taking inventory: antibacterial agents currently at or beyond Phase I. Curr Opin Microbiol. 2004;7:466–76. doi: 10.1016/j.mib.2004.08.013. doi:10.1016/j.mib.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Yamakawa T, Mitsuyama J, Hayashi K. In vitro and in vivo antibacterial activity of T-3912, a novel non-fluorinated topical quinolone. J Antimicrob Chemother. 2002;49:455–65. doi: 10.1093/jac/49.3.455. doi:10.1093/jac/49.3.455. [DOI] [PubMed] [Google Scholar]
- 22.Tien HC, Battad A, Bryce EA, et al. Multi-drug resistant Acinetobacter infections in critically injured Canadian forces soldiers. BMC Infect Dis. 2007;7:95. doi: 10.1186/1471-2334-7-95. doi:10.1186/1471-2334-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith ME, Ceremuga JM, Ellis MW, et al. Acinetobacter skin carriage among US army soldiers deployed in Iraq. Infect Control Hosp Epidemiol. 2007;28:720–2. doi: 10.1086/518966. doi:10.1086/518966. [DOI] [PubMed] [Google Scholar]
- 24.Cisneros JM, Rodriguez-Bano J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect. 2002;8:687–93. doi: 10.1046/j.1469-0691.2002.00487.x. doi:10.1046/j.1469-0691.2002.00487.x. [DOI] [PubMed] [Google Scholar]
- 25.Boerlin P, Eugster S, Gaschen F, et al. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet Microbiol. 2001;82:347–59. doi: 10.1016/s0378-1135(01)00396-0. doi:10.1016/S0378-1135(01)00396-0. [DOI] [PubMed] [Google Scholar]
- 26.Morgan DJ, Liang SY, Smith CL, et al. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns and hands of healthcare workers. Infect Control Hosp Epidemiol. 2010;31:716–21. doi: 10.1086/653201. doi:10.1086/653201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otvos L., Jr Antibacterial peptides and proteins with multiple cellular targets. J Pept Sci. 2005;11:697–706. doi: 10.1002/psc.698. doi:10.1002/psc.698. [DOI] [PubMed] [Google Scholar]
- 28.Hancock RE. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. 2001;1:156–64. doi: 10.1016/S1473-3099(01)00092-5. doi:10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 29.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. doi:10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 30.Wakabayashi H, Takase M, Tornita M. Lactoferricin derived from milk protein lactoferrin. Curr Pharm Res. 2003;9:1277–87. doi: 10.2174/1381612033454829. doi:10.2174/1381612033454829. [DOI] [PubMed] [Google Scholar]
- 31.Zaleski KJ, Kolodka T, Cywes-Bentley C, et al. Hyaluronic binding peptides prevent experimental staphylococcal wound infection. Antimicrob Agents Chemother. 2006;50:3856–60. doi: 10.1128/AAC.00082-06. doi:10.1128/AAC.00082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svenson J, Brandsdal B-O, Stensen W, et al. Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J Med Chem. 2007;50:3334–9. doi: 10.1021/jm0703542. doi:10.1021/jm0703542. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Johansson J, Agerberth B, et al. The antimicrobial peptide LL-37 binds to the human plasma protein apolipoprotein A-I. Rapid Commun Mass Spectrom. 2004;18:588–9. doi: 10.1002/rcm.1361. doi:10.1002/rcm.1361. [DOI] [PubMed] [Google Scholar]
- 34.Ogata M, Fletcher MF, Kloczewiak M, et al. Effects of anticoagulants on binding and neutralization of lipopolysaccharide by the peptide immunoglobulin conjugate CAP18106-138-immunoglobulin G in whole blood. Infect Immun. 1997;65:2160–7. doi: 10.1128/iai.65.6.2160-2167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kragol G, Lovas S, Varadi G, et al. The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry. 2001;40:3016–26. doi: 10.1021/bi002656a. doi:10.1021/bi002656a. [DOI] [PubMed] [Google Scholar]
- 36.Jenssen H, Aspmo SI. Serum stability of peptides. In: Otvos L, editor. Peptide-Based Drug Design. Totowa: Humana Press; 2008. pp. 177–86. [Google Scholar]
- 37.Powell MF, Grey H, Gaeta F, et al. Peptide stability in drug development: a comparison of peptide reactivity in different biological media. J Pharm Sci. 1992;81:731–5. doi: 10.1002/jps.2600810802. doi:10.1002/jps.2600810802. [DOI] [PubMed] [Google Scholar]
- 38.Chertov O, Biragyn A, Kwak LW, et al. Organic solvent extraction of proteins and peptides from serum as an effective preparation for detection and identification of biomarkers by mass spectrometry. Proteomics. 2004;4:1195–203. doi: 10.1002/pmic.200300677. doi:10.1002/pmic.200300677. [DOI] [PubMed] [Google Scholar]
- 39.Cassone M, Otvos L., Jr Synergy among antimicrobial peptides and between peptides and small molecule antibiotics. Expert Rev Anti Infect Ther. 2010;8:703–16. doi: 10.1586/eri.10.38. doi:10.1586/eri.10.38. [DOI] [PubMed] [Google Scholar]
- 40.Cassone M, Frith N, Vogiatzi P, et al. Induced resistance to the designer proline-rich antimicrobial peptide A3-APO does not involve changes in the intracellular target DnaK. Int J Pept Res Ther. 2009;15:121–8. doi:10.1007/s10989-009-9176-1. [Google Scholar]