Abstract
Background: Central nervous system histaminergic tone is thought to play a role in appetite regulation. In animal models, histamine receptor 1 (HRH1) agonists and histamine receptor 3 (HRH3) antagonists decrease food intake.
Objective: The objective of this study was to examine the acute effects of betahistine hydrochloride (an HRH1 agonist and HRH3 antagonist) on food intakes and appetites.
Design: The study was a proof-of-concept, randomized, double-blinded, placebo-controlled, dose-ranging study performed to examine the effects of betahistine in women with class I or II obesity [body mass index (BMI; in kg/m2) of 30–39.99]. After a 24-h placebo run-in period, subjects received a placebo (n = 19) or 48 (n = 19), 96 (n = 17), or 144 (n = 21) mg betahistine/d for 24 h. Treatment was followed by a buffet test meal to assess energy intake. Hunger, satiety, and desire to eat were measured after consuming the meal by using visual analog scales. Data were analyzed by using regression models with the assumption that there would be an increasing effect of betahistine doses. Analyses were adjusted for age, log fat and lean mass, food preferences, and intake during a buffet test meal obtained during the placebo run-in period.
Results: Of the 79 obese women (mean ± SD age: 42 ± 11 y; BMI: 35 ± 3) enrolled in the study, 76 women completed the study. The betahistine dose did not significantly change intakes from those observed during the run-in period of the buffet test meal (P = 0.78). Hunger, fullness, and desire to eat (all P > 0.62) similarly showed no differences according to the betahistine dose.
Conclusions: Betahistine did not produce an effect on food intakes or appetites. More potent histaminergic modulators may be required to elucidate the possible role of histaminergic pathways in human obesity. This trial was registered at clinicaltrials.gov as NCT00459992
INTRODUCTION
The prevalence of obesity [body mass index (BMI; in kg/m2) ≥30] in adult women in the United States is estimated to exceed 35% (1). Obesity disproportionately affects women; extreme obesity (BMI ≥40) is more common in women than in men (6.9% of women compared with 2.8% of men). Obesity is a risk factor for diabetes, hyperlipidemia, and hypertension in women (2–4), all of which are associated with an increased risk of cardiovascular disease and mortality. Thus, there is great interest in developing new pharmacotherapeutic approaches to alleviate obesity that are efficacious for women.
The histaminergic neuronal system plays a role in appetite regulation in rodent models. Of the 4 known histaminergic receptors, 2 histaminergic receptors, histamine receptor 1 (HRH1) and histamine receptor 3 (HRH3), appear to play a role in the regulation of energy intake (5). HRH1 is a postsynaptic receptor observed in the periventricular nucleus and the ventromedial hypothalamus. HRH3 is an inhibitory autoreceptor (6, 7) believed to regulate the secretion of histamine and other neurotransmitters. In animals, the administration of an intracerebroventricular histamine (8–10) or agents that inhibit histamine degradation (11) decrease energy intake. The inhibition of central nervous system histamine synthesis (12, 13) and blockage of HRH1 (14) increase feeding. Concordantly, HRH1-knockout animals showed mature-onset obesity associated with hyperphagia (15). In addition, the inhibition of HRH3-receptor activity decreases feeding. This effect is negated when animals are pretreated with chlorpheniramine, which indicates that increased histamine availability caused by the HRH3 antagonist may act at HRH1 receptors to suppress food intake (5). These data suggest medications with HRH1-agonist and HRH3-antagonist properties would be expected to inhibit food intake in humans.
In animal models, there is some evidence for sexual dimorphism in the histaminergic control of feeding. Female Wistar King A rats exposed to dietary histidine decreased their food intake more than do male rats or ovariectomized female rats (16). In another study, estrogen-induced suppression of feeding was partially blocked when rats were pretreated with α-fluoromethylhistidine (17). In addition, HRH1-knockout mice had less impressive estrogen-suppressed food intakes compared with those of wild-type mice.
Betahistine hydrochloride is an HRH1 agonist and HRH3 antagonist (18, 19) that has been used since the early 1960s for the treatment of Meniere disease (20). In animal models (21, 22), parenteral betahistine reduced food intake. Barak et al (23) reported that betahistine, at doses ranging from 16 to 48 mg betahistine/d, did not have a significant effect on weight loss relative to a placebo over a 12-wk interval in 281 adults. However, a subgroup post hoc analysis suggested weight loss occurred in non-Hispanic women ≤50 y old (23), which was consistent with a potential sexual dimorphism. Betahistine administered in an open-label fashion has also been reported to reduce olanzapine-associated weight gain in 3 schizophrenic patients (compared with in historical control subjects) (24).
Given these data, we performed a proof-of-concept, dose-ranging, randomized, double-blinded, placebo-controlled study to examine the short-term effects of betahistine on energy intake, hunger, and satiety. We studied only women because of the previously described data that suggested betahistine might be more efficacious in women than in men (16, 17, 23). We hypothesized that betahistine would suppress the desire to eat and decrease energy intakes of obese women in a dose-dependent manner.
SUBJECTS AND METHODS
Design overview
We conducted a single-center trial from 29 May 2007 to 27 February 2010 (www.clinicaltrials.gov, NCT00459992). After an outpatient screening visit, volunteers were admitted as inpatients for a 3-d and 2-night stay to participate in a randomized, double-blinded, placebo-controlled, dose-ranging study that examined the effects of betahistine hydrochloride on hunger, satiety, and energy intake during a buffet test meal (food array test). The trial was stopped once the planned number of participants were achieved.
Participants
Healthy women, aged 18–70 y, were recruited from Washington, DC, and the greater metropolitan area through flyers posted on local bulletin boards and advertisements in local newspapers. Respondents were eligible for inclusion in the study if they were in generally good health without chronic diseases that affected body weight, were menstruating regularly or menopausal (no menses for ≥3 mo), had a BMI between 30 and 40, and had maintained a stable weight for ≥3 mo. Reasons for exclusion were as follows: a history of asthma or allergic disease that requiring chronic antihistamine treatment; a history of peptic ulcer disease or use of histamine receptor 2 blockers; a history of cardiovascular disease, cardiac arrhythmias, or use of vasoactive medications, such as β-blockers, nitrates, and calcium-channel blockers; a history of anorexia or bulimia nervosa, or a binge-eating disorder; a history of another psychiatric disorder or use of antidepressants; specific dietary restrictions that would limit food choice in the standardized buffet test meal; a history of chronic kidney or liver disease; a history of diabetes; and pregnancy. The study was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Written consent was obtained from all participants. Subjects were compensated monetarily. An independent data-safety monitoring board oversaw the study.
Study protocol
Participants who were deemed eligible after phone screening were evaluated at an outpatient screening visit in the outpatient clinic of the National Institutes of Health (NIH) Hatfield Clinical Research Center (CRC). A physician or nurse practitioner performed detailed histories and physical examinations of subjects, and electrocardiograms and pulmonary function tests (pre- and postbronchodilator spirometry) were performed. Questionnaires (25–27) were administered to screen for the presence of eating disorders, and subjects completed a 50-item food-preference questionnaire that included, but was not limited to, all food and beverage items that would be presented in the study buffet test meals. Subjects who met any of the exclusion criteria (including the presence of an eating disorder) or who disliked ≥50% of the items that were presented in the buffet test meal were excluded. Subjects who qualified were invited to attend the inpatient visit.
On the day before admission to the CRC, subjects were instructed to avoid antihistamine medications as well as food items that are known to contain high amounts of histamine or histidine, such as red meats, seafood, cheeses, and caffeinated and alcoholic beverages. Subjects completed written 24-h food records to assess compliance with the dietary regulations, which were reviewed by study dietitians. Subjects were admitted to the CRC in the afternoon on the placebo run-in day. Assessments included an interview by using a standardized questionnaire that was specifically directed toward symptoms potentially related to the medication as well as an electrocardiogram and measurement of vital signs. After the 24-h, single-blind, placebo run-in period, participants entered the double-blinded, placebo-controlled, betahistine-treatment phase during which they were randomly assigned to one of 4 groups that received a placebo group or 16, 32, or 48 mg betahistine hydrochloride administered 3 times a day (at 2200, 0600, and 1400). The buffet test meal was administered at 1500 daily during the inpatient stay: first during the placebo run-in period and second after subjects had received 3 doses of study medication. Appetite was also assessed throughout the stay.
Randomization and dispensing of study medication
For the double-blinded portion of the study, investigators assigned consecutive code numbers to participants from prespecified lists stratified by race-ethnicity and degree of obesity (BMI of 30–34.99 and 35–39.99) into 6 strata. The NIH Clinical Center Pharmaceutical Development Section used permuted blocks with stratification to generate the allocations that translated code numbers into study-group assignments by using a pseudorandom number program. Betahistine hydrochloride (16-mg capsules) and matching placebo capsules, which were formulated to appear indistinguishable from the betahistine capsules, were provided by Obecure Ltd (Ramat Gan, Israel) under a cooperative research agreement with the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The NIH pharmacy staff, independent of the investigational team, packaged, labeled, and dispensed the study medication. No participant, investigator, or other medical or nursing staff who interacted with participants was aware of study-group assignments during the trial. During the placebo run-in period, all subjects received 3 doses, each of which consisted of 3 placebo capsules (at 2200, 0600, and 1400). During the double-blinded portion of the study, subjects were randomly assigned to receive either the placebo (3 capsules per administration) or one of 3 different doses of betahistine (16, 32, or 48 mg betahistine) 3 times daily (at 2200, 0600, and 1400). Because the betahistine was packaged as 16-mg capsules, subjects who were randomly assigned to receive 16- and 32-mg doses also received sufficient placebo capsules so that each subject always received 3 capsules at each scheduled time.
Diet, buffet test meals, and appetite assessment
Throughout their inpatient stay, subjects were prescribed diets intended to maintain energy intake appropriate for calculated energy requirements (isocaloric) according to the Mifflin St Jeor equation (28) with an activity factor of 1.3 (expecting that activity would be limited during the admission). During their admission, subjects received a standardized dinner with a macronutrient content consisting of 55% of energy from carbohydrates, 30% of energy from fat, and 15% of energy from protein. The dinner on the day of admission was designed to provide 40% of the estimated total daily energy requirements. On the second day of the admission, the energy content of each subject's dinner was set at the subject's calculated total daily energy requirement minus the energy consumed at breakfast and at the lunch buffet test meal (with a minimum of 300 kcal for dinner). Breakfast on days 2 and 3 were standardized and contained ≈15% of predicted total daily energy requirements (300–400 kcal).
Subjects ate twice from a lunch buffet test meal administered at 1500 (29), once on the placebo run-in day, and once on the randomized study day. Subjects ate alone and unobserved in a room free of food stimuli. The buffet supplied a total of ≈11,000 kcal and contained palatable food and beverage items that varied in macronutrient and energy contents (Table 1). Participants were instructed, “Let yourself go and eat as much as you would like. You may eat as much of anything as you would like to, but you do not have to eat anything that you do not like.” The consumed amount of food and beverage was measured as the weight difference of each item before and after eating. The caloric and nutritional composition of each food item was calculated by using data from the US Department of Agriculture National Nutrient Database for Standard Reference (US Department of Agriculture, Agricultural Research Service, Beltsville, MD), and the food-manufacturer nutrient information was obtained from food labels.
TABLE 1.
Items served at buffet test meals
| Item | Weight | Energy |
| g | kcal | |
| Dairy | ||
| American cheese | 240 | 758 |
| 2% Milk | 850 | 468 |
| Blueberry yogurt | 720 | 713 |
| Dessert and snacks | ||
| Shortbread cookies | 192 | 960 |
| Potato chips | 120 | 656 |
| Peanut butter candy1 | 120 | 596 |
| Pretzels | 150 | 570 |
| Jelly beans | 120 | 439 |
| Fruit | ||
| Apples | 414 | 215 |
| Bananas | 354 | 315 |
| Grapes | 250 | 168 |
| Vegetable | ||
| Baby carrots | 200 | 70 |
| Lettuce | 50 | 8 |
| Tomatoes | 200 | 36 |
| Bread | ||
| Kaiser rolls | 113 | 340 |
| Multigrain bread | 228 | 572 |
| White bread | 174 | 497 |
| Meat | ||
| Bologna | 180 | 569 |
| Ham | 180 | 214 |
| Turkey | 180 | 193 |
| Condiment | ||
| Jelly | 120 | 300 |
| Mayonnaise | 90 | 257 |
| Mustard | 90 | 60 |
| Peanut butter | 120 | 713 |
| Ranch dressing | 90 | 232 |
| Beverage | ||
| Apple juice | 850 | 432 |
| Orange juice | 850 | 383 |
| Water | 850 | 0 |
| Total | 8095 | 10,734 |
Reese's Pieces (The Hershey Co, Hershey, PA).
Appetite was assessed by using visual analog scales (VASs) (30) in which subjects rated their degree of hunger, fullness, and desire to eat on a 100-mm scale. These assessments were administered before breakfast and every hour thereafter until the buffet test meal. VAS tests were again performed immediately after the buffet test meal and again at 1600.
The total body fat and lean mass for each subject was assessed during the inpatient stay by using dual-energy X-ray absorptiometry (Hologic 4500A software, version 11.2; Hologic, Bedford, MA).
Monitoring of adverse events
At the conclusion of the study, each subject was asked to report any new symptoms by using the same standardized questionnaire that was administered on the day of inpatient admission. Vital signs (ie, heart rate, blood pressure, and temperature) were also monitored throughout the study.
Statistical analyses and power calculations
The primary endpoint was the change in measured energy intake (in kcal) (ie, the intake on the placebo run-in day minus the intake on the randomized study day) from the buffet test meal. From previous buffet test-meal studies that examined energy intake in our laboratory, we determined one SD as 450 kcal. With the use of Gpower power-calculation software (version 2.0; Department of Psychology, Bonn University, Bonn, Germany) (31), we calculated that 58 subjects would be needed to detect a dose-response relation in multiple regression analysis where 15% of the variance in change in intake was attributable to the betahistine dose with a power of 0.80 and α = 0.05. Thus, we sought ≤80 subjects to allow for up to one-quarter of subjects who might not complete the study.
For the primary outcome analysis of the mean change in energy intake, we used a multiple linear-regression model to determine the effect of escalating doses of betahistine; initial independent variables were age and race. We added log fat and lean mass, the percentage of items disliked from the buffet test meal, and the percentage of actual relative to predicted total daily energy requirements on the run-in day to adjust for other variables that may influence energy intake. A secondary analysis of the primary outcome was performed by using analysis of covariance to seek differences between the placebo and different doses of betahistine with the same covariates as in the primary analysis. A one-factor analysis of variance was also performed with post hoc analysis by using Dunnett's method with adjustments for multiple comparisons, and the effects of betahistine on individual groups were examined. All subjects with complete energy-intake data were used for the primary outcome analysis. All subjects who received a single dose of study medication were included in the safety analyses. Safety was examined by using contingency-table analysis.
For analyses of changes in hunger, fullness, and desire to eat throughout the randomized study medication day, we used repeated-measures analysis of covariance with adjustments for age, race, log fat and lean mass, and the percentage of items disliked from the buffet test meal.
P < 0.05 was considered significant for the primary-outcome analysis of change in energy intake. For the secondary-outcome analysis with multiple measures (ie, changes in macronutrient intake and appetite assessments), we used a Bonferroni correction with P < 0.017 considered significant. However, nominal P values are supplied.
RESULTS
Randomization and baseline characteristics
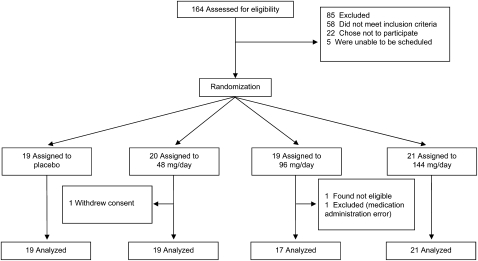
Flow through the study is shown in Figure 1. Seventy-nine women were randomly assigned to receive a placebo or one of the 3 betahistine doses. There were no significant differences in age, percentage body fat, BMI, or biochemical indexes for subjects who were randomly assigned to receive the placebo than for subjects who were randomly assigned to receive betahistine (Table 2). Three subjects were excluded from the outcome analyses as follows: one subject was excluded because of a prolonged corrected electrocardiographic QT interval that was detected only after randomization (but before medication administration), one subject was excluded who received incorrect medication, and one subject was excluded who withdrew consent to participate in the study.
FIGURE 1.
Flow of participants through the trial.
TABLE 2.
Characteristics of subjects who were randomly assigned1
| Betahistine dose |
|||||
| Placebo (n = 19) | 48 mg/d (n = 20) | 96 mg/d (n = 19) | 144 mg/d (n = 21) | P | |
| Age (y) | 38 ± 112 | 46 ± 11 | 44 ± 13 | 42 ± 12 | 0.23 |
| Race [n (%)]3 | 0.99 | ||||
| African American | 11 (57.9) | 11 (55) | 11 (57.9) | 12 (57.1) | |
| White | 6 (31.6) | 6 (30) | 5 (26.3) | 6 (28.6) | |
| Other | 2 (10.5) | 3 (15) | 3 (15.8) | 3 (14.3) | |
| Height (cm) | 164 ± 6 | 163 ± 6 | 162 ± 6 | 162 ± 8 | 0.79 |
| Weight (kg) | 95 ± 11 | 93 ± 13 | 91 ± 10 | 93 ± 12 | 0.77 |
| BMI (kg/m2) | 35.2 ± 3.1 | 34.8 ± 3.2 | 34.9 ± 28 | 35.2 ± 2.4 | 0.97 |
| Lean mass (kg)4 | 50.7 ± 6.0 | 50.9 ± 6.3 | 51.3 ± 6.3 | 50.5 ± 5.9 | 0.98 |
| Fat mass (kg)4 | 42.4 ± 7.3 | 40.2 ± 8.0 | 39.0 ± 5.7 | 40.4 ± 6.1 | 0.48 |
| Body fat percentage (%)4 | 44.3 ± 4.4 | 42.8 ± 3.5 | 42.1 ± 3.8 | 43.2 ± 2.6 | 0.32 |
| Corrected QT interval (ms) | 434 ± 21 | 424 ± 19 | 433 ± 23 | 430 ± 20 | 0.39 |
| Glucose (mg/dL) | 86 ± 6 | 88 ± 10 | 91 ± 10 | 91 ± 9 | 0.27 |
| Insulin (μIU/mL) | 10 ± 5 | 10 ± 6 | 12 ± 8 | 13 ± 7 | 0.25 |
| Triglycerides (mg/dL) | 94 ± 52 | 93 ± 34 | 101 ± 44 | 110 ± 66 | 0.70 |
| Hb A1C (%) | 5.4 ± 0.5 | 5.6 ± 0.3 | 5.6 ± 0.5 | 5.5 ± 0.5 | 0.63 |
Hb A1c, glycated hemoglobin. values represent ANOVA across groups.
Mean ± SD (all such values).
Race was self-reported.
Measurements obtained by using dual-energy X-ray absorptiometry.
Buffet test-meal energy intake
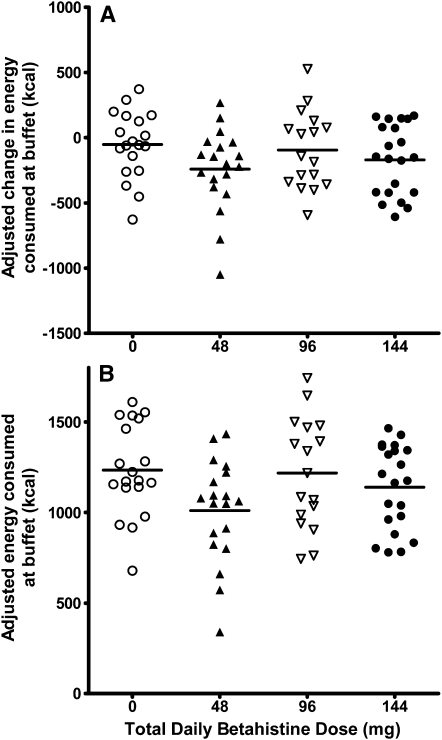
The primary outcome of the mean change in energy intake during the buffet test meal from the placebo run-in day to the betahistine-treatment day, with adjustment for age and race, did not differ significantly in women who received 0, 48, 96, or 144 mg betahistine/d [effect of dose: standardized β coefficient (95% CI) was −0.03 (−1.6, 1.2); P = 0.78]. The next analysis, which accounted for age, race, log fat and lean mass, the percentage of items disliked from the buffet test meal, and the percentage of actual relative to predicted total daily energy requirements on the run-in day similarly showed no significant effect attributable to the betahistine dose [effect of dose: standardized β coefficient (95% CI) was −0.08 (−1.7, 0.8); P = 0.49] (Figure 2A). We also performed a post hoc subgroup analysis in women ≤50 y of age; the betahistine dose again showed no significant effect on energy intake (effect of dose: P = 0.76). Similarly, the total energy intake on the betahistine-treatment day, with intake controlled for on the placebo run-in day, was not different in women who received the placebo or 48, 96, or 144 mg betahistine/d (effect of dose: P = 0.76) (Figure 2B), while accounting for the same covariates as above. There was also no significant difference observed when all subjects who received any dose of betahistine were compared to subjects who received the placebo (P = 0.16). An exploratory analysis of variance with repeated measures that was performed without any covariates showed no significant dose (P = 0.47) or dose × time interaction (P = 0.19). The post hoc multiple-comparisons analysis against the placebo group, which was adjusted by using Dunnett's method, showed no effect in any of the groups (P = 0.35 for the 48-mg betahistine/d group, P = 0.99 for the 96-mg betahistine/d group, and P = 0.98 for the 144-mg betahistine/d group).
FIGURE 2.
Energy intake during buffet test meal. Change in caloric intake from baseline on the betahistine-treatment day (Δ kcal; A: linear regression model adjusted for age, race, log fat and lean mass, percentage of items disliked from buffet test meal, and percentage of actual relative to predicted total daily energy requirements on the run-in day, with dose as a continuous variable; P = 0.49) and actual caloric intake on the betahistine-treatment day (total buffet kcal; B: linear regression model adjusted for age, race, caloric intake during buffet test meal on the run-in day, log fat and lean mass, percentage of items disliked from buffet test meal, and percentage of actual relative to predicted total daily energy requirements on the run-in day, with dose as a continuous variable; P = 0.76). ○, placebo (n = 19); ▴, 48 mg/d (n = 19); Δ, 96 mg/d (n = 17); •, 144 mg/d (n = 21).
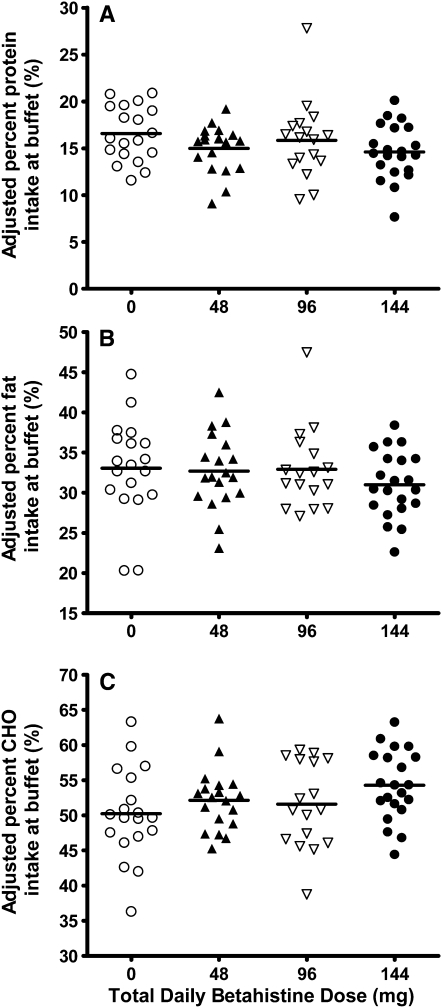
Macronutrient energy intake, which was expressed as a percentage of total energy intake (Figure 3), was not different between placebo and treatment groups for protein (P = 0.10) (Figure 3A), fat (P = 0.15) (Figure 3B), or carbohydrate (nominal P = 0.02) (Figure 3C). Despite the significant nominal P value for carbohydrate intake, after using a Bonferroni adjustment, the P value only indicated a trend toward significance; subjects who received the medication tended to have a greater percentage energy intake from carbohydrates. This model was adjusted for age, race, log fat and lean mass, the percentage of items disliked on the buffet test meal, and the corresponding percentage macronutrient energy intake on the placebo run-in day.
FIGURE 3.
Percentage macronutrient intake during buffet test meal. Percentage protein (A; P = 0.1), fat (B; P = 0.15), and carbohydrate (CHO) (C; P = 0.02) linear regression model adjusted for age, race, corresponding percentage macronutrient intake during the buffet test meal on the run-in day, log fat and lean mass, and percentage of items disliked from buffet test meal, with dose as a continuous variable. ○, placebo (n = 19); ▴, 48 mg/d (n = 19); Δ, 96 mg/d (n = 17); •, 144 mg/d (n = 21).
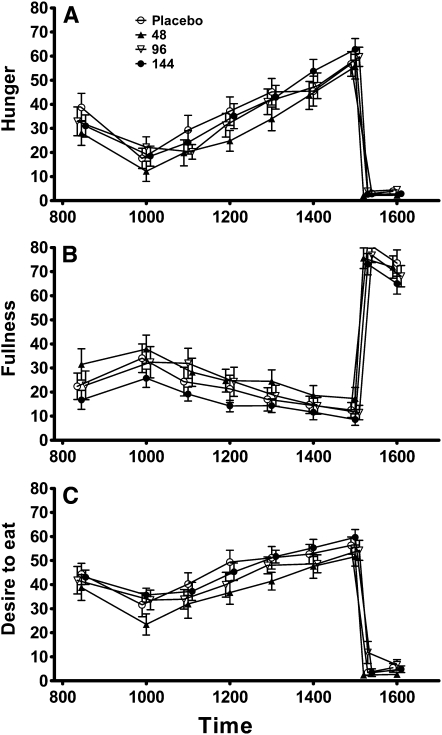
Appetite
Ratings of hunger, fullness, and desire to eat are shown in Figure 4. After the buffet test meal, there was no significant effect of treatment on the ratings of hunger (P = 0.66), fullness (P = 0.62), or desire to eat (P = 0.90). These models were adjusted for log fat and lean mass, the percentage of items disliked on the buffet, and the corresponding VAS rating after the buffet test meal on the placebo run-in day. Likewise, ratings of hunger (P = 0.63), fullness (P = 0.88), and desire to eat (P = 0.30) were not significantly associated with the betahistine dose at any time point during the day (Figure 3).
FIGURE 4.
Mean (±SD) values of appetite assessment during the test day. Hunger (A; P = 0.66), fullness (B; P = 0.62), and desire to eat (C; P = 0.90) were assessed during day 2 by using a visual analog scale (in mm). Repeated-measures ANCOVA adjusted for age, race, log fat and lean mass, and percentage of items disliked from the buffet test meal.
Adverse events
No subjects reported a serious or life-threatening adverse event. Overall, nonserious adverse events were similar between women in the placebo and betahistine groups. The most common adverse event was a headache, the frequency of which did not differ between treatment and placebo groups (Table 3). There was no difference between groups in changes in corrected electrocardiographic QT interval.
TABLE 3.
Percentage of subjects reporting adverse events
| Betahistine dose |
|||||
| Adverse event | Placebo (n = 19) | 48 mg/d (n = 20) | 96 mg/d (n = 19) | 144 mg/d (n = 21) | All subjects (n = 79) |
| Headache | 52.6 | 36.8 | 31.6 | 47.6 | 43.0 |
| Increased urination | 26.3 | 25.0 | 26.3 | 19.0 | 25.3 |
| Nausea | 10.5 | 5.3 | 15.8 | 9.5 | 10.1 |
| Excessive fatigue/tiredness | 10.5 | 0 | 15.8 | 4.8 | 7.6 |
| Diarrhea | 5.3 | 10.5 | 5.3 | 4.8 | 6.4 |
| Rash | 5.3 | 5.3 | 0 | 9.5 | 5.1 |
| Bloating | 0 | 5.3 | 5.3 | 9.5 | 5.1 |
| Abdominal pain | 10.5 | 5.3 | 0 | 0 | 3.8 |
| Blurred vision | 5.3 | 0 | 5.3 | 0 | 2.5 |
| Shortness of breath | 0 | 0 | 0 | 5.3 | 1.3 |
| Pain in the chest area | 0 | 0 | 0 | 1.3 | 1.3 |
| Vomiting | 0 | 0 | 0 | 0 | 0 |
| Depressed mood | 0 | 0 | 0 | 0 | 0 |
DISCUSSION
Despite compelling evidence in animals (21, 22) that implicated the central nervous system histaminergic tone in energy homeostasis, betahistine, an HRH1 agonist and HRH3 antagonist, showed no acute dose-dependent effects that suggested it potently suppressed eating. Measures of appetite (ie, hunger, satiety, and desire to eat) were similar between placebo and betahistine groups, and concordantly, there was no significant betahistine dose-dependent difference in energy intake observed during the buffet test meal.
In a post hoc subgroup analysis, Barak et al (23) reported somewhat greater weight loss (−4.24 ± 3.87 compared with −1.65 ± 2.96 kg) in non-Hispanic white women aged ≤50 y who took 48 mg betahistine/d rather than a placebo during a 12-wk randomized controlled trial. Such findings, in addition to animal studies that observed an effect of sex and estrogens on the histaminergic-suppression of food intake (16, 17), suggested betahistine might be effective as an appetite suppressant uniquely in women. Our findings in the current study, which enrolled only women, did not support this hypothesis, because all subjects combined and subjects <50 y of age did not show a significant dose-dependent response to betahistine treatment.
After multiple comparisons were controlled for, there was a nonsignificant trend toward a betahistine effect on carbohydrate intake, with an increased percentage of carbohydrate intake observed among subjects given higher doses of betahistine. We observed no corresponding decrease in fat and protein percentage consumption. The significance of these findings remains unclear; larger clinical studies are required to confirm this result.
Strengths of the current study included the randomized design, the inpatient tightly monitored setting, the sample size that was adequate to detect a 15% change in intake attributable to the betahistine dose, and the use of a placebo run-in period to acclimate subjects to the experimental situation. A limitation for the generalizability of the current study is in the exclusion of men. However, we purposefully intended to examine the effect on women given the limited data that suggested the possibility of a more robust response in women. It is also possible that effects of betahistine might have been more readily detected in lean women who might be anticipated to have energy intake regulatory systems with fewer genetic or environmentally induced alterations that might diminish responsiveness. We did not examine the long-term effect of betahistine on food intake, and it remains possible that the administration of higher doses for a longer period of time could induce some effect. Animal studies have shown effects at somewhat higher doses. For example, 4 and 8 mg betahistine/kg body weight diminished energy intakes in pygmy goats (21), whereas the highest dose in the current study was ≈1.3 mg betahistine/kg body weight. This maximum dose was selected because it was the highest dose for which prior human safety data were published (20). Finally, there are data consistent with a role for central nervous-system histaminergic tone as a modulator of the weight gained after exposure to antipsychotics such as olanzapine (24, 32–34). Our study was limited to obese, otherwise healthy women, and thus, we cannot exclude an effect of betahistine on antipsychotic-induced weight gain.
In conclusion, we showed no evidence that betahistine acutely decreases energy intake or suppresses appetite. It remains possible that a longer period of administration or higher dose may be necessary to show the effect of this compound on energy intake. Medications more potent than betahistine may be required to show conclusively whether histaminergic tone plays a role in human energy homeostasis.
Acknowledgments
We thank the volunteers who participated for their help in completing these studies.
The authors' responsibilities were as follows—LBY, KAC, MK, and JAY: conception and design of the study; AHA, LBY, AC, MK, and JAY: analysis and interpretation of data and drafting of the manuscript; and all authors: collection and assembly of data, critical revision of the manuscript for content, and approval of the final version of the manuscript. NB is an employee of Obecure Ltd. JAY reported research support from Roche for another obesity-related pharmacotherapy study. JAY and MK are commissioned officers in the US Public Health Service, Department of Health and Human Services. AHA, LBY, EAS, AA, AC, MK, SMB, KAC, JCR, and MKC reported no conflicts of interest.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 2.Hu FB. Overweight and obesity in women: health risks and consequences. J Womens Health (Larchmt) 2003;12:163–72 [DOI] [PubMed] [Google Scholar]
- 3.Ryan D. Obesity in women: a life cycle of medical risk. Int J Obes (Lond) 2007;31(suppl 2):S3–7; discussion S31–2 [DOI] [PubMed] [Google Scholar]
- 4.Zavorsky GS. Cardiopulmonary aspects of obesity in women. Obstet Gynecol Clin North Am 2009;36:267–84, viii [DOI] [PubMed] [Google Scholar]
- 5.Sakata T, Yoshimatsu H, Kurokawa M. Hypothalamic neuronal histamine: implications of its homeostatic control of energy metabolism. Nutrition 1997;13:403–11 [DOI] [PubMed] [Google Scholar]
- 6.Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983;302:832–7 [DOI] [PubMed] [Google Scholar]
- 7.Van Cauwenberge PB, De Moor SE. Physiopathology of H3-receptors and pharmacology of betahistine. Acta Otolaryngol Suppl 1997;526:43–6 [DOI] [PubMed] [Google Scholar]
- 8.Clineschmidt BV, Lotti VJ. Histamine: intraventricular injection suppresses ingestive behavior of the cat. Arch Int Pharmacodyn Ther 1973;206:288–98 [PubMed] [Google Scholar]
- 9.Itowi N, Nagai K, Nakagawa H, Watanabe T, Wada H. Changes in the feeding behavior of rats elicited by histamine infusion. Physiol Behav 1988;44:221–6 [DOI] [PubMed] [Google Scholar]
- 10.Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T. Central infusion of histamine reduces fat accumulation and upregulates UCP family in leptin-resistant obese mice. Diabetes 2001;50:376–84 [DOI] [PubMed] [Google Scholar]
- 11.Lecklin A, Tuomisto L, MacDonald E. Metoprine, an inhibitor of histamine N-methyltransferase but not catechol-O-methyltransferase, suppresses feeding in sated and in food deprived rats. Methods Find Exp Clin Pharmacol 1995;17:47–52 [PubMed] [Google Scholar]
- 12.Sakata T. Hypothalamic neuronal histamine modulates energy balance in rats. Agents Actions Suppl 1991;33:91–6 [DOI] [PubMed] [Google Scholar]
- 13.Ookuma K, Sakata T, Fukagawa K, et al. Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res 1993;628:235–42 [DOI] [PubMed] [Google Scholar]
- 14.Fukagawa K, Sakata T, Shiraishi T, et al. Neuronal histamine modulates feeding behavior through H1-receptor in rat hypothalamus. Am J Physiol 1989;256:R605–11 [DOI] [PubMed] [Google Scholar]
- 15.Masaki T, Chiba S, Yasuda T, et al. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes 2004;53:2250–60 [DOI] [PubMed] [Google Scholar]
- 16.Kasaoka S, Kawahara Y, Inoue S, et al. Gender effects in dietary histidine-induced anorexia. Nutrition 2005;21:855–8 [DOI] [PubMed] [Google Scholar]
- 17.Gotoh K, Masaki T, Chiba S, et al. Hypothalamic neuronal histamine signaling in the estrogen deficiency-induced obesity. J Neurochem 2009;110:1796–805 [DOI] [PubMed] [Google Scholar]
- 18.Arrang JM, Garbarg M, Quach TT, Dam Trung Tuong M, Yeramian E, Schwartz JC. Actions of betahistine at histamine receptors in the brain. Eur J Pharmacol 1985;111:73–84 [DOI] [PubMed] [Google Scholar]
- 19.Fossati A, Barone D, Benvenuti C. Binding affinity profile of betahistine and its metabolites for central histamine receptors of rodents. Pharmacol Res 2001;43:389–92 [DOI] [PubMed] [Google Scholar]
- 20.Jeck-Thole S, Wagner W. Betahistine: a retrospective synopsis of safety data. Drug Saf 2006;29:1049–59 [DOI] [PubMed] [Google Scholar]
- 21.Rossi R, Del Prete E, Scharrer E. Effect of the H1-histamine receptor agonist betahistine on drinking and eating behavior in pygmy goats. Physiol Behav 1999;66:517–21 [DOI] [PubMed] [Google Scholar]
- 22.Szelag A, Trocha M, Merwid-Lad A. Betahistine inhibits food intake in rats. Pol J Pharmacol 2001;53:701–7 [PubMed] [Google Scholar]
- 23.Barak N, Greenway FL, Fujioka K, Aronne LJ, Kushner RF. Effect of histaminergic manipulation on weight in obese adults: a randomized placebo controlled trial. Int J Obes (Lond) 2008;32:1559–65 [DOI] [PubMed] [Google Scholar]
- 24.Poyurovsky M, Pashinian A, Levi A, Weizman R, Weizman A. The effect of betahistine, a histamine H1 receptor agonist/H3 antagonist, on olanzapine-induced weight gain in first-episode schizophrenia patients. Int Clin Psychopharmacol 2005;20:101–3 [DOI] [PubMed] [Google Scholar]
- 25.Spitzer RL, Yanovski S, Wadden T, et al. Binge eating disorder: its further validation in a multisite study. Int J Eat Disord 1993;13:137–53 [PubMed] [Google Scholar]
- 26.Gluck ME, Geliebter A, Satov T. Night eating syndrome is associated with depression, low self-esteem, reduced daytime hunger, and less weight loss in obese outpatients. Obes Res 2001;9:264–7 [DOI] [PubMed] [Google Scholar]
- 27.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 28.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7 [DOI] [PubMed] [Google Scholar]
- 29.Yanovski SZ, Leet M, Yanovski JA, et al. Food selection and intake of obese women with binge-eating disorder. Am J Clin Nutr 1992;56:975–80(Published erratum appears in Am J Clin Nutr 1993;57:456.) [DOI] [PubMed] [Google Scholar]
- 30.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24:38–48 [DOI] [PubMed] [Google Scholar]
- 31.Faul F, Erfelder E. GPOWER: A priori, post-hoc, and compromize power analyses for MS-DOS. Bonn, Germany: Bonn University Department of Psychology, 1992 [Google Scholar]
- 32.Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 2003;28:519–26 [DOI] [PubMed] [Google Scholar]
- 33.Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA 2007;104:3456–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirshing DA, Wirshing WC, Kysar L, et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 1999;60:358–63 [PubMed] [Google Scholar]