Abstract
Background: The optimal diet for pregnancy that is complicated by excessive weight is unknown.
Objective: We aimed to examine the effects of a low–glycemic load (low-GL) diet in overweight and obese pregnant women.
Design: We randomly assigned 46 overweight or obese pregnant women to receive a low-GL or a low-fat diet. Participants received carbohydrate-rich foods, fats, and snack foods through home delivery or study visits. The primary outcome was birth weight z score. Other endpoints included infant anthropometric measurements, gestational duration, maternal weight gain, and maternal metabolic parameters.
Results: There were no significant differences in birth weight z score or other measures of infant adiposity between groups. However, in the low-GL compared with the low-fat group, gestational duration was longer (mean ± SD: 39.3 ± 1.1 compared with 37.9 ± 3.1 wk; P = 0.05) and fewer deliveries occurred at ≤38.0 wk (13% compared with 48%, P = 0.02; with exclusion of planned cesarean deliveries: 5% compared with 53%; P = 0.002). Adjusted head circumference was greater in the low-GL group (35.0 ± 0.8 compared with 34.2 ± 1.3 cm, P = 0.01). Women in the low-GL group had smaller increases in triglycerides [median (interquartile range): 49 (19, 70) compared with 93 (34, 129) mg/dL; P = 0.03] and total cholesterol [13 (0, 36) compared with 33 (22, 56) mg/dL, P = 0.04] and a greater decrease in C-reactive protein [−2.5 (−5.5, −0.7) compared with −0.4 (−1.4, 1.5) mg/dL, P = 0.007].
Conclusions: A low-GL diet resulted in longer pregnancy duration, greater infant head circumference, and improved maternal cardiovascular risk factors. Large-scale studies are warranted to evaluate whether dietary intervention during pregnancy aimed at lowering GL may be useful in the prevention of prematurity and other adverse maternal and infant outcomes. This trial is registered at clinicaltrials.gov as NCT00364403.
INTRODUCTION
Even under normal conditions, pregnancy is associated with insulin resistance and features of the metabolic syndrome (1, 2). In the third trimester, insulin sensitivity may decrease by ≥50% (3), a physiologic process that allows for more efficient transfer of nutrients across the placenta (3, 4). Insulin resistance antagonizes glucose uptake and promotes lipolysis in the mother, enhancing availability of carbohydrate and fat for fetal energy requirements and growth (3). Consequently, serum triglycerides may increase by 2- to 4-fold during normal pregnancy (5).
Obesity or excessive weight gain during pregnancy exacerbates insulin resistance and related metabolic aberrations. In addition to dyslipidemia, obese pregnant women frequently develop hallmarks of the metabolic syndrome, including hyperglycemia, hypertension, chronic inflammation, and endothelial dysfunction (6). Obese women are at increased risk of diabetes, preeclampsia, and preterm delivery, and these complications are associated with maternal cardiovascular disease over the long term (3, 6, 7). Fetal macrosomia may cause obstetric complications for the mother and neonate, including physical injury and postpartum hemorrhage. Moreover, the hormonal and metabolic abnormalities associated with an obese pregnancy appear to alter intrauterine development in ways that raise lifetime risk of chronic disease in the offspring (8–10).
Because of concern for teratogenic effects on the fetus, pharmacologic treatment of metabolic abnormalities during pregnancy has a limited role, highlighting the importance of dietary therapy. Unfortunately, there are few studies of specific diets during pregnancy (11–14). Most focus on preventing excessive weight gain, often including other therapeutic components (eg, physical activity) and lacking an active control. Consequently, there is virtually no evidence to recommend any specific diet.
A low–glycemic load (low-GL) diet offers an attractive approach for obese pregnant women. Glycemic index (GI) is a property of carbohydrate-containing food that describes the rise of blood glucose that occurs after a meal, and GL is the mean dietary GI × amount of carbohydrate consumed (15, 16). In pregnancy, a low-GL diet may exert beneficial effects by lowering gestational weight gain, decreasing insulin resistance independently of body weight, or directly ameliorating components of the metabolic syndrome. Clapp (17) showed that women consuming a low-GI diet throughout pregnancy experience no significant change in glycemic response to a mixed meal compared with a 75% increase in mid- and late gestation for those consuming a high-GI diet. Clapp also counseled 12 lean, physically active women on a low-GI diet before pregnancy to consume either a low-GI diet or a high-GI diet (6 per group) beginning in the first trimester of pregnancy (18). Women who stayed with the low-GI diet gained less weight and had infants with lower birth weight. Moses et al (19) observed similar findings in a larger cohort alternately assigned to receive low- and high-GI diets. The aim of our study was to examine the effects of a low-GL diet in overweight and obese pregnant women with the provision of specific foods to enhance treatment fidelity. We hypothesized that consumption of a low-GL diet would have more favorable effects on maternal and infant health, including lower birth weight and improved maternal metabolic risk factors.
SUBJECTS AND METHODS
Study overview
We conducted a randomized controlled pilot trial (www.clinicaltrials.gov; NCT00364403) comparing 2 diets during pregnancy on maternal and infant health outcomes. The intervention involved nutrition education, dietary counseling, and food provision beginning in the second or third trimester; physical activity recommendations; and counseling strategies with comparable treatment intensity across groups. The primary endpoint was birth weight z score, and other endpoints included maternal weight gain and body composition, maternal cardiovascular disease risk factors, gestational duration and infant anthropometric measurements. Repeated 24-h dietary recall interviews were used to collect process measures. The study was conducted at Beth Israel Deaconess Medical Center, Boston, MA, and Children's Hospital Boston, Boston, MA, and was approved by the institutional review boards of both institutions. The study was supervised by a Data and Safety Monitoring Board that comprised an obstetrician, a pediatric endocrinologist, and a statistician who were not otherwise involved with the study.
Subjects
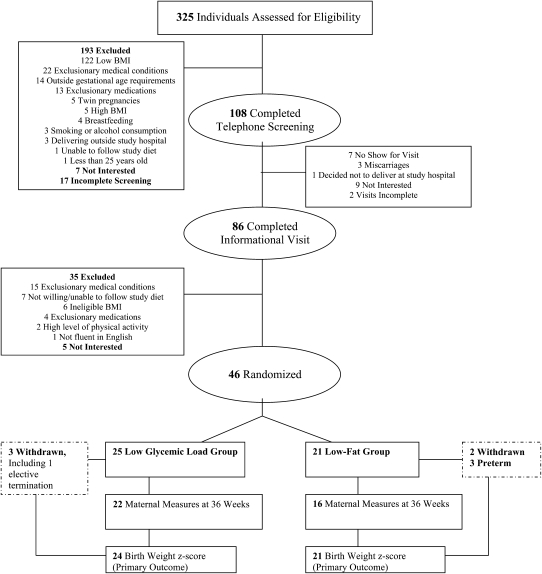
Subjects were pregnant women with a prepregnancy or first-trimester body mass index (BMI; in kg/m2) of ≥25 but <45. First-trimester body weight was obtained from the obstetrician, and self-reported prepregnancy body weight was used if the former was unavailable. Women needed to be ≥25 y old with a singleton pregnancy, who could begin the study by 13–28 wk gestation, and who were willing to consume the diets throughout the remainder of their pregnancy. Subjects were required to be fluent in English. Exclusion criteria included smoking or alcohol consumption during pregnancy; major comorbid health issues that might affect maternal or fetal weight gain (eg, hypothyroidism, diabetes, hypertension); use of prescription medications, dietary supplements, or herbal products known to affect body weight; plans to deliver outside of the study medical center; high levels of physical activity (defined as >1 h/d of moderate-vigorous activity for ≥4 d/wk); lactation in the preceding 3 mo; or being a first-degree relative of a current or prior subject or a family member with a lesser degree of relatedness living in the same household as a current or prior subject. Screening was a 2-part process spanning from January 2007 through June 2009 (Figure 1). Initial screening was conducted by telephone, and potentially eligible subjects were then interviewed in person.
FIGURE 1.
Study flow diagram.
Informed consent was obtained at the first visit. Random assignment was then performed by the study dietitian. Random assignment was stratified by history of prior pregnancies (at or beyond 13 wk gestation) and prepregnancy or first-trimester BMI >30. Within each of the 4 strata, subjects were assigned to the 2 diet groups in a 1:1 ratio. Separate random assignment envelopes for each stratum were prepared in advance by the Children's Hospital Boston Clinical Research Program in randomly permuted blocks of 2 and 4, preventing anticipation of future assignments.
Dietary intervention
Subjects were randomly assigned to receive either a low-fat or low-GL diet. Subjects began consuming the diet upon enrollment in the second or third trimester (13–28 wk gestation). The low-fat diet was designed to meet current recommendations for pregnancy from the American Dietetic Association and the Institute of Medicine (20), which include consumption of a moderately low-fat, low-saturated-fat, high complex carbohydrate diet without consideration of GI (21–23). Consequently, such diets tend to be moderately high in GL. Our low-fat diet was designed to be similar in GL to prevailing norms (24, 25), with a target macronutrient composition of 55% carbohydrate, 25% fat, and 20% protein. The low-GL diet was designed to be lower in GL, which was achieved by both moderately reducing total carbohydrate and replacing higher GI carbohydrates with lower GI carbohydrates. Target macronutrient composition for the low-GL diet was 45% carbohydrate, 35% fat, and 20% protein. Thus, we aimed to study the potential advantages of decreasing GL from prevailing norms.
The dietary intervention was introduced at the baseline visit and further detailed through two 1-h in-person counseling sessions. Subjects had in-person maintenance visits at 2–4-wk intervals. The final visit for measurements was at 36 wk. One in-person and weekly phone counseling sessions were offered after 36 wk. Structured written guides were used to ensure differentiation between interventions and consistency in delivery of intervention messages, thereby fostering treatment fidelity. In addition, we used a patient-centered counseling approach—considering the individual's perspectives, life experiences, circumstances, and resources—to promote dietary behavior change. Dietary modules available for both groups were developed to address anticipated dietary challenges during pregnancy (eg, inadequate calcium intake, eating out), and adherence to intervention diets was discussed at weekly reviews of counseling sessions with the study director. To further ensure differentiation in dietary intake between groups, we provided subjects with the majority of their carbohydrate-rich foods [eg, Cheerios (General Mills Inc, Minneapolis, MN) and Kashi GoLean cereals (Kashi Co, La Jolla, CA) for the low-fat and low-GL diets, respectively], healthful sources of fat (eg, reduced-fat peanut butter and regular peanut butter for the low-fat and low-GL diets, respectively), and snacks [eg, Nature Valley granola bars (General Mills Inc) and SoLo low-GI snack bars (SoLo GI Nutrition Inc, Kelowna, Canada) for the low-fat and low-GL diets, respectively] either through monthly delivery from a supermarket to their homes or at in-person visits. The study dietitians arranged deliveries on the basis of assigned diet and subjects’ food preferences. Subjects were instructed to eat ad libitum and to consume provided foods to maintain macronutrient balance. All subjects received routine prenatal care and were advised to take a standard prenatal multivitamin.
Assessment of outcomes
At each study visit, maternal measurements included weight, blood pressure, and urine analysis for ketones. Weight was obtained in 2 hospital gowns without shoes by using an electronic scale (Acme Medical Scale Model 2515; Acme Scale Co, San Leandro, CA; Scale-Tronix Model 5002; Scale-Tronix, White Plains, NY, beginning late May 2009). A single blood pressure was obtained with an automated system (Critikon Dinamap; GE Healthcare, Waukesha, WI) after the subject was seated quietly for 5 min. Research dietitians not involved in diet implementation assessed maternal body composition by bioelectrical impedance using a multi-frequency bioelectrical impedance analyzer (RJL Systems Inc, Clinton Township, MI), skinfold thickness at the biceps and thigh (Lange Skinfold Calipers Model 68902; Country Technology, Gays Mills, WI), and wrist circumference. Height was obtained at the screening visit by using a portable stadiometer (Model PE-AIM-101; Perspective Enterprises, Portage, MI).
At baseline and at the 36-wk visits, subjects had blood drawn by venipuncture after an overnight fast of ≈10 h. Blood was immediately sent for analysis of plasma glucose, lipids, and glycated hemoglobin. Additional blood was centrifuged and stored at −80°C for analysis of serum insulin and C-reactive protein (CRP) on study completion. Homeostasis model assessment of insulin resistance (HOMA-IR) {[glucose (mmol/L) × insulin (μU/mL)]/22.5}, a measure of insulin resistance (26), was then calculated. HOMA-IR has been validated as an estimate of insulin resistance during early, mid-, and late pregnancy (27).
Estimated due date, by either last menstrual period or ultrasound, was provided by the subject's obstetrician at enrollment. Gestational age at delivery was then calculated as 40 wk minus the difference between the estimated due date and actual delivery date. Birth weight was obtained in the delivery room by obstetric nursing staff by using a standard, calibrated beam scale (Lifeline Medical Inc, Danbury, CT). Birth weight z score was calculated by using sex-specific, intrauterine growth curves from a large, racially diverse US sample (28). Macrosomia (birth weight >4000g), large for gestational age (LGA; birth weight >90th percentile of weight for gestational age), and ponderal index [birth weight (kg)/birth length (m)3] were secondary outcomes. Between 12 and 48 h of life, postnatal infant measurements included length, head circumference, and abdominal circumference. Skinfold thickness at the triceps, thigh, suprailiac, and subscapular sites were obtained by using Harpenden skinfold calipers (Baty International, West Sussex, United Kingdom).
The following staff were blinded to group assignment: obstetricians who provided clinical care to subjects; nurses who measured maternal body weight and blood pressure, collected and processed maternal blood samples, and analyzed urinalyses; labor and delivery room nurses who obtained birth weight; laboratory staff who analyzed maternal blood; and staff who performed data entry. Staff who performed maternal body composition analysis, 24-h dietary recalls, and infant anthropometric measurements were predominantly, but not always, blinded due to logistical considerations. Formal blinding of subjects was not possible, although subjects were not informed of their group assignments.
Process evaluation
Dietary intake on one weekday and one weekend day was assessed by unannounced, telephone-administered, 24-h dietary recall interviews conducted between 32 and 36 wk gestation. Data were collected by using the Nutrition Data System for Research Software (NDS-R, versions 2006 and 2008; Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Final calculations were completed by using NDS-R, version 2009, with the recognition that the time-related database updates analytic data while maintaining nutrient profiles true to the version used for data collection. Dietary variables of interest for this report included carbohydrate, protein, total and saturated fat, GI, GL, fiber, and energy. Daily GI was calculated by summing the weighted GI value for each recalled food item:
Subsequently, GL was calculated as the product of GI and total carbohydrate intake, adjusted for energy intake:
Particular attention was directed toward GI values when using the NDS-R to quantify dietary intake from each food that was provided to participants as a component of the respective interventions. We systematically evaluated the macronutrient composition and processing of each provided food to ensure selection of an option from NDS-R with a GI value reflecting published data for the same, or comparable, product (29). Every time that a provided food was reported, we selected the same option from NDS-R to ensure consistency in GI values.
Power and sample size
Power calculations for this pilot study were based on a planned Student's t test with a 2-sided type I error rate (critical P value) of 0.05. The protocol assumed the SD of birth weight to be 500 g and specified 30 subjects per treatment group, providing 80% power to detect a difference of 367 g in birth weight or 0.74 in birth weight z score. Due to the extended recruitment period and limits of funding, the ultimate sample, which comprised 21 low-fat and 24 low-GL subjects with an actual SD of 574 g in birth weight, provided 80% power to detect a group difference of 487 g in birth weight or 0.85 in z score. There was a total of 18 infant outcomes, and 18 maternal outcomes considered. For each group of outcomes, the expected number of type I errors with critical P value of ≤0.05 was less than 1.
Statistical analysis
All analyses used the intention-to-treat principle, classifying each subject according to her randomly assigned diet regardless of duration or compliance. All tests were 2-tailed with a significance level of P ≤ 0.05. SAS software (version 9.2; SAS Institute, Cary, NC) was used for all computations.
Baseline measurements were compared between treatment groups by Student's t test or by Wilcoxon's rank-sum test for measures with strongly skewed distributions. Categorical baseline characteristics were compared by Fisher's exact test. Dietary intakes during the intervention period, infant characteristics at delivery, and postnatal infant measurements were compared similarly. Infant variables at delivery were adjusted by analysis of covariance for gestational age at birth, and postnatal infant measurements were further adjusted for cesarean section, postnatal age at measurement, and maternal history of prior pregnancy.
Maternal anthropometric measurements and metabolic parameters were obtained at baseline and 36 wk. Overall changes in weight, BMI, body composition, and blood pressure (36 wk–baseline) were compared between treatment groups by Student's t test. Changes in metabolic variables, many of which were markedly skewed in distribution, were compared by Wilcoxon's rank-sum test.
RESULTS
Subject characteristics
As shown in Figure 1, 325 women were assessed for eligibility; 46 were enrolled; and 5 individuals (11%) withdrew. There were 21 women randomly assigned to the low-fat group and 25 to the low-GL group, with study participation spanning from March 2007 to November 2009. Baseline characteristics of subjects did not differ between intervention groups (Table 1) except for CRP, which was lower in the low-fat group (P = 0.04). In general, subjects represented a moderately high risk obstetric population of overweight and obese women, with 37% (17/46) considered to have advanced maternal age (≥35 y). Subjects who withdrew from the study allowed collection of the infants’ birth information from the medical record. Therefore, the primary outcome, birth weight z score, and gestational duration were available for all but one participant, who had an elective, second-trimester termination. Three subjects (1 low-fat, 2 low-GL) were found to have exclusionary diagnoses after being randomly assigned (2 thyroid disease, 1 irritable bowel syndrome), and one (low-GL) was taking exclusionary medications. All 4 continued study participation. Omitting data from these subjects in the analyses had negligible effect on infant and maternal findings; thus, data from these subjects were included in the final results.
TABLE 1.
Maternal characteristics at baseline, compared by study arm1
| Low-GL diet (n = 25) | Low-fat diet (n = 21) | P | |
| Demographic characteristics | |||
| Age (y) | 33.7 ± 3.92 | 33.2 ± 3.7 | 0.67 |
| Education, BA or higher [n (%)] | 18 (72) | 16 (76) | 1.0 |
| Race-ethnicity [n (%)] | |||
| Non-Hispanic white | 14 (56) | 11 (52) | |
| Non-Hispanic black | 3 (12) | 2 (10) | |
| Hispanic | 5 (20) | 3 (14) | 0.77 |
| Asian | 0 (0) | 1 (5) | |
| Non-Hispanic mixed | 2 (8) | 1 (5) | |
| Other/unknown | 1 (4) | 3 (14) | |
| Measures | |||
| Gestational age3 (wk) | 19.8 ± 5.0 | 19.6 ± 4.3 | 0.90 |
| Weight (kg) | 88.4 ± 14.2 | 82.6 ± 10.1 | 0.12 |
| BMI (kg/m2) | 32.1 ± 4.6 | 31.2 ± 3.1 | 0.43 |
| Systolic BP (mm Hg) | 111 ± 12 | 109 ± 11 | 0.64 |
| Diastolic BP (mm Hg) | 65 ± 8 | 64 ± 7 | 0.76 |
| Heart rate (beats/min) | 78 ± 9 | 81 ± 12 | 0.44 |
| Body temperature (°F) | 97.6 ± 0.7 | 97.8 ± 0.4 | 0.26 |
| Fat mass (kg) | 34.6 ± 10.2 | 29.8 ± 6.3 | 0.07 |
| Fat-free mass (kg) | 53.8 ± 5.1 | 52.8 ± 5.0 | 0.50 |
| Total body water (L) | 39.4 ± 3.7 | 38.6 ± 3.7 | 0.50 |
| Biceps skinfold thickness (mm) | 20 ± 6 | 19 ± 4 | 0.50 |
| Thigh skinfold thickness (mm) | 51 ± 6 | 49 ± 6 | 0.25 |
| Wrist circumference (cm) | 16.2 ± 1.0 | 15.8 ± 0.7 | 0.09 |
| Plasma concentrations | |||
| Glucose (mg/dL) | 74.6 ± 6.4 | 78.6 ± 9.9 | 0.11 |
| Insulin4 (μIU/mL) | 5.9 (4.5, 11.1)5 | 7.9 (5.3, 12.1) | 0.19 |
| HOMA-IR4 | 1.1 (0.8, 2.2) | 1.7 (1.0, 2.2) | 0.22 |
| C-reactive protein (mg/dL) | 7.3 (3.7, 9.4) | 4.1 (2.1, 5.5) | 0.04 |
| Hb A1C (%) | 5.3 ± 0.3 | 5.3 ± 0.3 | 0.93 |
| Total cholesterol (mg/dL) | 218 ± 32 | 215 ± 34 | 0.73 |
| HDL cholesterol (mg/dL) | 77 ± 14 | 77 ± 20 | 0.92 |
| LDL cholesterol (mg/dL) | 111 ± 24 | 108 ± 28 | 0.66 |
| Triglycerides (mg/dL) | 144 (108, 176) | 140 (95, 180) | 0.94 |
GL, glycemic load; BA, Bachelor of Arts degree; BP, blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; Hb A1c, glycated hemoglobin. Means ± SDs were compared by Student's t test. Skewed distributions (median, interquartile range) were compared by Wilcoxon's rank-sum test. Categorical variables [n (%)] were compared by Fisher's exact test.
Mean ± SD (all such values).
Gestational age at the start of the intervention.
Low-GL group, n = 24.
Median; interquartile range in parentheses (all such values).
Process evaluation
Attendance at counseling sessions for subjects who did not withdraw was ≈100% (one subject missed session 2). A total of 95% (39/41) of these subjects had ≥2 maintenance sessions, and 93% (38/41) completed the 36-wk visit. Dietary recall data are presented in Table 2. Mean percentage of energy from fat in the low-fat group approximated the target prescription of 25%. GI and GL were significantly lower in the low-GL group, whereas fiber intake was modestly higher.
TABLE 2.
Maternal diet during intervention, compared by study arm1
| Low-GL diet (n = 22) | Low-fat diet (n = 19) | P | |
| Carbohydrate (% of energy) | 48.8 ± 8.1 | 52.3 ± 9.6 | 0.21 |
| Protein (% of energy) | 21.9 ± 4.3 | 21.2 ± 5.0 | 0.62 |
| Fat (% of energy) | 30.5 ± 8.1 | 27.9 ± 6.8 | 0.27 |
| Saturated fat (% of energy) | 9.6 ± 2.8 | 9.1 ± 2.6 | 0.59 |
| Glycemic index2 | 51.8 ± 6.9 | 58.0 ± 4.3 | 0.002 |
| GL2 (g/1000 kcal) | 56.3 ± 15.2 | 69.1 ± 11.9 | 0.005 |
| Fiber (g/1000 kcal) | 16.5 ± 5.0 | 13.4 ± 4.5 | 0.05 |
| Energy (kcal) | 1641 ± 461 | 1665 ± 266 | 0.84 |
Values are mean ± SDs compared by Student's t test. GL, glycemic load. Dietary intake was assessed by 24-h dietary recall interview administered between 32 and 36 wk of pregnancy.
See Subjects and Methods for more details.
Birth outcomes
There was no significant difference in birth weight z score between the 2 groups (Table 3). Although not significant, all 4 infant skinfold-thickness measurements were lower in the low-GL group, and these infants tended to be longer (49.6 ± 3.0 compared with 47.1 ± 3.5 cm, P = 0.07). Head circumference was greater (35.0 ± 0.8 compared with 34.2 ± 1.3 cm, P = 0.01) in the low-GL group, and this difference remained significant after adjustment for postnatal age at measurement, gestational duration, maternal history of prior pregnancy, and mode of delivery.
TABLE 3.
Infant characteristics, compared by study arm1
| Low-GL diet | Low-fat diet | P values | ||||
| n | Values | n | Values | Unadjusted | Adjusted2 | |
| At delivery | ||||||
| Weight (g) | 24 | 3507 ± 4123 | 21 | 3133 ± 671 | 0.03 | 0.22 |
| Weight4 (z score) | 24 | 0.35 ± 0.81 | 21 | 0.19 ± 0.84 | 0.52 | — |
| Macrosomia5 [n (%)] | 24 | 2 (8) | 21 | 1 (5) | 1.0 | — |
| Large for gestational age6 [n (%)] | 24 | 2 (8) | 21 | 3 (14) | 0.65 | — |
| Ponderal index7 (kg/m3) | 22 | 27.9 ± 2.0 | 20 | 26.7 ± 3.7 | 0.19 | 0.44 |
| Gestational age (wk) | 24 | 39.3 ± 1.1 | 21 | 37.9 ± 3.1 | 0.05 | — |
| Preterm8 [n (%)] | 24 | 1 (4) | 21 | 4 (19) | 0.17 | — |
| Gestational age ≤38.0 wk: all [n (%)] | 24 | 3 (13) | 21 | 10 (48) | 0.02 | — |
| Excluding planned cesarean sections [n (%)] | 20 | 1 (5) | 17 | 9 (53) | 0.002 | — |
| Cesarean delivery [n (%)] | 24 | 21 | ||||
| No | 18 (75) | 12 (57) | ||||
| Planned9 | 4 (17) | 4 (19) | 0.30 | — | ||
| Unplanned | 2 (8) | 5 (24) | ||||
| Early postnatal measures | ||||||
| Age at measurement (h) | 21 | 26.5 ± 10.2 | 18 | 30.3 ± 15.2 | 0.37 | — |
| Length (cm) | 22 | 49.6 ± 3.0 | 18 | 47.1 ± 3.5 | 0.02 | 0.07 |
| Length (z score) | 22 | −0.26 ± 1.18 | 18 | −1.01 ± 1.23 | 0.06 | 0.1010 |
| Head circumference (cm) | 22 | 35.0 ± 0.8 | 18 | 34.2 ± 1.3 | 0.01 | 0.01 |
| Head circumference (z score) | 22 | 0.56 ± 0.57 | 18 | 0.13 ± 0.70 | 0.04 | 0.0110 |
| Abdominal circumference (cm) | 22 | 32.8 ± 1.5 | 18 | 32.3 ± 2.0 | 0.46 | 0.47 |
| Skinfold thickness (mm) | ||||||
| Triceps | 22 | 4.3 ± 0.7 | 18 | 4.8 ± 1.0 | 0.10 | 0.21 |
| Subscapular | 22 | 4.0 ± 0.8 | 18 | 4.4 ± 1.1 | 0.19 | 0.43 |
| Suprailiac | 22 | 3.5 ± 0.8 | 18 | 3.6 ± 1.1 | 0.69 | 0.97 |
| Thigh | 22 | 5.3 ± 1.0 | 18 | 5.6 ± 1.6 | 0.52 | 0.63 |
GL, glycemic load. Means ± SDs were compared by Student's t test (unadjusted) and by ANCOVA (adjusted). Categorical variables [n (%)] were compared by Fisher's exact test.
For delivery measures, ANCOVA adjusted for gestational age; for early postnatal measures, ANCOVA additionally adjusted for postnatal age at measurement, maternal history of prior pregnancy, and cesarean delivery.
Mean ± SD (all such values).
z score accounts for gestational age.
Weight >4000 g.
Defined as >90th percentile of weight for gestational age.
Calculated by using length measurement obtained at delivery.
Gestational age <37.0 wk.
Planned cesarean delivery defined as a cesarean delivery known to be required in advance of hospitalization for delivery (eg, elective repeat, breech, placenta previa).
ANCOVA not adjusted for gestational age because z score accounts for gestational age.
There were 13 births before 38 wk gestation, of which 4 were induced for medical reasons [HELLP sydrome (ie, hemolysis, elevated liver enzymes, low platelet count), preeclampsia, hypertension, and oligohydramnios]. Gestational duration was about 10 d longer in the low-GL group (39.3 ± 1.1 compared with 37.9 ± 3.1 wk, P = 0.05), and significantly fewer pregnancies delivered at ≤38 wk gestation in this group (13% compared with 48%, P = 0.02). There were 8 planned cesarean sections (Table 3). Mean gestational duration for infants with planned cesarean section was 38.2 ± 0.8 wk in the low-GL group and 39.0 ± 1.2 wk in the low-fat group. After excluding planned cesarean sections, individuals in the low-GL group were 10-fold less likely to have gestational duration ≤38 wk (5% compared with 53%, P = 0.002). The study lacked power to examine group effects on frank prematurity (delivery at <37 wk), which occurred at a nonsignificantly lower frequency in the low-GL group (4% compared with 19%, P = 0.17).
Maternal outcomes
There was no significant difference between groups in the change in maternal weight or anthropometric measurements from baseline to 36 wk (Table 4). The low-GL group showed a smaller mean increase in fat mass and a greater mean increase in fat-free mass than did the low-fat group, but these differentials were nonsignificant. There were no group differences in change in systolic or diastolic blood pressure. Women in the low-GL group had significantly lesser increases in triglycerides (median: 49 compared with 93 mg/dL; P = 0.03) and total cholesterol (median: 13 compared with 33 mg/dL; P = 0.04). CRP decreased significantly more in the low-GL group (median: −2.5 compared with −0.4 mg/dL; P = 0.007).
TABLE 4.
Maternal changes late in pregnancy, compared by study arm1
| Low-GL diet | Low-fat diet | ||||
| n | Values | n | Values | P | |
| Change in measure, baseline to 36 wk2 | |||||
| Weight (kg) | 22 | 6.4 ± 4.53 | 16 | 6.9 ± 4.2 | 0.74 |
| BMI (kg/m2) | 22 | 2.3 ± 1.6 | 16 | 2.6 ± 1.5 | 0.59 |
| Systolic BP (mm Hg) | 22 | 0 ± 9 | 16 | 2 ± 14 | 0.47 |
| Diastolic BP (mm Hg) | 22 | 1 ± 5 | 16 | 3 ± 6 | 0.31 |
| Fat mass (kg) | 22 | 2.7 ± 3.6 | 16 | 4.1 ± 3.1 | 0.23 |
| Fat-free mass (kg) | 22 | 3.5 ± 3.3 | 16 | 1.8 ± 4.7 | 0.18 |
| Biceps skinfold thickness (mm) | 22 | 0 ± 7 | 16 | 3 ± 6 | 0.33 |
| Wrist circumference (cm) | 22 | 0.4 ± 0.7 | 16 | 0.3 ± 0.5 | 0.94 |
| Thigh skinfold thickness (mm) | 22 | 3 ± 6 | 16 | 4 ± 6 | 0.62 |
| Change in plasma concentration, baseline to 36 wk4 | |||||
| Glucose (mg/dL) | 18 | −0.5 (–3.0, 4.0)5 | 14 | −0.5 (–2.0, 4.0) | 0.92 |
| Insulin (μIU/mL) | 20 | 2.9 (0.0, 5.6) | 16 | 2.5 (0.2, 4.2) | 0.59 |
| HOMA-IR | 17 | 0.4 (0.0, 1.1) | 14 | 0.6 (0.0, 0.8) | 0.91 |
| C-reactive protein (mg/dL) | 22 | −2.5 (–5.5, –0.7) | 16 | −0.4 (–1.4, 1.5) | 0.007 |
| Hb A1C (%) | 22 | 0.2 (0.0, 0.3) | 16 | 0.2 (0.1, 0.3) | 0.94 |
| Total cholesterol (mg/dL) | 22 | 13 (0, 36) | 16 | 33 (22, 56) | 0.04 |
| HDL cholesterol (mg/dL) | 22 | −7 (–11, 2) | 16 | −7 (–13, 6) | 0.86 |
| LDL cholesterol (mg/dL) | 22 | 18 (–1, 33) | 16 | 31 (17, 47) | 0.19 |
| Triglycerides (mg/dL) | 21 | 49 (19, 70) | 16 | 93 (34, 129) | 0.03 |
GL, glycemic load; BP, blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; Hb A1c, glycated hemoglobin.
P values derived from Student's t test comparing mean changes.
Mean ± SD (all such values).
P values derived from Wilcoxon's rank-sum test comparing distributions of change in plasma concentration; this test was used because the majority of distributions showed marked skew, differing mean and median.
Median; interquartile range in parentheses (all such values).
Adverse events
There were 19 serious adverse events (SAEs) associated with 13 subjects (9 low-fat and 4 low-GL). None were deemed directly due to study participation. Maternal events included 7 unplanned cesarean sections (5 low-fat and 2 low-GL) related to HELLP syndrome, oligohydramnios, failed induction, and preterm premature rupture of membranes. Other SAEs included hospitalizations for vaginal bleeding, premature contractions, and premature rupture of membranes; precipitous vaginal delivery with severe postpartum hemorrhage; and preeclampsia with premature delivery. Five infant SAEs associated with 4 infants included 3 premature deliveries (<37 wk) requiring prolonged hospitalization (all low-fat), one neonatal death due to sepsis (low-fat), and one aggressive resuscitation for a tight nuchal cord and drop in heart rate (low-GL).
Anticipated events deemed possibly related to the study intervention included one subject (low-fat) with glycated hemoglobin >6% at 36 wk; one subject (low-fat) with elevated triglycerides at 36 wk; 4 subjects (2 low-fat, 2 low-GL) with moderate-large ketones; 3 subjects (all low-GL) with glucosuria at study visits; 5 subjects (3 low-fat, 2 low-GL) with lightheadedness or nausea at a fasting visit with phlebotomy; and 3 subjects (all low-GL) subjects with weight loss for 2 consecutive study visits.
DISCUSSION
Overall, we observed no significant differences in birth weight z score or other measures of body composition among infants of overweight and obese women receiving a low-GL or low-fat diet during pregnancy. Although not statistically significant, length was greater and all 4 skinfold-thickness measurements were smaller among infants in the low-GL group. Our results differ somewhat from both Clapp (18) and Moses et al (19) who observed a lower birth weight among infants of women consuming a low-GI diet. However, compared with those studies, subjects in our study were heavier and started the intervention later in pregnancy. Taken together, these trials suggest a possible effect of dietary GL during pregnancy on infant body composition that may be greater when dietary intervention is initiated earlier in pregnancy.
We unexpectedly found that gestational duration was longer, and the proportion of pregnancies delivered ≤38 wk gestation was substantially less, in the low-GL group. Even within the normal range for gestational age, infants born between 37 and 38 wk have 1.5- to 2-fold higher risk of adverse outcomes (30, 31) and are more likely to experience problems in school performance at age 10 y (32), compared with infants born between 39 and 40 wk. This cited difference in gestational age is comparable to the difference between the 2 intervention groups in the present study. Moses et al (19) reported a similar effect on gestational duration comparing low- and high-GI diets (39.5 compared with 38.9 wk, P = 0.07) that, although not statistically significant, may increase confidence in our finding.
The specific mechanisms through which dietary composition might influence gestational duration remain speculative and understudied but could involve inflammation (33–35). A proinflammatory profile of cytokines has been described in maternal serum and amniotic fluid in preterm labor (34), and observational studies have identified CRP, a serum marker of inflammation, in early pregnancy as an important predictor of preterm delivery (36, 37). Consistent with other reports (38, 39), CRP decreased markedly in our study in the low-GL group relative to the low-fat diet group. Whereas the low-GL group had a higher CRP at baseline, analysis of change in CRP by repeated measures did not alter the conclusions for CRP. The improvement in dyslipidemia with the low-GL diet may also have contributed to the lengthening of gestational duration, possibly mediated by beneficial effects on endothelial dysfunction. High serum cholesterol and triglycerides, in particular, have been associated with risk of preterm birth (40). However, future research will be necessary to clarify the effect of these relations and nutrition during pregnancy. Because prematurity accounts for an estimated $15.5 billion in annual medical costs (31) and significant morbidity and mortality, dietary treatment to extend gestational duration among overweight and obese women could have major public health significance.
Another unexpected finding was the larger head circumference in the low-GL group. If reproducible, this finding may also have public health significance because infant head circumference is a significant predictor of brain volume (41) and early-childhood IQ (42, 43). Although little is known about the relation between maternal diet and head circumference in a well-nourished population, hormones regulated by diet (eg, insulin, insulin-like growth factors, growth hormone, and cortisol) could theoretically influence neural growth directly or by altering availability of nutrients needed for central nervous system growth (43). Alternatively, intrinsic dietary components, such as the amount and type of fat, might underlie this finding. In a possibly related line of investigation, several studies have identified an association between GI or GL and neural tube defects (44–46), a disorder of neuronal differentiation.
In addition to a potential role mediating infant outcomes, improvements in maternal triglycerides, total cholesterol, CRP, and perhaps adiposity on the low-GL diet may benefit the mother by reducing immediate and long-term risk of cardiovascular disease (3, 7). Although we did not observe a significant group difference in maternal insulin resistance, studies in other clinical settings have reported beneficial effects of a low-GI or low-GL diet on related measures (47–49). Our methods, which used an indirect assessment of insulin resistance late in pregnancy, may have been insufficiently sensitive to address this issue.
This study is one of a very few to examine the independent effects of dietary composition during pregnancy on maternal and infant outcomes by ensuring comparable treatment intensity across groups and with control for other potential behavioral and dietary (ie, total energy, protein) confounders. In addition, we used a comparison diet consistent with current recommendations, provided key foods to improve differentiation between dietary groups, and obtained process measures to assess treatment adherence. Other strengths of the study include excellent subject compliance with study protocol, low drop-out rate, and collection of the primary endpoint for all pregnancies resulting in a live birth. The most notable limitation is the small sample size, providing both insufficient power to assess some endpoints of interest and limiting generalizability. Lack of baseline dietary data precluded assessment of within-individual change in dietary intake, and we did not have a formal assessment of physical activity during study participation. Whereas fasting glucose and glycated hemoglobin were assessed at baseline and 36 wk, an oral-glucose-tolerance test was not conducted as part of the study protocol (although all subjects had standard clinical screening tests for gestational diabetes as part of their prenatal care). Finally, we recognize that study results cannot be exclusively attributed to the effects of GL, because changes in other measured or unmeasured dietary variables unrelated to GL may be causally involved. However, this conceptual limitation generally applies to all outpatient studies of specific diets. Moreover, lack of proof regarding causal mechanisms would not negate the potential public health significance of the findings.
In conclusion, in this pilot study, we found that a low-GL diet in overweight and obese pregnant women did not significantly lower birth weight z score or other measures of infant body adiposity when compared with a low-fat diet. However, several major maternal cardiovascular disease risk factors improved, including triglycerides, total cholesterol, and CRP. These and other beneficial metabolic and hormonal effects of a low-GL diet may have contributed to an increase in gestational duration and infant head circumference in the low-GL group. Larger-scale trials and mechanistically oriented studies are needed to examine whether a low-GL diet during pregnancy may be useful in the prevention of prematurity and other adverse maternal and infant outcomes.
Acknowledgments
We thank Linda Seger-Shippee for conducting dietary recalls; Meredith Beard for handling the administrative aspects of the study; the staff at the Beth Israel Deaconess Medical Center General Clinical Research Center, labor and delivery unit, and postpartum units for their care, cooperation and support; our Data and Safety Monitoring Board (Joseph Majzoub, Hope Ricciotti and Shiva Gautam) for their time and feedback; Handan Titiz in the Clinical Research Program at Children's Hospital Boston for developing our study database; Anne Barbara and Suzanne Rostler for their contribution to the dietary intervention; Abigail Machson-Carter, Pamela Krajewski, and Erica Garcia-Lago for their important contribution to study recruitment, data collection, and data management; and Solo GI Nutrition Inc, British Columbia, Canada for supplying the Solo low-GI snack bars used in the study.
The authors’ responsibilities were as follows—ETR, DBP, TCT, CBE, HAF, and DSL: study conception and design; ETR, DBP, TCT, MM Lovesky, EAC, and MM Leidig: acquisition of data; ETR, DBP, TCT, CBE, HAF, and DSL: analysis and interpretation of data; ETR, HAF, and DSL: drafting the article; DBP, TCT, CBE, MM Lovesky, EAC, MM Leidig: critical revision of manuscript for important intellectual content; ETR, CBE, HAF: statistical analysis; DBP, HAF, and DSL: obtained funding; ETR, DBP, MM Leidig, MM Lovesky, EAC, and DSL: administrative, technical, and material support; and ETR, TCT, and DSL: supervision. ETR and DSL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ETR was formerly Chief Medical Officer for Pediatric Weight Management Centers, LLC's Great Moves! Program, a company privately owned and operated in collaboration with the physicians of Children's Hospital Boston. ETR provided contracted clinical and administrative services for the company but neither had nor has equity or other economic interest in the business. The remaining authors reported no disclosures.
REFERENCES
- 1.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 1991;165:1667–72 [DOI] [PubMed] [Google Scholar]
- 2.Dahlgren J. Pregnancy and insulin resistance. Metab Syndr Relat Disord 2006;4:149–52 [DOI] [PubMed] [Google Scholar]
- 3.Siega-Riz AM, King JC. Position of the American Dietetic Association and American Society for Nutrition: obesity, reproduction, and pregnancy outcomes. J Am Diet Assoc 2009;109:918–27 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol 1990;162:1008–14 [DOI] [PubMed] [Google Scholar]
- 5.Huda SS, Brodie LE, Sattar N. Obesity in pregnancy: prevalence and metabolic consequences. Semin Fetal Neonatal Med 2009;15:70–6 [DOI] [PubMed] [Google Scholar]
- 6.Paramsothy P, Knopp RH. Metabolic syndrome in women of childbearing age and pregnancy: recognition and management of dyslipidemia. Metab Syndr Relat Disord 2005;3:250–8 [DOI] [PubMed] [Google Scholar]
- 7.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 2001;357:2002–6 [DOI] [PubMed] [Google Scholar]
- 8.Oken E, Gillman MW. Fetal origins of obesity. Obes Res 2003;11:496–506 [DOI] [PubMed] [Google Scholar]
- 9.Junien C, Nathanielsz P. Report on the IASO Stock Conference 2006: early and lifelong environmental epigenomic programming of metabolic syndrome, obesity and type II diabetes. Obes Rev 2007;8:487–502 [DOI] [PubMed] [Google Scholar]
- 10.Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gynecol Clin North Am 2009;36:285–300 [DOI] [PubMed] [Google Scholar]
- 11.Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol 2009;113:305–12 [DOI] [PubMed] [Google Scholar]
- 12.Dodd JM, Crowther CA, Robinson JS. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: a systematic review. Acta Obstet Gynecol Scand 2008;87:702–6 [DOI] [PubMed] [Google Scholar]
- 13.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010;91:373–80 [DOI] [PubMed] [Google Scholar]
- 14.Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord 2002;26:1494–502 [DOI] [PubMed] [Google Scholar]
- 15.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23 [DOI] [PubMed] [Google Scholar]
- 16.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6 [DOI] [PubMed] [Google Scholar]
- 17.Clapp JF., III Effect of dietary carbohydrate on the glucose and insulin response to mixed caloric intake and exercise in both nonpregnant and pregnant women. Diabetes Care 1998;21(suppl 2):B107–12 [PubMed] [Google Scholar]
- 18.Clapp JF., III Diet, exercise, and feto-placental growth. In: Grundlagenreferate. Arch Gynecol Obstet 1997;261:101–8 [Google Scholar]
- 19.Moses RG, Luebcke M, Davis WS, et al. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am J Clin Nutr 2006;84:807–12 [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine Dietary Reference Intakes for energy, carbohydrate, fiber, fat, protein and amino acids (macronutrients). Washington, DC: National Academy Press, 2005 [Google Scholar]
- 21.Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2002;25:148–98 [DOI] [PubMed] [Google Scholar]
- 22.Kaiser LL, Allen L. Position of the American Dietetic Association: nutrition and lifestyle for a healthy pregnancy outcome. J Am Diet Assoc 2002;102:1479–90 [DOI] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services, US Department of Agriculture Dietary Guidelines for Americans, 2005. 6th ed.Washington, DC: US Government Printing Office, 2005 [Google Scholar]
- 24.Liu S, Manson JE, Stampfer MJ, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr 2001;73:560–6 [DOI] [PubMed] [Google Scholar]
- 25.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30 [DOI] [PubMed] [Google Scholar]
- 26.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95 [DOI] [PubMed] [Google Scholar]
- 27.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001;24:1602–7 [DOI] [PubMed] [Google Scholar]
- 28.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics 2010;125:e214–24 [DOI] [PubMed] [Google Scholar]
- 29.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tita AT, Landon MB, Spong CY, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med 2009;360:111–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green NS, Damus K, Simpson JL, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol 2005;193:626–35 [DOI] [PubMed] [Google Scholar]
- 32.Kirkegaard I, Obel C, Hedegaard M, Henriksen TB. Gestational age and birth weight in relation to school performance of 10-year-old children: a follow-up study of children born after 32 completed weeks. Pediatrics 2006;118:1600–6 [DOI] [PubMed] [Google Scholar]
- 33.Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol 2010;30:441–6 [DOI] [PubMed] [Google Scholar]
- 34.Orsi NM. Cytokine networks in the establishment and maintenance of pregnancy. Hum Fertil (Camb) 2008;11:222–30 [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohsoonthorn V, Qiu C, Williams MA. Maternal serum C-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin Biochem 2007;40:330–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol 2005;162:1108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr 2002;75:492–8 [DOI] [PubMed] [Google Scholar]
- 39.Pereira MA, Swain JS, Goldfine AB, Rifai N, Ludwig DS. Effects of a low glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004;292:2482–90 [DOI] [PubMed] [Google Scholar]
- 40.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol 2007;166:1312–9 [DOI] [PubMed] [Google Scholar]
- 41.Lindley AA, Benson JE, Grimes C, Cole TM, III, Herman AA. The relationship in neonates between clinically measured head circumference and brain volume estimated from head CT-scans. Early Hum Dev 1999;56:17–29 [DOI] [PubMed] [Google Scholar]
- 42.Gale CR, O'Callaghan FJ, Bredow M, Martyn CN. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 2006;118:1486–92 [DOI] [PubMed] [Google Scholar]
- 43.Broekman BF, Chan YH, Chong YS, et al. The influence of birth size on intelligence in healthy children. Pediatrics 2009;123:e1011–6 [DOI] [PubMed] [Google Scholar]
- 44.Shaw GM, Quach T, Nelson V, et al. Neural tube defects associated with maternal periconceptional dietary intake of simple sugars and glycemic index. Am J Clin Nutr 2003;78:972–8 [DOI] [PubMed] [Google Scholar]
- 45.Carmichael SL, Witte JS, Shaw GM. Nutrient pathways and neural tube defects: a semi-Bayesian hierarchical analysis. Epidemiology 2009;20:67–73 [DOI] [PubMed] [Google Scholar]
- 46.Yazdy MM, Liu S, Mitchell AA, Werler MM. Maternal dietary glycemic intake and the risk of neural tube defects. Am J Epidemiol 2010;171:407–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clapp JF, Lopez B. Low-versus high-glycemic index diets in women: effects on caloric requirement, substrate utilization and insulin sensitivity. Metab Syndr Relat Disord 2007;5:231–42 [DOI] [PubMed] [Google Scholar]
- 48.Rizkalla SW, Taghrid L, Laromiguiere M, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care 2004;27:1866–72 [DOI] [PubMed] [Google Scholar]
- 49.Laaksonen DE, Toppinen LK, Juntunen KS, et al. Dietary carbohydrate modification enhances insulin secretion in persons with the metabolic syndrome. Am J Clin Nutr 2005;82:1218–27 [DOI] [PubMed] [Google Scholar]