Abstract
Background: Circulating 25-hydroxyvitamin D [25(OH)D] and parathyroid hormone (PTH) concentrations have been shown to be associated with insulin sensitivity; however, adiposity may confound this relation. Furthermore, African Americans (AAs) have lower insulin sensitivity and 25(OH)D concentrations than do European Americans (EAs); whether these differences are associated in a cause-and-effect manner has not been determined.
Objectives: The objectives of this study were to examine the relation of 25(OH)D and PTH concentrations with whole-body insulin sensitivity and to determine whether lower 25(OH)D concentrations in AAs compared with EAs contribute to the lower insulin sensitivity of AAs relative to that of EAs.
Design: This was a cross-sectional study of 25 AA and 25 EA women. We determined the whole-body insulin sensitivity index (SI) with an intravenous glucose tolerance test and minimal modeling. Percentage body fat was determined with dual-energy X-ray absorptiometry, and intraabdominal adipose tissue (IAAT) was determined with computed tomography.
Results: Multiple linear regression analysis indicated that 25(OH)D and PTH concentrations were independent determinants of SI [standardized β = 0.24 (P = 0.04) and −0.36 (P = 0.002), respectively] after adjustment for age, race, and IAAT. The mean ethnic difference in SI decreased from 2.70 [· 10−4 · min−1/(μIU/mL)] after adjustment for IAAT and percentage body fat to 1.80 [· 10−4 · min−1/(μIU/mL)] after further adjustment for 25(OH)D and PTH concentrations.
Conclusions: 25(OH)D and PTH concentrations were independently associated with whole-body insulin sensitivity in a cohort of healthy women, which suggested that these variables may influence insulin sensitivity through independent mechanisms. Furthermore, ethnic differences in 25(OH)D concentrations may contribute to ethnic differences in insulin sensitivity.
INTRODUCTION
Vitamin D may play a role in maintaining insulin sensitivity (1), the loss of which has been implicated in the progression of type 2 diabetes (2). Cross-sectionally, 25-hydroxyvitamin D [25(OH)D] concentrations are generally shown to be associated with insulin sensitivity (1, 3–6). However, because vitamin D may become sequestered in adipose tissue (7) and obesity is associated with vitamin D deficiency (8, 9) and insulin resistance (2), adiposity may be a confounder of these relations (10). Parathyroid hormone (PTH) concentrations are often elevated in vitamin D–deficient states (11) and may further confound the relation between 25(OH)D concentrations and insulin sensitivity because PTH has also been shown to be positively associated with insulin sensitivity (12, 13). Indexes of insulin sensitivity derived from fasting values of insulin and glucose, such as the homeostatic model assessment of insulin resistance (HOMA-IR), are commonly used to investigate the associations between 25(OH)D concentrations and insulin sensitivity (1). These indexes tend to reflect more hepatic insulin resistance (14) as opposed to whole-body insulin sensitivity, which encompasses both hepatic and peripheral insulin sensitivity (15). In addition, the accuracy of indexes such as HOMA-IR in estimating whole-body insulin sensitivity may vary by ethnicity and obesity status (16). To our knowledge, no previous study has examined the relation between 25(OH)D concentrations and a direct measure of whole-body insulin sensitivity after adjusting for a robust measure of adiposity and PTH concentrations.
African Americans (AAs) have a disproportionately greater risk of insulin resistance and type 2 diabetes than do European Americans (EAs) (17). The causes of ethnic differences in insulin sensitivity are not clear and have not been explained by differences in adiposity and body composition (17). AAs also generally have lower serum 25(OH)D concentrations than EAs (9). To our knowledge, it has not been examined whether lower serum 25(OH)D concentrations in AAs relative to those in EAs contribute to ethnic differences in insulin sensitivity.
The overall objective of this study was to examine the relations of serum 25(OH)D and PTH concentrations with insulin sensitivity, independent of adiposity, in healthy women by using a frequently sampled intravenous glucose tolerance test, dual-energy X-ray absorptiometry (DXA), and computed tomography (CT). The use of these robust measures allowed for a thorough investigation of the relations of 25(OH)D and PTH concentrations with whole-body insulin sensitivity, body composition, and fat distribution. Furthermore, this study aimed to determine whether lower 25(OH)D concentrations in AAs contributed to their lower insulin sensitivity.
SUBJECTS AND METHODS
Subjects
Subjects were 50 healthy, premenopausal and postmenopausal women of AA and EA ethnicity identified from 2 studies conducted at the University of Alabama at Birmingham (UAB). Exclusion criteria for both studies were type 1 or type 2 diabetes, polycystic ovary syndrome, disorders of glucose or lipid metabolism, use of medications known to affect body composition or glucose metabolism (including antihypertensive medications), tobacco use, or abnormal glucose tolerance. All premenopausal women had regular menstrual cycles. A portion of the premenopausal women (n = 13) had been previously subjected to a weight-loss intervention (18) and were in the 2-y follow-up phase of the study (2 y after weight loss). Subject selection for this substudy was based on a sufficient quantity of sera to assay 25(OH)D and PTH, as well as available insulin sensitivity, DXA, and CT measurements. The mean age, body mass index (BMI; in kg/m2), percentage body fat, intraabdominal adipose tissue (IAAT), and insulin sensitivity index (SI) of this substudy did not significantly differ from the respective parent studies, with mean differences <10% of mean values from the parent studies. All subjects were recruited between February 2001 and February 2007. Both studies were approved by the UAB Institutional Review Board for Human Use, and written informed consent was obtained from all subjects before testing.
Protocol
The protocol for each study was previously reported (18–20). In brief, subjects were admitted as in patients to the UAB General Clinical Research Center, and insulin sensitivity and body-composition testing was performed. Weight stability in the post-weight-loss women was ascertained before testing via a 4-wk weight-maintenance diet with weights checked 3 times/wk and food provided in the last 2 wk. Testing in all premenopausal women was conducted during the follicular phase of the menstrual cycle. Metabolic tests were conducted year round.
Insulin sensitivity testing
Insulin sensitivity was measured by using an insulin-modified intravenous glucose tolerance test and minimal modeling after a 12-h overnight fast, the details of which were previously described (19, 20). In brief, a catheter was placed in the antecubital space of both arms, and fasted blood samples were drawn for subsequent analyses of lipids and hormones. Glucose (50% dextrose) was injected intravenously at time zero, and multiple subsequent blood draws were taken over a period of 3–4 h. Insulin (0.02 U insulin/kg) was administered at time 20 relative to glucose administration. Samples were processed and stored at −80°C until ready for analysis. SI was determined from insulin and glucose values entered into the MINMOD computer program (version 3; Richard N Bergman, University of Southern California, Los Angeles, CA) (15). This index has been validated against and correlates well with the gold-standard euglycemic clamp (21). HOMA-IR was calculated as follows:
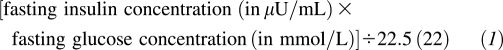 |
Laboratory analyses
Glucose, insulin, and lipid profiles were assayed in the UAB Metabolism Core Laboratory of the General Clinical Research Center, Clinical Nutrition Research Center, Diabetes Research Training Center, and Center for Clinical and Translational Science. Glucose was measured in 10 μL sera with an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics, Rochester, NY). The mean intra- and interassay CVs for glucose analysis in the Core Laboratory were 0.61% and 1.45%, respectively. Insulin concentrations were determined in duplicate 100-μL aliquots with double-antibody radioimmunoassays (Linco Research Inc, St Charles, MO). The insulin assay has a sensitivity of 3.35 μIU/mL in the Core Laboratory and mean intra- and interassay CVs of 3.49% and 5.57%, respectively. Serum 25(OH)D concentrations were assayed with a liquid chromatography-tandem mass spectrometry technique (Quest Diagnostics Nichols Institute, San Juan Capistrano, CA). Values are reported as total serum 25(OH)D concentrations [including 25(OH)D2 and 25(OH)D3]. Vitamin D deficiency was defined as serum 25(OH)D concentrations ≤20 ng/mL, and vitamin D insufficiency was defined as serum 25(OH)D concentrations <30 ng/mL (11). Serum PTH concentrations were assessed by a2-site immunochemiluminometric assay that detected intact PTH and the aminoterminally truncated PTH fragments, and serum calcium concentrations were determined by using spectrophotometry (Quest Diagnostics Nichols Institute). The PTH value for one subject was >3 SD above the mean and, therefore, was excluded from the analyses.
Body composition and fat distribution
Percentage body fat was determined by using DXA (Lunar Prodigy densitometer; GE Healthcare Lunar, Madison, WI) in the UAB Department of Nutrition Sciences. Subjects were scanned in light clothing and in the supine position with their arms to the sides. IAAT and subcutaneous abdominal adipose tissue were determined by using CT (23) with a HiLight/Advantage Scanner (General Electric, Milwaukee, WI) in the UAB Department of Radiology. Subjects were scanned in the supine position with their arms above their heads. A 5-mm scan was taken at the level of the umbilicus (approximately the L4–L5 intervertebral space) and later analyzed for the cross-sectional area (in cm2) of adipose tissue with a density-contour computer program (NIH Image; NIH, Bethesda, MD; http://rsb.info.nih.gov/nih-image/) with Hounsfield units for adipose tissue set at −190 to −30.
Statistical analyses
Descriptive characteristics are reported as means (±SDs). The distribution of all continuous variables was examined, and variables that deviated from a normal distribution were log10 transformed (fasting insulin concentrations, SI, 25(OH)D concentrations, IAAT, and HOMA-IR). Partial correlation analyses, with adjustment for ethnicity, were used to investigate the relations of 25(OH)D and PTH concentrations with indexes of insulin sensitivity. Potential interactions of ethnic group with 25(OH)D and PTH concentrations were investigated with multiple linear regression analysis (MLR). Interaction terms were not statistically significant for any indexes of insulin sensitivity (P > 0.18 for all); thus, data are reported with both ethnic groups pooled together. Forward stepwise MLR was used to explore the best independent predictors of the dependent variable SI. The following variables were tested as predictors of SI: race, IAAT, subcutaneous abdominal adipose tissue, age, percentage body fat, and serum concentrations of PTH, 25(OH)D, and calcium. A significance probability value of 0.15 was used as the criteria for entry into and exit out of the model. Variables observed to be significant in the stepwise analysis were subsequently used in the MLR analysis for SI. To explore the possibility of a threshold effect of vitamin D concentration on SI, MLR analyses were repeated in subjects characterized as vitamin D insufficient. Ethnic differences in continuous variables were determined with 2-group t tests. Ethnic differences in mean SI, adjusted for IAAT and percentage body fat, were determined with analyses of covariance and compared with means that were further adjusted for 25(OH)D and PTH concentrations. Statistical analyses were 2-tailed with a significance level of 0.05 and were performed with SAS software (version 9.1; SAS Institute, Cary, NC).
RESULTS
Descriptive and metabolic characteristics for all women and by ethnic group are presented in Table 1. A majority of the women were premenopausal (78%) and overweight or obese (53% of women with a BMI >25). Ages ranged from 18.9 to 67.4 y. The mean 25(OH)D concentration was 22.3 ± 13.7 ng/mL with 54% of women classified as vitamin D deficient, and 72% of women were classified as vitamin D insufficient. Two-group t tests indicated that AAs had lower 25(OH)D (P < 0.001) concentrations and SI (P < 0.001) than EAs and had greater fasting insulin (P = 0.001) concentrations, HOMA-IR (P = 0.005), and PTH concentrations (P = 0.07). 25(OH)D concentrations did not differ by the season of blood draw [P = 0.28 (analysis of variance)].
TABLE 1.
Descriptive and metabolic characteristics1
| Variable | All (n = 50) | European Americans (n = 25) | African Americans (n = 25) | 2 |
| Age (y) | 38.2 ± 13.13 | 39.1 ± 13.3 | 37.4 ± 13.0 | 0.64 |
| Premenopausal status [n (%)] | 39 (78) | 19 (76) | 20 (80) | 0.73 |
| BMI (kg/m2) | 26.4 ± 4.7 | 25.1 ± 3.9 | 27.7 ± 5.0 | 0.05 |
| Percentage body fat | 39.0 ± 7.9 | 38.6 ± 7.2 | 39.4 ± 8.6 | 0.74 |
| IAAT (cm2)4 | 76.6 ± 43.5 | 81.4 ± 40.6 | 71.8 ± 46.6 | 0.25 |
| SAAT (cm2) | 278.1 ± 150.1 | 251.8 ± 140.0 | 305.4 ± 157.8 | 0.21 |
| Fasting glucose (mg/dL) | 91.2 ± 9.3 | 91.2 ± 8.3 | 91.3 ± 10.4 | 0.98 |
| 25(OH)D (ng/mL)4 | 22.3 ± 13.7 | 29.3 ± 13.5 | 15.4 ± 10.0 | <0.001 |
| PTH (pg/mL) | 44.5 ± 17.7 | 40.0 ± 16.7 | 49.2 ± 17.85 | 0.07 |
| Serum calcium (mg/dL) | 9.5 ± 0.5 | 9.6 ± 0.6 | 9.4 ± 0.3 | 0.23 |
| Fasting insulin (μIU/mL)4 | 11.0 ± 5.0 | 8.6 ± 3.4 | 13.3 ± 5.3 | 0.001 |
| SI [· 10minus4 · minminus1/(μIU/mL)]4 | 4.3 ± 2.7 | 5.7 ± 2.7 | 2.8 ± 1.6 | <0.001 |
| HOMA-IR4 | 2.5 ± 1.2 | 1.97 ± 0.84 | 3.06 ± 1.36 | 0.005 |
IAAT, intraabdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; SI, insulin sensitivity index; HOMA-IR, homeostatic model assessment of insulin resistance.
P for ethnic differences measured by a 2-group t test or a chi-square test.
Mean ± SD (all such values).
Log10 transformed for analysis.
n = 24.
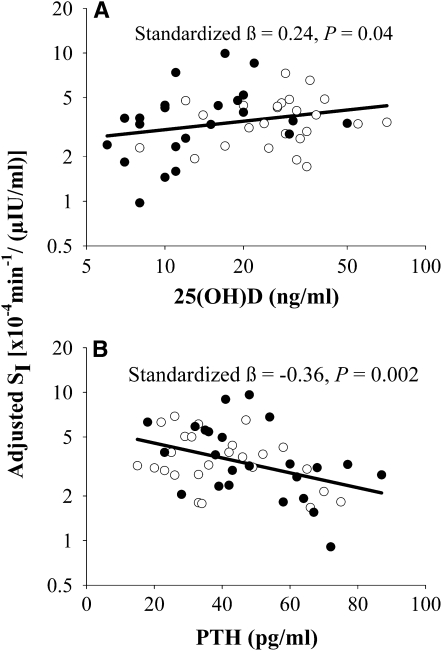
Both 25(OH)D and PTH concentrations were significantly associated with SI in ethnic group–adjusted partial correlation analyses, (r = 0.31, P = 0.03 and r = −0.37, P = 0.009, respectively). Neither 25(OH)D nor PTH concentrations were significantly associated with fasting glucose or fasting insulin concentrations or HOMA-IR (P > 0.05 for all). The correlation between PTH and 25(OH)D concentrations was r = −0.25 (P = 0.08). Age was significantly associated with PTH concentrations (r = 0.29, P = 0.04) but not with 25(OH)D concentrations (r = 0.02, P = 0.89). Variables identified in stepwise MLR analysis as significant independent predictors of SI, and subsequently used in MLR analyses, were race, IAAT, age, and PTH and 25(OH)D concentrations. The final model for the dependent variable SI is shown in Table 2A and indicates that both 25(OH)D and PTH concentrations had independent associations with SI. The linear relations of 25(OH)D and PTH concentrations with SI, which were adjusted for all other variables in the model, are depicted in Figure 1. Subset MLR analysis in subjects with vitamin D insufficiency only is provided in Table 2B. Ethnic group was removed from the reduced model because the majority of subjects were AAs (n = 21), and race was not a significant correlate.
TABLE 2.
Multiple linear regression for the dependent variable insulin sensitivity index (SI)1
| Independent variable | Variable estimate ± SEE | Partial r2 | P |
| A. All subjects (n = 49)2 | |||
| 25(OH)D | 0.28 ± 0.13 | 0.09 | 0.04 |
| IAAT | −0.56 ± 0.13 | 0.29 | <0.001 |
| PTH | −0.006 ± 0.002 | 0.21 | 0.002 |
| Age | 0.01 ± 0.003 | 0.21 | 0.002 |
| Ethnic group | 0.11 ± 0.04 | 0.19 | 0.003 |
| B. Vitamin D insufficient (n = 35)3 | |||
| 25(OH)D | 0.90 ± 0.17 | 0.48 | <0.001 |
| IAAT | −0.40 ± 0.15 | 0.19 | 0.01 |
| PTH | −0.007 ± 0.002 | 0.31 | 0.001 |
| Age | 0.006 ± 0.004 | 0.07 | 0.14 |
25(OH)D, 25-hydroxyvitamin D; IAAT, intraabdominal adipose tissue; PTH, parathyroid hormone. Variables that were log10 transformed for analyses were SI, 25(OH)D, and IAAT. Ethnic group was coded such that European American = 0 and African American = 1. Vitamin D–insufficient subjects were those with 25(OH)D concentrations <30 ng/mL.
R2 = 0.60, P < 0.001.
R2 = 0.62, P < 0.001.
FIGURE 1.
Relations with the insulin sensitivity index (SI). A: 25-Hydroxyvitamin D [25(OH)D] with SI adjusted for intraabdominal adipose tissue (IAAT), age, ethnicity, and parathyroid hormone (PTH). B: PTH with SI adjusted for IAAT, age, ethnicity, and 25(OH)D. Filled circles represent African Americans; open circles represent European Americans. Standardized βs and P values were measured with multiple linear regression analysis (n = 49). Axes containing SI or 25(OH)D were log10 transformed.
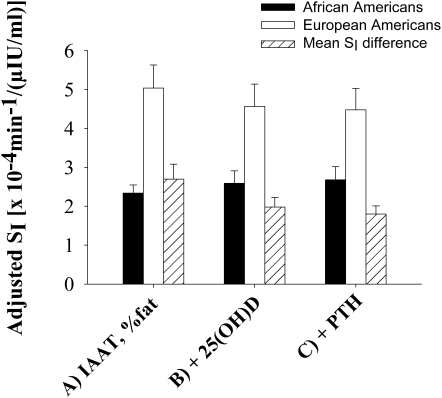
The ethnic difference in SI, which was adjusted for IAAT and percentage fat, was 2.70 [· 10−4 · min−1/(μIU/mL)] (adjusted mean SI was 5.04 ± 0.59 and 2.34 ± 0.21 [· 10−4 · min−1/(μIU/mL)] for EAs and AAs, respectively) as shown in Figure 2 (P for ethnic difference <0.001). Adjustment for 25(OH)D concentrations reduced the ethnic difference to 1.98 [· 10−4 · min−1/(μIU/mL)] (P for ethnic difference = 0.003). Adjustment for both 25(OH)D and PTH concentrations reduced the ethnic difference in SI by 33% to 1.80 [· 10−4 · min−1/(μIU/mL)] (adjusted mean SI was 4.48 ± 0.55 and 2.68 ± 0.34 [· 10−4 · min−1/(μIU/mL)] for EAs and AAs, respectively; P for ethnic difference = 0.008).
FIGURE 2.
Insulin sensitivity index (SI) in African Americans (AAs) and European Americans (EAs) adjusted for intraabdominal adipose tissue (IAAT) and percentage body fat (n = 25 AAs and 25 EAs) (A), further adjusted for 25-hydroxyvitamin D [25(OH)D] (n = 25 AAs and 25 EAs) (B), and further adjusted for 25(OH)D and parathyroid hormone (PTH) (n = 24 AAs and 25 EAs) (C). The ethnic difference in SI was reduced from 2.70 to 1.80 [· 10−4 · min−1/(μIU/mL)] (a 33% decrease) with adjustment for 25(OH)D and PTH. ANCOVA was used to determine adjusted means.
DISCUSSION
This study aimed to investigate the relations of circulating 25(OH)D and PTH concentrations with insulin sensitivity by using direct measures of insulin sensitivity after accounting for robust measures of body composition and fat distribution. Results indicated that both 25(OH)D and PTH concentrations were independently associated with a measure of whole-body insulin sensitivity. In addition, the magnitude of lower insulin sensitivity in AAs was reduced by adjustment for 25(OH)D and PTH concentrations. These observations suggest that vitamin D and PTH may play independent roles in the regulation of metabolic health, and that vitamin D supplementation may be useful for minimizing ethnic disparities at risk of type 2 diabetes.
Several studies have shown 25(OH)D concentrations to be associated with insulin sensitivity and resistance (1); however, the majority of studies relied on proxy indexes of insulin sensitivity, used crude measures of adiposity as adjusting variables, and/or have not accounted for PTH. We show, for the first time to our knowledge, that 25(OH)D concentrations were positively associated with a robust measure of insulin sensitivity, independent of percentage body fat, IAAT, and PTH concentrations in a sample of AA and EA women. Our use of DXA-derived percentage body fat and CT-derived IAAT as covariates, as opposed to BMI, provided accurate adjustment for both body composition and abdominal fat accumulation. Nevertheless, our results are in accordance with studies by Lind et al (6) and Chiu et al (3) who showed that 25(OH)D concentrations were positively associated with clamp-derived measures of insulin sensitivity, independent of BMI, in middle-aged Swedish men and in relatively young men and women of varying ethnicity, respectively. However, this is in contrast to a recent study in middle-aged Italian men and women that reported that 25(OH)D concentrations were associated with euglycemic clamp-derived insulin sensitivity but not after adjustment for BMI (24). It is likely that differences in study populations lead to varying outcomes. The relation between 25(OH)D concentrations and SI was strengthened when only vitamin D insufficient subjects were analyzed (Table 2B). A comparison of the partial contribution of 25(OH)D to SI in the whole group compared with the vitamin D insufficient subset (9% compared with 48%) suggested that 25(OH)D may be more clinically relevant to insulin sensitivity in vitamin D–insufficient subjects.
In contrast to observations with whole-body insulin sensitivity, 25(OH)D were not significantly associated with HOMA-IR, which is a surrogate measure that primarily reflects hepatic insulin resistance (14). The minimal model-derived SI encompasses peripheral and hepatic insulin sensitivity (15). In vitro studies have shown a direct effect of vitamin D concentrations on the up-regulation of human insulin-receptor gene expression and subsequent enhancement of insulin-mediated glucose transport (25, 26), as well as the promotion of insulin-mediated glucose uptake secondary to increased tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) in muscle cells (C2C12) subjected to free fatty acid–induced insulin resistance (27). In addition, vitamin D3 supplementation in Asian-Indian men improved an index of insulin sensitivity derived from an oral glucose tolerance test but did not improve HOMA-IR (28). Taken together, these findings suggest that vitamin D is more related to insulin-stimulated peripheral glucose uptake as opposed to insulin-mediated inhibition of hepatic glucose production, although glucose tracer studies are required to confirm this hypothesis.
In this study, we observed an association between PTH concentrations and SI, independent of 25(OH)D and adiposity. Several lines of evidence support a direct role of PTH in impeding insulin sensitivity. For example, compared with control subjects, patients with primary hyperparathyroidism are insulin resistant, and insulin sensitivity improves after parathyroidectomy in these patients (29). In rat adipocytes, insulin-stimulated glucose uptake declined secondary to a PTH-induced elevation in intracellular calcium both in vivo and in vitro (30). In addition, PTH promoted serine phosphorylation of insulin receptor substrate-1 in 3T3-L1 adipocytes, which resulted in reduced insulin-stimulated glucose uptake (31). As with 25(OH)D concentrations, we did not find PTH concentrations to be related to HOMA-IR. Few clinical studies examined the relation of PTH concentrations with insulin sensitivity and took into account the potential confounding effects of 25(OH)D concentrations and/or adiposity (4, 12, 13, 24) or considered the relations of PTH concentrations with hepatic compared with peripheral insulin sensitivity. Therefore, to our knowledge, we present novel clinical findings that suggested that PTH concentrations are related to whole-body insulin sensitivity, independent of 25(OH)D and adiposity, and may specifically influence insulin-stimulated glucose uptake.
Although vitamin D deficiency is often accompanied by compensatory increases in serum PTH concentrations (32), our data suggest that the individual roles of vitamin D and PTH in insulin sensitivity are independent of each other. Their interaction in the regulation of calcium homeostasis is well characterized, with PTH concentrations promoting the up-regulation of renal 1α-hydroxylase expression and subsequent conversion of 25(OH)D to 1,25(OH)2D (11). However, it is possible that, as calcium concentrations normalize, the interaction of vitamin D and PTH shifts from one of synergism concerning bone metabolism to one of independence regarding effects on extraskeletal metabolism.
Our results suggest that 25(OH)D and PTH may be contributors to the ethnic difference in SI. Lower 25(OH)D and greater PTH concentrations in AAs did not fully explain their lower SI relative to EAs. However, adjustment for 25(OH)D and PTH concentrations reduced the magnitude of the ethnic difference in SI, suggesting that 25(OH)D and PTH concentrations explain a portion of this difference. Lower 25(OH)D and elevated PTH concentrations in AAs are likely a result of decreased cutaneous vitamin D synthesis (33) and low vitamin D and calcium intakes (34–36). Vitamin D supplementation is a feasible, safe, and relatively inexpensive approach to elevate 25(OH)D concentrations and reduce PTH concentrations in AAs (37). We previously showed that dietary vitamin D and calcium were positively associated with insulin sensitivity in AA women (38). Vitamin D and calcium interventions in AAs may thus help to reduce ethnic health disparities in insulin resistance and type 2 diabetes.
Major strengths of this study were the use of sophisticated, robust methodology to measure insulin sensitivity, fat distribution, and body composition. The predictors of SI identified in this study [25(OH)D and PTH concentrations, IAAT, age, and ethnicity] explained 60% of the variance in SI. In addition, measurement of both 25(OH)D and PTH concentrations provided some insight into the independent roles of these hormones on metabolic health. Limitations of the study were that we did not have measures of dietary intake or physical activity in a majority of the women and, thus, could not determine the influence of these factors, and the cross-sectional nature of this study limited our ability to infer cause-and-effect relations. Furthermore, the relatively small sample size in the current study may have limited our power to detect significant ethnic differences in the relations of 25(OH)D and PTH concentrations with metabolic outcomes. Given previous suggestions that relations of dietary vitamin D and 25(OH)D concentrations with metabolic variables may differ in ethnic groups (38, 39), further study by using an appropriate methodology and sufficient sample size is warranted.
In conclusion, circulating 25(OH)D and PTH concentrations were independently associated with whole-body insulin sensitivity and may be more closely related to peripheral rather than hepatic insulin sensitivity. Although vitamin D and PTH are closely connected in the regulation of skeletal health, they likely influence metabolic health through independent mechanisms. Furthermore, lower 25(OH)D and greater PTH concentrations in AAs compared with EAs may contribute to the ethnic disparities in insulin resistance and type 2 diabetes.
Acknowledgments
We thank Maryellen Williams and Cindy Zeng for their continued laboratory support.
The authors’ responsibilities were as follows—JAA and APA: conceived of the project and developed the overall research plan; JAA, APA, and BAG: designed the study and interpreted the analyses; JAA: analyzed the data and was responsible for overall drafting of the manuscript; GRH: provided statistical expertise; APA, GRH, and BAG: provided critical review of the manuscript; and all authors: contributed to data collection and read and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010:351–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu KC, Chu A, Go VLW, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr 2004;79:820–5 [DOI] [PubMed] [Google Scholar]
- 4.Kamycheva E, Jorde R, Figenschau Y, Haug E. Insulin sensitivity in subjects with secondary hyperparathyroidism and the effect of a low serum 25-hydroxyvitamin D level on insulin sensitivity. J Endocrinol Invest 2007;30:126–32 [DOI] [PubMed] [Google Scholar]
- 5.Kayaniyil S, Vieth R, Retnakaran R, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010;33:1379–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lind L, Pollare T, Hvarfner A, Lithell H, Sørensen OH, Ljunghall S. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res 1989;11:141–7 [PubMed] [Google Scholar]
- 7.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3 [DOI] [PubMed] [Google Scholar]
- 8.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007;85:860–8 [DOI] [PubMed] [Google Scholar]
- 9.Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 2002;76:187–92 [DOI] [PubMed] [Google Scholar]
- 10.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 12.Chiu KC, Chuang LM, Lee NP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism 2000;49:1501–5 [DOI] [PubMed] [Google Scholar]
- 13.Richart T, Thijs L, Nawrot T, et al. The metabolic syndrome and carotid intima-media thickness in relation to the parathyroid hormone to 25-OH-D3 ratio in a general population. Am J Hypertens (Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 14.Hoffman RP. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatr Diabetes 2008;9:57–61 [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–77 [DOI] [PubMed] [Google Scholar]
- 16.Alvarez JA, Bush NC, Hunter GR, Brock DW, Gower BA. Ethnicity and weight status affect the accuracy of proxy indices of insulin sensitivity. Obesity (Silver Spring) 2008;16:2739–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffner SM, D'Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 1996;45:742–8 [DOI] [PubMed] [Google Scholar]
- 18.Katsoulis K, Blaudeau TE, Roy JP, Hunter GR. Diet-induced changes in intra-abdominal adipose tissue and CVD risk in American women. Obesity (Silver Spring) 2009;17:2169–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandler-Laney PC, Phadke RP, Granger WM, et al. Adiposity and β-cell function: relationships differ with ethnicity and age. Obesity (Silver Spring) (Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gower BA, Hunter GR, Chandler-Laney PC, Alvarez JA, Bush NC. Glucose metabolism and diet predict changes in adiposity and fat distribution in weight-reduced women. Obesity (Silver Spring) 2010;18:1532–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 1987;79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 23.Kekes-Szabo T, Hunter GR, Nyikos I, Nicholson C, Snyder S, Berland L. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obes Res 1994;2:450–7 [DOI] [PubMed] [Google Scholar]
- 24.Muscogiuri G, Sorice GP, Prioletta A, et al. 25-Hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) (Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 25.Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 2000;47:383–91 [DOI] [PubMed] [Google Scholar]
- 26.Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D3. Cell Biochem Funct 2002;20:227–32 [DOI] [PubMed] [Google Scholar]
- 27.Zhou QG, Hou FF, Guo ZJ, Liang M, Wang GB, Zhang X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab Res Rev 2008;24:459–64 [DOI] [PubMed] [Google Scholar]
- 28.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med 2009;26:19–27 [DOI] [PubMed] [Google Scholar]
- 29.Kautzky-Willer A, Pacini G, Niederle B, Schernthaner G, Prager R. Insulin secretion, insulin sensitivity and hepatic insulin extraction in primary hyperparathyroidism before and after surgery. Clin Endocrinol (Oxf) 1992;37:147–55 [DOI] [PubMed] [Google Scholar]
- 30.Reusch JE, Begum N, Sussman KE, Draznin B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology 1991;129:3269–73 [DOI] [PubMed] [Google Scholar]
- 31.Chang E, Donkin SS, Teegarden D. Parathyroid hormone suppresses insulin signaling in adipocytes. Mol Cell Endocrinol 2009;307:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477–501 [DOI] [PubMed] [Google Scholar]
- 33.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;1:74–6 [DOI] [PubMed] [Google Scholar]
- 34.Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. J Bone Miner Res 2007;22(suppl 2):V34–8 [DOI] [PubMed] [Google Scholar]
- 35.Fulgoni V, III, Nicholls J, Reed A, et al. Dairy consumption and related nutrient intake in African-American adults and children in the United States: continuing survey of food intakes by individuals 1994-1996, 1998, and the National Health And Nutrition Examination Survey 1999-2000. J Am Diet Assoc 2007;107:256–64 [DOI] [PubMed] [Google Scholar]
- 36.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 2005;135:2478–85 [DOI] [PubMed] [Google Scholar]
- 37.Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr 2007;86:1657–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez JA, Bush NC, Choquette SS, et al. Vitamin D intake is associated with insulin sensitivity in African American, but not European American, women. Nutr Metab (Lond) 2010;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–8 [DOI] [PubMed] [Google Scholar]