Abstract
Background: We previously showed that thermal injury depletes plasma vitamin E in pediatric burn patients; however, plasma changes may reflect immediate alterations in vitamin E nutriture. Adipose tissue α-tocopherol concentrations are generally accepted to reflect long-term vitamin E status.
Objective: To test the hypothesis that thermal injury depletes body stores of vitamin E, α-tocopherol concentrations were measured in adipose tissue samples.
Design: Pediatric patients (n = 8) were followed up to 1 y after burn injury. Surgically obtained samples were collected at various intervals and stored at −80°C in a biorepository. α- and γ-Tocopherols, cholesterol, and triglycerides were measured in the same tissue aliquot.
Results: During the first week after injury, adipose tissue α-tocopherol concentrations were within the expected normal range of 199 ± 40 nmol/g adipose tissue but were substantially lower at weeks 2 and 3 (133 ± 13 and 109 ± 8 nmol/g adipose tissue, respectively). Individual rates of decrease, estimated by linear regression, showed that adipose tissue α-tocopherol decreased by an average of 6.1 ± 0.6 nmol/g daily. During the first month after injury, adipose tissue triglyceride concentrations also decreased, whereas no changes in cholesterol concentrations occurred.
Conclusions: These data emphasize that the burn injury experienced by these pediatric patients altered their metabolism such that vitamin E status diminished during the month after injury. Further studies are needed to evaluate the mechanism and consequences of the observed vitamin E depletion. This trial was registered at clinicaltrials.gov as NCT00675714.
INTRODUCTION
In the United States, ≈100,000 people require hospitalization and 5000 deaths occur each year because of burn injury (1), and the likelihood of death from the combination of burn and smoke inhalation injury is increased (2). This combined injury is typically associated with a systemic inflammatory response and increased production of reactive oxygen species (ROS) (3–5). Indeed, we previously reported that plasma antioxidants, especially α-tocopherol, are severely depleted in such patients; moreover, prolonged plasma α-tocopherol depletion is associated with increased mortality (6). The ROS increase created by thermal injury up-regulates inflammatory responses that further prolong radical-mediated damage beyond that caused by the initial injury (7). Prolonged disturbances in both glucose and lipid metabolism have also been reported (8), including a cycle of adipose tissue breakdown with increased free fatty acid turnover (8). Moreover, studies in experimental animals have described adipocyte apoptosis in response to severe burn injury (9).
The assessment of vitamin E status is difficult because vitamin E is fat soluble; therefore, low plasma concentrations could reflect a variety of factors, including low circulating lipid concentrations, low vitamin E intakes, or high oxidative stress levels. Moreover, tissue vitamin E concentrations are dependent on the transport of α-tocopherol in plasma lipoproteins and the mechanisms of delivery of lipoproteins and lipids (10); thus, alterations in lipid metabolism could alter vitamin E status. Furthermore, the hypermetabolic state of burn patients promotes tissue catabolism, which can be alleviated by insulin treatments (11). These factors emphasize the necessity of measuring tissue vitamin E concentrations.
No tissue acts as a storage site that releases α-tocopherol on demand, but rather tissues appear to have α-tocopherol concentrations in relation to their lipid contents (12). Adipose tissue has been used to assess long-term vitamin E status (13), and typical adipose tissue α-tocopherol concentrations in adults are ≈100–300 μg/g (200–700 nmol/g) (14–16). Moreover, adipose tissue α-tocopherol concentrations have been used to demonstrate the adequacy of vitamin E supplementation in previously vitamin E–deficient subjects (17), and adipose tissue α-tocopherol concentrations have been shown to be predictive of peripheral nerve concentrations in vitamin E–deficient humans (18). This finding is import because vitamin E deficiency in humans causes peripheral neuropathy due to a dying back of large caliber fibers in sensory neurons (19, 20). Remarkably, peripheral neuropathy in burn patients is also common (21), but the possible etiologies are many.
The proposed study was carried out to test the hypothesis that severe burn injuries in children are sufficient to alter tissue vitamin E status, potentially severe enough to cause extreme vitamin E depletion. Therefore, vitamin E concentrations were measured in adipose tissue biopsies that were obtained from children with burn injuries at various intervals and were stored in a biorepository.
SUBJECTS AND METHODS
Study design and patient information
The protocol for this study was approved by the Institutional Review Board of The University of Texas Medical Branch; only de-identified samples were sent to Linus Pauling Institute, Oregon State University, for analysis. All subjects, or their legal guardians, signed an informed consent document to allow samples to be used in research studies. The initial recruitment date for the study subjects reported herein was 2003–2004.
Subjects were selected from a biorepository databank of archived, frozen samples given the criterion that they had adipose tissue samples taken within the first week of injury and at several times during the first month of injury. Subject characteristics are shown in Table 1, and the biopsy sites are shown in Table 2. Routinely, within 48 h of admission, patients receive enteral feedings of VIVONEX T.E.N. (Total Enteral Nutrition; Novartis Medical Nutrition, Fremont, MI). The amount of enteral feeding solution given is based on body weight, burn size, and severity. The patient's resting energy expenditure is measured weekly and the feedings are adjusted depending on the results. The vitamin E content of VIVONEX T.E.N. is 15 IU (d-α-tocopheryl acetate) per 1000 kcal.
TABLE 1.
Subject characteristics at admission to the Shriners Hospital for Children, Galveston, TX1
| Subject | Age | Sex | Total body surface area of burn | Third-degree burn | Hematocrit |
| y | % | % | % | ||
| A | 2 | M | 70 | 70 | 45 |
| B | 11 | M | 93 | 93 | 23 |
| C | 5 | M | 56 | 56 | 40 |
| D | 7 | M | 72 | 51 | — |
| E | 3 | F | 71 | 71 | 21 |
| F | 16 | M | 74 | 70 | 49 |
| G | 4 | M | 63 | 29 | 57 |
| H | 9 | M | 45 | 35 | 40 |
Subjects were selected from a biorepository databank of archived, frozen samples with the criterion that they had adipose tissue samples taken within the first week of injury and at several times during the first month of injury.
TABLE 2.
Individual adipose tissue sampling sites and lipid concentrations1
| Subject and specimen location | Time since burn | Cholesterol | Triglyceride | Cholesterol per triglyceride |
| d | mg/g wet wt | mg/g wet wt | μg/mg wet wt | |
| A | ||||
| Arm | 2 | 0.41 | 228 | 1.82 |
| Leg | 9 | 0.37 | 455 | 0.81 |
| Arm | 12 | 0.37 | 318 | 1.15 |
| Arm | 33 | 0.53 | 301 | 1.77 |
| Arm | 103 | 0.66 | 435 | 1.51 |
| Arm | 165 | 0.49 | 296 | 1.66 |
| B | ||||
| Leg | 3 | 0.59 | 312 | 1.88 |
| Leg | 10 | 0.69 | 409 | 1.69 |
| Leg | 24 | 1.08 | 314 | 3.43 |
| Leg | 40 | 1.76 | 252 | 6.99 |
| Leg | 172 | 0.97 | 421 | 2.29 |
| C | ||||
| Chest | 5 | 0.32 | 371 | 0.87 |
| Abdomen | 12 | 0.43 | 254 | 1.68 |
| Arm | 19 | 0.54 | 89 | 6.05 |
| Arm | 89 | 0.37 | 390 | 0.94 |
| Arm | 175 | 0.93 | 465 | 2.00 |
| D | ||||
| Leg | 8 | 0.66 | 453 | 1.45 |
| Abdomen | 15 | 1.17 | 344 | 3.40 |
| Abdomen | 23 | 1.28 | 342 | 3.73 |
| E | ||||
| Right arm | 7 | 0.95 | 398 | 2.40 |
| Left arm | 17 | 1.03 | 388 | 2.64 |
| Left buttock | 31 | 0.64 | 257 | 2.47 |
| F | ||||
| Leg | 6 | 0.78 | 272 | 2.87 |
| Arm | 22 | 0.65 | 338 | 1.93 |
| Arm | 36 | 0.73 | 387 | 1.90 |
| Lower abdomen | 50 | 0.51 | 248 | 2.05 |
| Arm | 56 | 0.79 | 406 | 1.94 |
| G | ||||
| Left buttock | 6 | 1.29 | 478 | 2.70 |
| Left shoulder/arm | 22 | 1.78 | 221 | 8.06 |
| Right arm | 30 | 1.63 | 193 | 8.44 |
| H | ||||
| Leg | 2 | 0.61 | 436 | 1.39 |
| Abdomen | 14 | 0.75 | 422 | 1.77 |
| Abdomen | 21 | 0.90 | 381 | 2.35 |
| Chest | 29 | 0.47 | 343 | 1.38 |
| Arm | 196 | 0.45 | 468 | 0.96 |
Adipose tissue was collected at various intervals (≥3) from 8 pediatric patients. Specimens were collected from live tissue, at the indicated locations, that was exposed during surgery. The tissue selected was neither involved in the wound nor thermally injured. Samples were snap-frozen in liquid nitrogen and stored at −80°C until analyzed.
Adipose tissue was collected at various intervals (≥3) from 8 pediatric patients over the first 30 d after burn injury for all of the patients shown and up to 1 y in some subjects. Specimens were collected from live tissue that was exposed during the surgery that was neither involved in the wound nor thermally injured. Samples were snap-frozen in liquid nitrogen and stored at −80°C until analyzed.
Adipose tissue measurements
Both α- and γ-tocopherols were measured after tissue saponification, extraction, and analysis by HPLC with electrochemical detection, as described previously (22). Cholesterol in the same extract was measured by using an Amplex Red kit (Molecular Probes, Invitrogen, Carlsbad, CA). In the same saponified adipose tissue sample, triglycerides were estimated from the fatty acids extracted from the acidified aqueous phase of the samples by using a spectrophotometric method, as described previously (13).
Statistics
Data are expressed as means ± SEs. Linear rates of disappearance of tocopherol concentrations were calculated by using individual concentrations versus days after injury by using Excel (Excel 2008 for Mac version 12.2.5; Microsoft, Redmond, WA). Nonlinear fitting was carried out by using Prism 4 for Macintosh (version 4.0b; GraphPad, La Jolla, CA).
RESULTS
Adipose tissue alpha- and gamma-tocopherol, triglyceride, and cholesterol concentrations
The adipose tissue concentrations of tocopherols, cholesterol, and triglycerides were estimated from the same aliquot of the biopsy sample. This procedure has the advantage that cholesterol is in the same extract as the tocopherol; therefore, cholesterol can be used as an alternative to wet weight as a correction factor. However, the term “triglycerides” is a misnomer. The fatty acids were extracted from the saponified tissue; thus, extracts include fatty acids released from triglycerides, cholesterol, and phospholipids, and a calculation was made assuming that all fatty acids are from triglyceride and that there are 3 fatty acids per triglyceride. Given that the adipose tissue is largely triglyceride, this measure has been used as an acceptable surrogate (13, 18). It was anticipated that the adipose tissue cholesterol and triglycerides per wet weight would remain relatively constant per subject.
The adipose tissue α-tocopherol concentrations (Table 3) in the initial biopsy taken from the pediatric patients within the first week after injury were on average within the normal range observed in healthy adults (16). However, by the third week after insult, the average α-tocopherol concentration had decreased by ≈55%, from 199 ± 43 to 109 ± 9 nmol/g. A similar pattern of decrease was observed for γ-tocopherol concentrations. Not all subjects had samples taken for every interval shown in Table 3, and some subjects were noted to have a more rapid depletion of their adipose tissue α-tocopherol concentrations than did others. Therefore, the individual data were examined, and nonlinear fitting of data were performed (Figure 1). Only those subjects with initial (on admittance to the Shriners Hospital for Children) α-tocopherol concentrations >150 nmol/g adipose tissue were observed to have decreasing tocopherol concentrations. Interestingly, the 2 subjects (F and G) with the highest adipose tissue α-tocopherol concentrations also had the highest γ-tocopherol concentrations.
TABLE 3.
Adipose tissue α- and γ-tocopherol concentrations1
| Time after injury | Time after injury | Subject | No. of subjects | α-Tocopherol | γ-Tocopherol |
| d | nmol/g | nmol/g | |||
| 1 wk | 0–7 | A, B, C, E, F, G, H | 7 | 199 ± 432 | 49 ± 14 |
| 2 wk | 8–14 | A, B, C, D, H | 5 | 126 ± 14 | 24 ± 3 |
| 3 wk | 15–21 | C, D, E, H | 4 | 109 ± 9 | 23 ± 4 |
| 4 wk | 22–28 | B, D, F, G | 4 | 167 ± 40 | 49 ± 18 |
| 5 wk | 29–36 | A, E, F, G, H | 5 | 142 ± 20 | 35 ± 12 |
| 6–12 wk | 37–89 | B, C, F | 3 | 131 ± 18 | 28 ± 10 |
| 15–28 wk | 103–196 | A, B, C, H | 4 | 167 ± 27 | 24 ± 5 |
Because not every subject had a sample taken at every time point, no statistical analyses were performed. Cholesterol, α- and γ-tocopherols, and triglycerides were measured in the same biopsy sample shown in Table 2.
Mean ± SE (all such values).
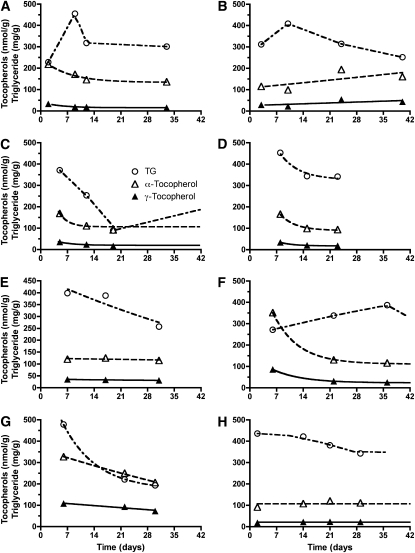
FIGURE 1.
A–H: Adipose tissue concentrations of α-tocopherol (△), γ-tocopherol (▴), and triglycerides (TG; ○) were estimated from the saponified biopsy sample at each time point for each individual (n = 8 subjects, described in Tables 1 and 2). The tocopherol and triglyceride concentrations were fit by using nonlinear techniques; lines are shown starting with the first actual data point and ending with the last data point for each individual, except where the last point was beyond 42 d.
Depletion of adipose tissue α- and γ-tocopherol concentrations was observed in 5 subjects (A, C, D, F, and G), with initial concentrations >150 nmol α-tocopherol/g adipose tissue. Given that various subjects were represented at the time points examined, the behavior of each person's tocopherol and triglyceride concentrations were examined individually and are shown for each of the subjects (Figure 1). In the first biopsy sample, adipose tissue α-tocopherol concentrations in subjects B (115 nmol/g), E (120 nmol/g), and H (92 nmol/g) did not decrease and were below the lowest values reached by the remaining subjects. Importantly, the disappearance rates of tocopherol were similar for α- and γ-tocopherol in each subject (exponential for initial α-tocopherol concentrations of >150 nmol/g adipose tissue and constant for concentrations <150 nmol/g adipose tissue), which suggested a common rapid depletion mechanism for both tocopherols until minimum tissue concentrations are achieved (Figure 1). In contrast, the triglyceride disappearance rate differed from that of the tocopherols (Figure 1), which suggested a tocopherol-depletion mechanism independent of that of triglyceride. Moreover, the cholesterol content of the adipose tissue did not appear to change and was 0.8 ± 0.1 mg/g wet weight (mean ± SE; n = 8 subjects). This lack of change in adipose tissue cholesterol emphasizes the importance of the alterations in the tocopherol and triglyceride concentrations.
Estimates of the linear rates of decrease in α-tocopherol/g adipose tissue are shown for each of the subjects (A, C, D, F, and G). On average, adipose tissue α-tocopherol decreased at a rate of 6.1 ± 0.6 nmol/g per day (Table 4). α-and γ-Tocopherol concentrations are often presented as both nmol/g tissue and per cholesterol or per triglyceride concentrations. However, because triglyceride concentrations decreased, but cholesterol did not, only the ratios per cholesterol are reported (Table 4). These data also confirm the depletion of both α- and γ-tocopherols from adipose tissue. Over the first 4 wk of the study, α-tocopherol decreased by 12.3 ± 3.4 μmol/g cholesterol per day, and γ-tocopherol decreased by 2.7 ± 0.7 μmol/g cholesterol per day.
TABLE 4.
Rates of tocopherol disappearance1
| Measured concentration during week 1 | Daily rate | Estimated concentration on day 0 | R2 | |
| α-Tocopherol (nmol/g wet wt) | ||||
| Subject | ||||
| A | 218 | −7.2 | 233 | 0.997 |
| C | 169 | −5.5 | 190 | 0.917 |
| D | 166 | −4.7 | 192 | 0.771 |
| F | 352 | −8.0 | 370 | 0.827 |
| G | 327 | −5.0 | 358 | 0.999 |
| Mean ± SE | 247 ± 39 | −6.1 ± 0.6 | 269 ± 40 | — |
| α-Tocopherol (μmol/g cholesterol) | ||||
| Subject | ||||
| A | 527 | −8.6 | 533 | 0.972 |
| C | 528 | −25.5 | 625 | 0.921 |
| D | 254 | −11.7 | 317 | 0.764 |
| F | 453 | −9.9 | 482 | 0.882 |
| G | 254 | −5.6 | 281 | 0.946 |
| Mean ± SE | 403 ± 62 | −12.3 ± 3.4 | 448 ± 65 | — |
| γ-Tocopherol (nmol/g wet wt) | ||||
| Subject | ||||
| A | 33 | −1.6 | 36 | 0.959 |
| C | 35 | −1.3 | 41 | 0.982 |
| D | 34 | −1.0 | 40 | 0.806 |
| F | 86 | −2.0 | 92 | 0.855 |
| G | 108 | −1.4 | 118 | 0.935 |
| Mean ± SE | 59 ± 16 | −1.5 ± 0.2 | 65 ± 17 | — |
| γ-Tocopherol (μmol/g cholesterol) | ||||
| Subject | ||||
| A | 79 | −1.4 | 72 | 0.841 |
| C | 109 | −5.5 | 132 | 0.956 |
| D | 52 | −2.5 | 65 | 0.776 |
| F | 111 | −2.6 | 120 | 0.906 |
| G | 84 | −1.7 | 92 | 0.974 |
| Mean ± SE | 87 ± 11 | −2.7 ± 0.7 | 96 ± 13 | — |
Only subjects with an initial α-tocopherol concentration >150 nmol/g (n = 5) are shown. Rates of disappearance of α-tocopherol and γ-tocopherol concentrations were calculated separately by using individual concentrations and at the days after injury shown in Figure 1.
DISCUSSION
The most dramatic finding in this study was the severity of the α-tocopherol depletion from adipose tissue that occurred within a few weeks of burn injury in pediatric patients. Importantly, adipose tissue reflects long-term vitamin E status, and changes in adipose tissue α-tocopherol generally take years to occur (23). The data reported herein emphasize that severe burn injury induces a hypercatabolic state (11) that causes a redistribution, if not an outright depletion, of α-tocopherol and further suggests that inadequate vitamin E is being supplied to these children despite the enteral feedings provided to the subjects. The Recommended Dietary Allowance (RDA) (24) for vitamin E is 7 mg α-tocopherol/d for children with a reference weight of 20 kg and a body mass index (in kg/m2) of 15, who are aged 4–8 y (the mean age of the children in this study was 7 y). One suggested energy allowance for children with burns is 70–90 kcal/kg (25), which equals 1400–1800 kcal/d plus extra caloric input depending on the severity of the burn. The current practice of the Shriner's Hospital for Children is to determine the exact caloric need by determining the resting energy expenditure and multiplying it by 1.4. This is being done to equate for the basal metabolic needs plus a 1.4 times stress factor (eg, for dressing changes or tub room visits), which will cover the entire metabolic needs of these severely burned patients. The enteral feed used provides 15 IU α-tocopherol (10 mg) per 1000 kcal. It is expected that, based on the calculation of caloric requirements, the vitamin E intakes during the first weeks of hospitalization of all the children in this study met or exceeded the RDA for healthy children. In contrast, given the estimated daily rate of loss of 6 nmol/g (2.6 mg/kg) adipose tissue, assuming that adipose tissue is 50% fat, the estimated body composition of 17% fat and 24 kg body weight of the children [on the basis of Rennie et al (26)] equates to a loss of ≈5 mg α-tocopherol/d! Given that the absorption of vitamin E is <50% (27), it is clear that the vitamin E intakes of these pediatric burn patients are less than their requirements.
Most surveys of healthy children report that vitamin E intakes are well below the RDA (28–30), some of which estimate that 80–90% of children have vitamin E intakes below the estimated average requirement (30). Thus, it is likely that the children we studied had low dietary vitamin E intakes before injury, and that the intakes in hospital were at least at the RDA range. Thus, the increase in adipose tissue α-tocopherol observed after 3 wk was likely due to the improved health and metabolic status and to continued intakes of α-tocopherol at RDA levels.
The rapidity of the adipose tissue α-tocopherol depletion was also remarkable. Although lower adipose tissue α-tocopherol concentrations have been reported in vitamin E–deficient children, these are usually observed after years of inadequate vitamin E absorption, such as in children with cholestatic liver disease (19) or in those with a genetic defect in lipoprotein synthesis, eg, abetalipoproteinemia (17). Moreover, it is unlikely that the depletion in adipose tissue vitamin E is due to dietary changes because Handelman et al (23) studied adipose tissue tocopherol concentrations in adults before, during supplementation with 800 IU/d for 1 y, and after discontinuation of supplementation for 1 y. They found some increase in adipose tissue α-tocopherol with supplementation; however, on cessation of the supplement, no decrease in adipose tissue concentrations was detected, which led the authors to conclude that adipose tissue α-tocopherol concentrations change very slowly. It seems likely that the children who came to the hospital with low adipose tissue α-tocopherol (<150 nmol/g) were at the low end of normal because of prolonged low dietary vitamin E intakes. In support of this idea are the findings by Kim et al (31), who reported that US children who consume inadequate vitamin E–containing diets have plasma concentrations near deficient concentrations (<11 μmol/L). Unfortunately, plasma samples were not available from the thermally injured children described herein.
The adipose tissue tocopherol concentrations are also reported per cholesterol concentrations, because frequently this lipid is used as a denominator to express vitamin E concentrations in plasma or tissues. Vitamin E is a fat-soluble vitamin, so lipid-corrected values obviate the difficulty of using tissue weight, especially if the tissue is edematous. Despite this correction, relative to cholesterol, α-tocopherol decreased to nearly half the initial concentration within the first 3 wk after injury. The greater utility of the ratio of α-tocopherol per cholesterol was observed previously in patients receiving parenteral nutrition, in whom undernutrition caused a depletion of adipose tissue triglyceride concentrations (32). Underfeeding is not likely the cause of the depletion of adipose tissue tocopherol in the patients reported herein, because these patients were consistently provided enteral feedings containing recommended dietary amounts of vitamin E (33).
It should be noted that the pattern of alterations in adipose tissue triglyceride was not similar to that of the tocopherols (Figure 1). We emphasize that the disappearance rates of tocopherol were similar for α- and γ-tocopherol in each subject (exponential for initial α-tocopherol concentrations >150 nmol/g adipose tissue and constant for those <150 nmol/g adipose tissue), which suggests a common, rapid depletion mechanism for both tocopherols until minimum tissue concentrations are achieved (Figure 1), whereas the disappearance of the triglyceride clearly differed from that of the tocopherols, which suggests a tocopherol-depletion mechanism independent of that of triglyceride. It is well known that burn patients are in a hypercatabolic state and have an elevated metabolic rate (34) and that depletion of fat stores, massive lipolysis, and the development of fatty liver are all components of the disorder (34). The redistribution of tocopherol as described herein, however, was not previously observed in obese subjects consuming a weight-reduction diet that depleted triglycerides from the adipose tissue (35). Thus, the trauma of burn patients and its sequelae are different from that previously described in other patients. The findings in burn patients suggest that there is a labile pool of tocopherols that can be drawn on during stress. However, this suggestion is speculative and will need to be confirmed in future studies.
The depletion of α-tocopherol observed in the pediatric patients with burn injury described herein perhaps resulted not only from the mobilization of adipose tissue triglyceride and tocopherols but also from the oxidative stress the patients experienced. It is not clear from the available data whether the α-tocopherol is being redistributed to other sites or whether it is being depleted (oxidized) as a result of lipid peroxidation. A study in pediatric burn patients supplemented with a combination of antioxidants (average intakes of 900 mg vitamin C/d, 400 mg vitamin E/d, and 7 mg Zn/d) resulted in a significant increase in circulating vitamin E, a decrease in malondialdehyde, and a decrease in wound healing time (36). Our previous mechanistic studies in an ovine model of burn and smoke injury showed that the injury to the lung and skin is reflected in a higher depletion of liver α-tocopherol, which suggests mobilization of vitamin E from the liver (37). However, the kinetic studies that used deuterium-labeled vitamin E also suggested that, in addition to depletion of α-tocopherol from the lung and liver, other tissues were also depleted (37). The present investigation suggests that, during severe thermal injury in humans, adipose tissue can release α-tocopherol to protect other tissues. Given the observation that the patterns of tocopherol and triglyceride concentrations are not parallel, it appears that tocopherol depletion cannot be explained solely by tissue catabolism. The limited number of patients investigated limits the generalizability of the data; however, given the well-appreciated hypermetabolic and profoundly catabolic state and disordered lipid metabolism, the depletion of vitamin E may be a very significant problem in patients with burn injury.
The next step is to consider what effect additional vitamin E supplementation may have in ameliorating the oxidative stress of burn injury in patients. Vitamin E deficiency is manifested by peripheral neuropathy in noninjured patients (19, 20). In burn patients, peripheral neuropathy is common (21) and has many possible etiologies. We theorize that increased vitamin E supplementation may decrease neuropathy and improve this clinical problem. It should also be noted that studies of vitamin E supplementation in patients with a genetic defect in the α-tocopherol transfer protein have shown that vitamin E can slow or reverse neurologic symptoms, but only in the early stages of the disorder (38). Another central issue to be addressed is whether attenuation of vitamin E depletion is beneficial in terms of postburn outcomes. Future studies are planned to address these questions.
Acknowledgments
Mary Kelly provided excellent and expert assistance.
The authors’ responsibilities were as follows—MGT, JG, and DLT: formulated the specific hypothesis of the study, which was done within an ongoing larger randomized trial; JG, CCF, MGJ, and DH: participated in patient consent, sample collection, and follow-up; SWL, MGT, DLT, LDT, and GB: analyzed the data and drafted the manuscript; and SWL and MGT: performed measurements of vitamin E and its metabolites. All authors participated in preparing the final draft of the manuscript. No author had a financial or personal conflict of interest related to this research or its source of funding.
REFERENCES
- 1.Herndon DN, Spies M. Modern burn care. Semin Pediatr Surg 2001;10:28–31 [DOI] [PubMed] [Google Scholar]
- 2.Barrow RE, Spies M, Barrow L, Herndon D. Influence of demographics and inhalation injury on burn mortality in children. Burns 2004;30:72–7 [DOI] [PubMed] [Google Scholar]
- 3.Nuytinck HK, Offermans XJ, Kubat K, Goris JA. Whole-body inflammation in trauma patients. An autopsy study. Arch Surg 1988;123:1519–24 [DOI] [PubMed] [Google Scholar]
- 4.Traber DL, Herndon DN, Soejima K. The pathophysiology of inhalation injury Herndon DN.ed. Total burn care 2nd ed.New York, NY: WB Saunders, 2002:221–32 [Google Scholar]
- 5.Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008;34:6–17 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TT, Cox CS, Traber DL, et al. Free radical activity and loss of plasma antioxidants, vitamin E, and sulfhydryl groups in patients with burns: the 1993 Moyer Award. J Burn Care Rehabil 1993;14:602–9 [DOI] [PubMed] [Google Scholar]
- 7.Greenhalgh DG, Saffle JR, Holmes JH, IV, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007;28:776–90 [DOI] [PubMed] [Google Scholar]
- 8.Cree MG, Wolfe RR. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab 2008;294:E1–9 [DOI] [PubMed] [Google Scholar]
- 9.Yasuhara S, Kaneki M, Sugita H, et al. Adipocyte apoptosis after burn injury is associated with altered fat metabolism. J Burn Care Res 2006;27:367–76 [DOI] [PubMed] [Google Scholar]
- 10.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr 2007;27:347–62 [DOI] [PubMed] [Google Scholar]
- 11.Jeschke MG, Kulp GA, Kraft R, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med 2010;182:351–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton GW, Traber MG, Acuff RV, et al. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr 1998;67:669–84 [DOI] [PubMed] [Google Scholar]
- 13.Kayden HJ, Hatam LJ, Traber MG. The measurement of nanograms of tocopherol from needle aspiration biopsies of adipose tissue: normal and abetalipoproteinemic subjects. J Lipid Res 1983;24:652–6 [PubMed] [Google Scholar]
- 14.Kardinaal AF, van 't Veer P, Brants HA, van den Berg H, van Schoonhoven J, Hermus RJ. Relations between antioxidant vitamins in adipose tissue, plasma, and diet. Am J Epidemiol 1995;141:440–50 [DOI] [PubMed] [Google Scholar]
- 15.Su LC, Bui M, Kardinaal A, et al. Differences between plasma and adipose tissue biomarkers of carotenoids and tocopherols. Cancer Epidemiol Biomarkers Prev 1998;7:1043–8 [PubMed] [Google Scholar]
- 16.El-Sohemy A, Baylin A, Ascherio A, Kabagambe E, Spiegelman D, Campos H. Population-based study of alpha- and gamma-tocopherol in plasma and adipose tissue as biomarkers of intake in Costa Rican adults. Am J Clin Nutr 2001;74:356–63 [DOI] [PubMed] [Google Scholar]
- 17.Traber MG, Rader D, Acuff R, Brewer HB, Kayden HJ. Discrimination between RRR- and all rac-a-tocopherols labeled with deuterium by patients with abetalipoproteinemia. Atherosclerosis 1994;108:27–37 [DOI] [PubMed] [Google Scholar]
- 18.Traber MG, Sokol RJ, Ringel SP, Neville HE, Thellman CA, Kayden HJ. Lack of tocopherol in peripheral nerves of vitamin E-deficient patients with peripheral neuropathy. N Engl J Med 1987;317:262–5 [DOI] [PubMed] [Google Scholar]
- 19.Sokol RJ, Guggenheim MA, Heubi JE, et al. Frequency and clinical progression of the vitamin E deficiency neurologic disorder in children with prolonged neonatal cholestasis. Am J Dis Child 1985;139:1211–5 [DOI] [PubMed] [Google Scholar]
- 20.Sokol RJ, Bove KE, Heubi JE, Iannaccone ST. Vitamin E deficiency during chronic childhood cholestasis: presence of sural nerve lesion prior to 2 1/2 years of age. J Pediatr 1983;103:197–204 [DOI] [PubMed] [Google Scholar]
- 21.Kowalske K, Holavanahalli R, Helm P. Neuropathy after burn injury. J Burn Care Rehabil 2001;22:353–7 [DOI] [PubMed] [Google Scholar]
- 22.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols and ubiquinones. J Lipid Res 1996;37:893–901 [PubMed] [Google Scholar]
- 23.Handelman GJ, Epstein WL, Peerson J, Spiegelman D, Machlin LJ, Dratz EA. Human adipose alpha-tocopherol and gamma-tocopherol kinetics during and after 1 y of alpha-tocopherol supplementation. Am J Clin Nutr 1994;59:1025–32 [DOI] [PubMed] [Google Scholar]
- 24.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press, 2000 [PubMed] [Google Scholar]
- 25.Chan MM, Chan GM. Nutritional therapy for burns in children and adults. Nutrition 2009;25:261–9 [DOI] [PubMed] [Google Scholar]
- 26.Rennie KL, Livingstone MB, Wells JC, et al. Association of physical activity with body-composition indexes in children aged 6-8 y at varied risk of obesity. Am J Clin Nutr 2005;82:13–20 [DOI] [PubMed] [Google Scholar]
- 27.Bruno RS, Leonard SW, Park S-I, Zhao Y, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled α-tocopheryl acetate. Am J Clin Nutr 2006;83:299–304 [DOI] [PubMed] [Google Scholar]
- 28.Lockner DW, Crowe TK, Skipper BJ. Dietary intake and parents’ perception of mealtime behaviors in preschool-age children with autism spectrum disorder and in typically developing children. J Am Diet Assoc 2008;108:1360–3 [DOI] [PubMed] [Google Scholar]
- 29.Drewel BT, Giraud DW, Davy SR, Driskell JA. Less than adequate vitamin E status observed in a group of preschool boys and girls living in the United States. J Nutr Biochem 2006;17:132–8 [DOI] [PubMed] [Google Scholar]
- 30.Affenito SG, Thompson DR, Franko DL, et al. Longitudinal assessment of micronutrient intake among African-American and white girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Am Diet Assoc 2007;107:1113–23 [DOI] [PubMed] [Google Scholar]
- 31.Kim YN, Lora KR, Giraud DW, Driskell JA. Nonsupplemented children of Latino immigrants have low vitamin E intakes and plasma concentrations and normal vitamin C, selenium, and carotenoid intakes and plasma concentrations. J Am Diet Assoc 2006;106:385–91 [DOI] [PubMed] [Google Scholar]
- 32.Steephen AC, Traber MG, Ito Y, Lewis LH, Kayden HJ, Shike M. Vitamin E status of patients receiving long-term parenteral nutrition: is vitamin E supplementation adequate? JPEN J Parenter Enteral Nutr 1991;15:647–52 [DOI] [PubMed] [Google Scholar]
- 33.Products PM. Drugs for human use; drug efficacy study implementation; amendment. Fed Regist 2000;21200 [Google Scholar]
- 34.Williams FN, Jeschke MG, Chinkes DL, Suman OE, Branski LK, Herndon DN. Modulation of the hypermetabolic response to trauma: temperature, nutrition, and drugs. J Am Coll Surg 2009;208:489–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaefer EJ, Woo R, Kibata M, Bjornsen L, Schreibman PH. Mobilization of triglyceride but not cholesterol or tocopherol from human adipocytes during weight reduction. Am J Clin Nutr 1983;37:749–54 [DOI] [PubMed] [Google Scholar]
- 36.Barbosa E, Faintuch J, Machado Moreira EA, et al. Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: a randomized, double-blind, placebo-controlled pilot study. J Burn Care Res 2009;30:859–66 [DOI] [PubMed] [Google Scholar]
- 37.Traber MG, Shimoda K, Murakami K, et al. Burn and smoke inhalation injury in sheep depletes vitamin E: kinetic studies using deuterated tocopherols. Free Radic Biol Med 2007;42:1421–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mariotti C, Gellera C, Rimoldi M, et al. Ataxia with isolated vitamin E deficiency: neurological phenotype, clinical follow-up and novel mutations in TTPA gene in Italian families. Neurol Sci 2004;25:130–7 [DOI] [PubMed] [Google Scholar]