Abstract
Background: Iron deficiency anemia (IDA) affects many young women in sub-Saharan Africa. Its etiology is multifactorial, but the major cause is low dietary iron bioavailability exacerbated by parasitic infections such as malaria.
Objective: We investigated whether asymptomatic Plasmodium falciparum parasitemia in Beninese women would impair absorption of dietary iron or utilization of circulating iron.
Design: Iron absorption and utilization from an iron-fortified sorghum-based meal were estimated by using oral and intravenous isotope labels in 23 afebrile women with a positive malaria smear (asexual P. falciparum parasitemia; >500 parasites/μL blood). The women were studied while infected, treated, and then restudied 10 d after treatment. Iron status, hepcidin, and inflammation indexes were measured before and after treatment.
Results: Treatment reduced low-grade inflammation, as reflected by decreases in serum ferritin, C-reactive protein, interleukin-6, interleukin-8, and interleukin-10 (P < 0.05); this was accompanied by a reduction in median serum hepcidin of ≈50%, from 2.7 to 1.4 nmol/L (P < 0.005). Treatment decreased serum erythropoietin and growth differentiation factor 15 (P < 0.05). Clearance of parasitemia increased geometric mean dietary iron absorption (from 10.2% to 17.6%; P = 0.008) but did not affect systemic iron utilization (85.0% compared with 83.1%; NS).
Conclusions: Dietary iron absorption is reduced by ≈40% in asymptomatic P. falciparum parasitemia, likely because of low-grade inflammation and its modulation of circulating hepcidin. Because asymptomatic parasitemia has a protracted course and is very common in malarial areas, this effect may contribute to IDA and blunt the efficacy of iron supplementation and fortification programs. This trial was registered at clinicaltrials.gov as NCT01108939.
INTRODUCTION
See corresponding editorials on pages 1283 and 1285.
Iron deficiency anemia (IDA) affects many young women in sub-Saharan Africa and increases maternal and perinatal mortality and reduces work capacity (1). The etiology of IDA in Africa is multifactorial, but the major cause is low dietary iron bioavailability from monotonous cereal-based diets (2), which is exacerbated by chronic parasitic infections such as malaria (3). High-dose iron supplements can improve iron status in areas of endemic malaria (4). However, iron fortification of foods may be a more cost-effective, sustainable, and potentially safer strategy to improve iron intakes and reduce anemia (1, 5). Disappointingly, most trials of iron fortification in malarial-endemic areas of Africa have been ineffective (6) or have had only limited effect (7, 8). One reason for the blunted effect of iron fortification could be poor absorption due to chronic inflammation from parasite diseases, such as malaria.
In sub-Saharan Africa, 74% of the population lives in highly malarial endemic areas (9). In acute febrile malaria, the main mechanisms contributing to anemia are hemolysis, increased splenic clearance of erythrocytes, and, possibly, reduced erythropoiesis (10, 11). The intense inflammation of acute malaria increases cytokine concentrations, such as interleukin (IL)-6 (12, 13), which can stimulate hepatic hepcidin production. High circulating hepcidin can reduce iron absorption from the gut and increase iron sequestration in the reticuloendothelial system (RES) (14) by blocking the iron-transporter ferroportin; the resulting hypoferremia limits the iron available for erythropoiesis and contributes to anemia (15, 16). The use of stable isotopes showed that iron absorption was shown to be decreased in young children recovering from acute malaria (17). Growth differentiation factor 15 (GDF-15) produced by the marrow erythroid compartment inhibits hepcidin expression and can increase iron absorption (18); however, the interaction between GDF-15 and hepcidin in malaria is unclear (19).
However, acute febrile episodes are much less frequent than asymptomatic Plasmodium falciparum parasitemia, which, in areas of perennial transmission, can affect much of the population for most of the year (20). Cross-sectional studies have reported high prevalence rates of 74–95% for asymptomatic P. falciparum parasitemia among young women in West Africa (21, 22). It is unknown whether iron bioavailability is reduced by low-level, afebrile parasitemia, which has a protracted course and only a limited inflammatory response (19, 23). If afebrile parasitemia impairs iron absorption, its contribution to iron deficiency and anemia in populations may be more important than acute febrile malaria, which usually affects individuals for only a few days each year (24).
Therefore, measuring the effect of asymptomatic parasitemia on host iron absorption and utilization may provide insights into the etiology of anemia in the tropics. Our hypotheses were as follows: 1) asymptomatic P. falciparum parasitemia in young African women would impair both the absorption of dietary iron and utilization of circulating iron and 2) the mechanism of this effect would be the presence of low-grade inflammation affecting the balance of circulating hepcidin and GDF-15 concentrations.
SUBJECTS AND METHODS
Subjects
The study was carried out in young Beninese women recruited in Natitingou and Toucountouna in the Atacora department in Northern Benin. This is an area of endemic seasonal or perennial malaria transmission. The main transmission season is from May to November (25), during which time the percentage of subjects with a positive blood test result for malaria is from ≈60% to 80% (26). The inclusion criteria were as follows: 1) female aged 16–40 y, 2) body weight <65 kg, 3) not pregnant (confirmed by pregnancy testing) and not breastfeeding, 4) no chronic medical illnesses, 5) no medicinal iron at the time of entry into the study, 6) a positive malaria smear (asexual P. falciparum parasitemia; >500 parasites/μL blood), 7) no clinical symptoms (eg, headache, malaise) and lack of fever (<37.5°C) or self-reported fever in past 7 d, 8) no infection with soil-transmitted helminths (hookworms, bilharziosis, Ascaris lumbricoides, Trichuris trichiura), and 9) no severe anemia (hemoglobin <8.0 g/dL).
Sample size calculations indicated that ≥16 women should be included for paired comparisons based on 80% power to detect a 40% significant difference in iron absorption, an SD of 8.2% for log-transformed absorption data (based on previous Swiss Federal Institute of Technology Zurich studies), and a type I error rate of 5%. We anticipated that there could be a substantial dropout rate during the intensive 6-wk study; therefore, we enrolled 23 subjects. The subjects provided informed written consent. Ethical approval for the study was given by the ethical review committee at the Ministry of Health in Benin and the Swiss Federal Institute of Technology Zürich (Zurich, Switzerland).
Iron-absorption and utilization study
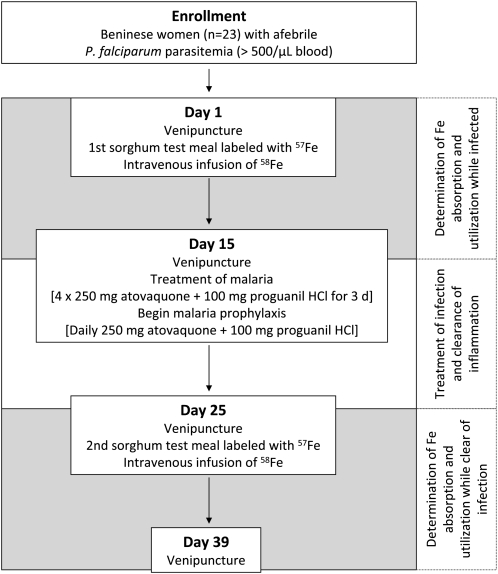
The women were studied while infected, then treated, and then restudied (Figure 1). Iron absorption and utilization were estimated by using stable-isotope techniques in which the incorporation into erythrocytes of an oral 57Fe dose and an intravenous 58Fe dose was measured 14 d after administration (27, 28). On day 1, a baseline venous blood sample was drawn after an overnight fast for determination of isotopic composition and iron status. The subjects then received a test meal (iron-fortified sorghum porridge; see below) labeled with 57Fe as NaFeEDTA, which was fed under standardized conditions and close supervision. Each test meal contained 3 mg labeled 57Fe. One hour later, 2 mL of an aqueous solution containing 100 μg 58Fe as iron citrate was taken into a syringe and, via a 250-mL infusion bag leading into a 0.9% saline drip, slowly infused over 50 min (29). At completion of the infusion, 10 mL normal saline was injected into the 250-mL bag, the bag was rinsed by rotation and inversion, and the saline was infused into the subject. This was done twice; we assumed there was no residual isotope remaining in the bags after this rinsing procedure. The rate of intravenous infusion of iron was based on the estimated 2 μg/min plasma appearance of iron normally absorbed from the gastrointestinal tract (30). No intake of food and fluids was allowed for 4 h after the test meal intake. Fourteen days later (day 15), a second venous blood sample was drawn after an overnight fast, and treatment and prophylaxis were started. To treat malaria, 4 tablets Malarone (250 mg atovaquone + 100 mg proguanil hydrochloride; GlaxoSmithKline, Middlesex, United Kingdom) were administered on 3 consecutive days. After treatment, one tablet Malarone was administered daily as prophylaxis until the study was completed. Before the women were restudied, there was a 10-d waiting period to allow infection-related inflammation to subside (Figure 1). During the 10 d, blood smears were repeated to check whether the women were free of malaria parasites. In addition, a stool smear was done to confirm the absence of soil-transmitted helminths. On day 25, a third venous blood sample was drawn after an overnight fast for determination of isotopic composition and confirmation of iron status. The subjects then received the second test meal and the second intravenous dose. Fourteen days later (day 39), a venous blood sample was drawn after an overnight fast. Because prophylaxis against bacterial and intestinal parasite infections is common in this area, the subjects received 500 mg ciprofloxacillin (Bayer, Leverkusen, Germany) every 2 d from day 1 (evening) through day 39 and 400 mg albendazole (GlaxoSmithKline) on days 0 and 21.
FIGURE 1.
Overview of the study design.
Test meal preparation
A reddish sorghum variety typically found in northern Benin was soaked for 12 h and then milled. The resulting sorghum flour was fermented in excess water for 24 h, dried at 37°C in an oven, and then milled again. To prepare test meal servings, 60 g of the fermented sorghum flour was mixed with 200 g mineral water, and the resulting slurry was added to 300 g previously boiled mineral water and then boiled for 6 min. During boiling, 18 g sugar was added with continual stirring. After cooling, portions based on 50 g dry weight of sorghum flour were weighed as test meal servings. All ingredients were purchased in bulk and used for the entire study. The food portions were prepared freshly on each study day, and the 3 mg fortification iron (as Na57FeEDTA) was added at the time of feeding after the porridge cooled.
Test meal analysis
Iron was analyzed by graphite-furnace atomic absorption spectrophotometry (GF-AAS, AA240Z; Varian, Palo Alto, CA) after mineralization by microwave digestion (MLS ETHOSplus, MLS GmbH; Leutkirch, Germany) using a mixture of HNO3 and H2O2. The phytic acid (PA) concentration was measured by using a modification of the Makower method (31), in which iron was replaced by cerium in the precipitation step. After the mineralization of food samples, inorganic phosphate was determined according to Van Veldhoven and Mannaerts (32) and converted into PA concentrations. The total polyphenol content was measured by using a modification of the Folin-Ciocalteau method, as suggested by Singleton (33) and was expressed as gallic acid equivalents.
Preparation of isotopically labeled iron
Na57FeEDTA was prepared in solution from 57Fe-enriched elemental iron. The metal was dissolved in 2 mL HCl, diluted with water, and stored in polytetrafluoroethylene containers. The resulting FeCl3 solution was mixed with a freshly prepared aqueous Na2EDTA solution (Na2EDTA · H2O2; Sigma Chemical Co, St Louis, MO) at a molar ratio of 1:1 (Fe:EDTA) 20 min before test meal administration. The resulting NaFeEDTA solution was added to the test meal (34). Iron citrate, enriched with 58Fe, was prepared for intravenous infusion from elemental 58Fe according to the method described previously (35). The solution was divided into ampules containing 100 μg Fe, sterilized, and checked for pyrogens. Enrichment of isotopic labels was 95.5% for 57Fe and 93.1% for 58Fe. The isotopic composition of the stable-isotope labels was measured by using negative thermal ionization–mass spectrometry (28).
Parasite diagnosis
Thick and thin blood smears were stained by using the Giemsa coloration technique and were examined independently by 2 experienced microscopists. During screening, one blood smear from the subjects was examined. After inclusion in the study, the subjects’ blood smears were examined in duplicate. Parasite density was quantified against leukocytes; fields containing ≥200 leukocytes were counted; if <10 parasites were identified, the counting continued to 500 leukocytes. These counts were converted to the number of parasites per μL blood, assuming 8000 leukocytes per 1-μL blood. One hundred high-power fields were examined before a slide was declared negative. Rapid malaria diagnostic tests (Paramax-3; MD Doctors Direct GmbH, Egg b, Zurich, Switzerland) were used to support the results of the blood smears. Two KatoKatz thick smears were prepared from each stool sample. The smears were examined immediately after preparation under a light microscope, and all hookworm eggs were counted. Subsequently, the smears were re-read, and the number of eggs of A. lumbricoides, Schistosoma mansoni, and T. trichiura was determined. Urine samples were collected for a pregnancy test (hCG Urine with OBC; Unipath Ltd, Bedford, United Kingdom), the detection of Schistosoma haematobium, and the detection of blood, glucose, and bilirubin. S. haematobium infection was assessed by using the syringe filtration technique. Ten milliliters of urine (collected between 1000 and 1400) was filtered with micropore filters, a drop of Lugol (Merck, Darmstadt, Germany) was placed on the filter, and the filter was examined under a microscope; the number of S. haematobium eggs was counted. Blood, glucose and bilirubin were detected by using test strips for urinalysis (Uriscan; MD Doctors Direct GmbH).
Blood analysis
Hemoglobin was measured in whole blood on the day of collection by using HemoCue hemoglobin 201+ (HemoCue AG, Wetzikon, Switzerland); anemia was defined as hemoglobin <12 g/dL (36). Serum was separated and frozen at −20°C with no freeze-thaw cycles. Serum ferritin (SF) and high-sensitivity C-reactive protein (CRP) were measured with an IMMULITE automatic system (DPC Bühlmann GmbH, Aschwil, Switzerland). Expected high-sensitivity CRP concentrations for healthy individuals were <3 mg/L. Serum transferrin receptor (TfR) was determined by using enzyme-linked immunosorbent assays (ELISA) (Ramco Laboratories Inc, Houston, TX); the normal range in healthy blood donors is 2.9–8.3 μg/mL. Iron deficiency was defined by an elevated sTfR (>8.3 μg/mL) and/or a low SF (<15 μg/L), although these indexes were likely confounded by the low-grade infection (37). IDA was defined as hemoglobin <12 g/dL and sTfR >8.3 μg/mL and/or SF <15 μg/L. Erythropoietin was measured by using ELISA (IBL ELISA; Immunobiological Laboratories, Hamburg, Germany); this assay has a reference range of 4–36 mU/mL in adults. GDF-15 was determined by ELISA for human GDF-15 (DuoSet; R&D Systems, Minneapolis, MN). Serum hepcidin measurements were performed by a combination of weak cation-exchange chromatography and time-of-flight mass spectrometry. An internal standard (synthetic hepcidin-24; Peptide International Inc, Louisville, KY) was used for quantification (38). Peptide spectra were generated on a Microflex LT matrix-enhanced laser desorption/ionization time-of-flight mass spectrometry platform (Bruker Daltonics, Bremen, Germany). Serum hepcidin-25 concentrations were expressed as nmol/L. The lower limit of detection of this method was 0.5 nmol/L; the average CVs were 2.7% (intrarun) and 6.5% (interrun). The median reference concentration of serum hepcidin-25 in healthy Dutch adults is 4.2 nmol/L (range: 0.5–13.9 nmol/L) (39). Total serum iron and transferrin concentrations were measured on an Aeroset (Abbott Laboratories, Abbott Park, IL) by using Roche and Abbott reagents, respectively; the transferrin concentration was converted to total-iron-binding capacity (TIBC) by using the equation transferrin × 25. Cytokines were analyzed in duplicate by using a Bio-Plex Pro assay (Bio-Rad Laboratories, Hercules, CA), which included: IL-2, IL-4, IL-6, IL-8, IL-10, and granulocyte macrophage colony-stimulating factor (GM-CSF), γ-interferon (IFN-γ), and tumor necrosis factor-α (TNF-α). The limit of detection for each assay was calculated as the concentration of analyte on the standard curve for which the corresponding fluorescence value was 2 SDs greater than the blank. Values below the limit of detection were reported as the limit of detection. Whole blood was mineralized by microwave digestion, and iron was separated by anion-exchange chromatography and a subsequent solvent-solvent extraction step into diethylether. Iron was analyzed by negative thermal ionization–mass spectrometry with a magnetic sector field mass spectrometer (Finnigan MAT 262; Thermo Finnigan, Bremen, Germany) equipped with a multicollector system for simultaneous ion beam detection; isotopic dilution calculations were done as described previously (28).
Data analysis
Data were analyzed by using SPSS 13.0 for Windows (SPSS, Chicago, IL) and Excel (XP 2002; Microsoft, Seattle, WA). The amount of 57Fe and 58Fe label present in the blood was calculated from isotope dilution (28). Circulating iron was calculated from the blood volume based on height and weight (40) and from the hemoglobin concentration (infection period: mean of days 1 and 15; infection-free period: mean of days 25 and 39). The amount of stable isotope administered was used to calculate the fractional 57Fe and 58Fe incorporation into erythrocytes after 14 d. The absorption of the oral iron was calculated by dividing the percentage of erythrocyte incorporation of the oral dose by the fractional erythrocyte incorporation of the intravenous dose (30, 35). Results of iron and inflammation indexes were presented as means ± SDs if normally distributed. If not normally distributed, the results were presented as medians with 25th–75th percentile ranges. Values were logarithmically transformed before statistical analysis and tested for normality with the Shapiro-Wilk test. If normally distributed after log transformation, paired t tests were used for comparisons between the 2 time points before and after treatment. If still not normally distributed after log transformation, Wilcoxon's signed-rank tests were used for comparison. Correlations were run by using log-transformed data. For normally distributed values, Pearson's correlations were performed. Spearman's rho correlations were used for values still not normally distributed after log transformation. Multiple linear regressions were done with log changes (day 25 to day 1) in iron absorption and utilization as the dependant variables and including the changes in log SF, log hepcidin, log TfR, log GDF-15, and log IL-10 as covariates. P < 0.05 was considered significant.
RESULTS
Four hundred thirty-six women were screened. The prevalence of S. mansoni and hookworm was 26% and 14%, respectively. The prevalence of asymptomatic parasitemia in women with a negative stool smear was 31%, but most of the positive smears had <500 parasites/μL blood.
The baseline characteristics of the women enrolled in the study (n = 23) are shown in Tables 1 and 2. The mean (± SD) age was 20.2 ± 4.8 (range: 16–35) y, and the mean (±SD) body mass index (in kg/m2) was 21.5 ± 2.4 (range: 17.4–24.8). During the study, no subjects had detectable soil-transmitted helminths, and none developed fever or other clinical signs of infection. The median parasite value at enrollment was 1320 counts/μL. However, at entry into the study on day 1 (1–2 d later), the median parasite count had decreased to 880 parasites/μL and by day 15, 14 of the women no longer had detectable asexual P. falciparum parasitemia. After treatment and continuous prophylaxis, no subject had detectable parasites on days 25 and 39.
TABLE 1.
Iron indexes in Beninese women (n = 23) before and after treatment of asymptomatic Plasmodium falciparum parasitemia
| Iron indexes | Day 1 (before treatment) | Day 25 (after treatment) | P |
| Hemoglobin (g/dL) | 13.4 ± 151 | 12.7 ± 12 | 0.0272 |
| Serum ferritin (μg/L) | 71 (29–99)3 | 37 (21–57) | 0.0014 |
| Serum transferrin receptor (mg/L) | 5.2 (4.8–7.3) | 5.6 (5.2–7.6) | 0.0072 |
| Total iron-binding capacity (mmol/L) | 68.0 (63.0–73.0) | 63.0 (57.5–70.5) | 0.0062 |
| Serum iron (μmol/L) | 13.3 ± 5.6 | 12.7 ± 6.0 | 0.4732 |
| Transferrin saturation (%) | 19.9 ± 9.1 | 20.7 ± 11.3 | 0.9122 |
| Serum erythropoietin (IU/L) | 11.2 (8.0–16.3) | 8.9 (7.2–13.6) | 0.0322 |
Mean ± SD (all such values).
Paired t test (log transformed).
Median; 25th–75th percentile in parentheses (all such values).
Wilcoxon's signed-rank test (log transformed).
TABLE 2.
Inflammation indexes in Beninese women (n = 23) before and after treatment of asymptomatic Plasmodium falciparum parasitemia1
| Inflammation indexes | Day 1 (before treatment) | Day 25 (after treatment) | P |
| Parasites (no./μL) | 880 (123–2760) | 0 (0) | 0.0012 |
| C-reactive protein (mg/dL) | 0.9 (0.4–5.7) | 0.30 (0.3–0.4) | 0.0012 |
| Hepcidin (nmol/L) | 2.7 (1.0–4.6) | 1.4 (0.7–2.4) | 0.0052 |
| GDF-15 (pg/mL) | 497 (400–612) | 381 (341–432) | 0.0032 |
| Interleukin-6 (pg/mL) | 1.32 (0.96–1.92) | 1.27 (0.70–1.32) | 0.0312 |
| Interleukin-8 (pg/mL) | 7.31 (4.58–10.03) | 4.18 (2.60–5.67) | 0.0013 |
| Interleukin-10 (pg/mL) | 7.38 (4.44–13.93) | 2.94 (1.91–2.94) | 0.0013 |
All values are medians; 25th–75th percentiles in parentheses. GDF-15, growth differentiation factor 15.
Wilcoxon's signed-rank test (log transformed).
Paired t test (log transformed).
Iron indexes before and after malarial treatment are shown in Table 1. At baseline (day 1), 22% of women were anemic, the prevalence of iron deficiency was 13%, and 9% had IDA. Clearance of parasitemia was associated with a marked decrease in SF and a smaller but still significant increases in sTfR and decreases in erythropoietin and GDF-15 (Tables 1 and 2). There was no significant change in serum iron or serum transferrin, whereas TIBC showed a small but significant decrease with malarial treatment (Table 1).
Infection/inflammation indexes before and after malarial treatment are shown in Table 2. Clearance of parasitemia was associated with a reduction in low-grade inflammation; there were significant decreases in CRP, IL-6, IL-8, and IL-10 between days 1 and 25. At entry into the study on day 1, 7 women had CRP concentrations >3 mg/L. After treatment, no subjects had CRP concentrations >3 mg/L. The reduction in low-grade inflammation was accompanied by a reduction in median hepcidin of ≈50%. Serum concentrations of IL-2, IL-4, GM-CSF, IFN-γ, and TNF-α (data not shown) for all subjects, except one, were below the detection limit at days 1 and 25. In the subject with detectable concentrations, concentrations on day 25 were 128.1, 1.5, 21.8, 324.2, and 40.4 pg/mL, respectively.
The native iron content of the sorghum used to prepare the test meals was 3.4 ± 0.2 mg/100 g, but the total iron content in the sorghum flour was 8.9 ± 0.5 mg/100 g due to the introduction of iron, which occurred during local milling. Phytate and polyphenol contents of the sorghum flour were 421 ± 24 mg and 37.5 ± 0.5 mg/100 g, respectively. The meal based on 50 g flour therefore contained 1.7 mg native Fe, 2.8 mg contaminant Fe, 3 mg Fe as NaFeEDTA, 211 mg phytate, and 18.8 mg polyphenols.
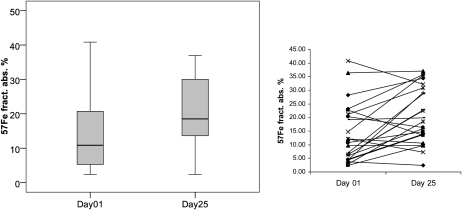
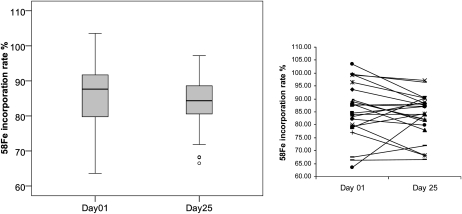
Iron absorption and utilization before and after malarial treatment are shown in Figures 2 and 3. There was a significant 70% increase in oral iron absorption with treatment: geometric mean absorption (95% CI) on days 1 and 25 were 10.2% (7.4–14.0%) and 17.6% (13.5–22.3%), respectively; 18 of 23 women showed an increase in absorption (Figure 2). In contrast, there was no significant change in systemic utilization of the intravenous iron; 14 of 23 women showed a decrease in utilization after treatment (Figure 3).
FIGURE 2.
Iron absorption in Beninese women (n = 23) who consumed a sorghum porridge labeled with 3 mg 57Fe as NaFeEDTA before (day 1) and after (day 25) malarial treatment. Iron absorption on day 1 was significantly lower than that on day 25 (Wilcoxon's signed-rank test, P = 0.008). The box plots show the median and 25th and 75th percentiles. Whiskers in the plots represent the highest and lowest values. The line graph shows individual iron absorption of the 23 women before and after treatment. fract. abs., fractional absorption.
FIGURE 3.
Erythrocyte iron utilization in Beninese women (n = 23) who received an intravenous dose of 100 μg 58Fe-labeled iron citrate before (day 1) and after (day 25) malarial treatment. Iron utilization did not differ significantly between the 2 d (Wilcoxon's signed-rank test, P = 0.107). The box plots show the median and 25th and 75th percentiles. Whiskers in the plots represent the highest and lowest values that are not outliers. Outliers (values that are between 1.5 and 3 times the interquartile range) are represented by circles beyond the whiskers. The line graph shows individual iron utilization of the 23 women before and after treatment.
Associations between iron absorption and iron and inflammation indexes are shown in Table 3. On day 1, during infection, SF, CRP, and GDF-15 showed significant negative correlations with iron absorption, whereas TfR and TIBC showed significant positive correlations. After malarial treatment (day 25), SF, serum iron, serum transferrin, and hepcidin showed significant negative correlations with iron absorption. On day 1, systemic iron utilization negatively correlated with GDF-15 (r = −0.578, P < 0.01); otherwise, there were no relevant significant associations between the measured indexes and systemic utilization. Change (Δ) in iron absorption (from days 25 to day 1) was negatively correlated with ΔSF (r = −0.493, P < 0.05), ΔCRP (r = −0.574, P < 0.01), and ΔIL-8 (r = −0.426, P = 0.05) and was positively correlated with ΔTIBC (r = 0.415, P < 0.05). ΔIron utilization (day 25 − day 1) was positively correlated with ΔIL-10 (r = 0.422, P < 0.05).
TABLE 3.
Univariate correlations of iron absorption with iron and inflammation indexes on day 1 (before malarial treatment) and day 25 (after malarial treatment) in Beninese women (n = 23) with asymptomatic Plasmodium falciparum parasitemia1
| Iron absorption on day 1 | Iron absorption on day 25 | |
| Serum ferritin2 | −0.5543 | −0.7843 |
| Serum transferrin receptor4 | 0.5763 | — |
| Total-iron-binding capacity4 | 0.4725 | — |
| Serum iron2 | — | −0.4735 |
| Transferrin saturation2 | — | −0.4565 |
| C-reactive protein2 | −0.4915 | — |
| Hepcidin2 | — | −0.6793 |
| GDF-154 | −0.4465 | — |
GDF-15, growth differentiation factor 15.
Spearman's rho correlations.
P < 0.01 (2-tailed).
Pearson's correlation coefficients.
P < 0.05 (2-tailed).
In multivariate models with log Δiron absorption and Δutilization as the dependent variables and including log ΔSF, Δhepcidin, ΔTfR, ΔGDF-15, and ΔIL-10 as covariates, ΔSF (P = 0.008), ΔGDF-15 (P = 0.017), and ΔTfR (P = 0.025) were significant predictors of Δiron absorption (r2 = 0.598), and ΔGDF-15 (P < 0.000) and ΔIL-10 (P < 0.001) were predictors of Δiron utilization (r2 = 0.621).
DISCUSSION
This study showed that afebrile malarial parasitemia, a common condition in endemic areas, decreases dietary iron absorption. This effect appears to be due to low-level inflammation modulation of serum hepcidin. This effect may help explain why iron supplements and iron fortification of staple foods or complementary foods may be less effective in malarial endemic areas (6–8). Fractional iron absorption in the present study was relatively high, which was likely due to the use of NaFeEDTA, an iron chelator, as the iron fortificant (41–45). The test meal in the present study included ≈2.8 mg contaminant Fe introduced by local milling; we assumed that its bioavailability in our test meal was negligible (46, 47).
Our study was the first to directly quantify iron incorporation into erythrocytes from dietary iron and systemic iron during, and after treatment, of asymptomatic malarial parasitemia. The advantage of using both an oral and an intravenous tracer is that it allows separation of dietary iron bioavailability into its 2 components: intestinal absorption and systemic utilization. Acute febrile malaria causes sequestration of RES iron and hypoferremia (48), which often coexists with normal or increased bone marrow iron (49). Release of iron from macrophages, like intestinal cells, is impaired with increased hepcidin and responsible for anemia by restricting iron supply to erythrocytes. In contrast with subjects with acute febrile malaria, our subjects with afebrile parasitemia had no evidence of hypoferremia during infection: the decrease in hepcidin with parasite clearance was not associated with a change in serum iron or in the percentage of serum transferrin. In contrast, asymptomatic parasitemia decreases dietary iron absorption, which suggests that iron export via ferroportin from enterocytes is sensitive to even small changes in hepcidin in the low-normal range. This finding is consistent with a previous cross-sectional study that used stable isotopes in healthy Swiss women, in which mean iron absorption from a test meal increased and serum hepcidin concentrations decreased slightly (50).
Previous studies of iron metabolism and hepcidin during malaria have focused on changes during treatment of acute febrile infection. Hepcidin regulation is complex during malaria infection: hepcidin production may be upregulated by inflammatory cytokines (15, 51, 52); however, at the same time, hypoxia associated with malarial anemia and possibly with erythropoietic factors such as GDF-15, may down-regulate hepcidin production (53, 54). The inflammatory stimuli appear to predominate, because studies have generally found a positive relation between malaria and circulating hepcidin (55). In anemic Tanzanian children aged <3 y of age with febrile P. falciparum malaria, urinary hepcidin strongly increased and was positively correlated with inflammation; antimalarial treatment led to a rapid decrease in urinary hepcidin, TNF-α, IL-6, and IL-10 and in a reversal of hypoferremia (48).
In contrast with previous studies, our Beninese subjects had lower levels of parasitemia, no clinical symptoms, and probable partial immunity from repeated exposure (56). Treatment of parasitemia decreased inflammation (CRP, SF, IL-6, IL-8, and IL-10), which was associated with a significant fall in serum hepcidin. Thus, our findings suggest that the low-grade inflammation in asymptomatic malaria is sufficient to trigger a small increase in serum hepcidin. Similarly, because SF is an acute phase protein, the resolution of low-grade inflammation likely explains the ≈48% decrease in SF associated with treatment of parasitemia (Table 1). Iron absorption was lower in those subjects with more inflammation (as reflected in higher SF and/or higher CRP concentrations) on day 1 or higher hepcidin on day 25 (Table 3). During experimental P. falciparum malaria infection in Dutch adults (57), low parasitemia and its treatment was associated with an increase in IL-6, a decline in serum iron and serum transferrin, and an increase in SF; there was an increase in serum hepcidin in 4 of 5 volunteers. Parasitemia and its treatment were associated with a transient decrease in reticulocyte hemoglobin content over 3 d, which was followed by a compensatory reticulocytosis, which suggests impaired iron incorporation into developing erythrocytes. In contrast, we directly measured systemic iron utilization by using labeled intravenous iron and found no effect of treatment of parasitemia. This suggests that parasitemia-associated inflammation may reduce iron release from macrophages rather than impair iron incorporation during erythropoiesis. Compared with previous studies, geometric mean incorporation of an intravenous iron dose in the present study (83–85%) was lower than in young Thai women (93%) (29), but was somewhat higher than in older British men (79%) (30).
In our study, there were small but significant decreases in both erythropoietin and GDF-15 with clearance of parasitemia. Acute malaria may impair iron homeostasis through a direct and/or indirect suppressive effect on erythropoiesis (11, 58). Increased hepcidin production may contribute by directly inhibiting erythroid progenitor proliferation and survival (59). Decreased erythropoietin production and/or sensitivity during malaria may also play a role (10, 60, 61), but most studies have found an adequate host erythropoietin response to malaria infection (62). In the study by Doherty et al (17), erythropoietin was significantly lower in children recovering from febrile malaria than in anemic children without malaria. In contrast, in our study of mostly nonanemic women, there was a decrease in erythropoietin with treatment, which suggested that erythropoietin was not limiting erythropoiesis during asymptomatic parasitemia. A small but significant fall in serum GDF-15 with clearance of parasitemia was observed. Because circulating GDF-15 fell along with hepcidin, the improvement in iron absorption did not appear to be the result of a “marrow” signal from GDF-15 to increase iron absorption, but rather to a decrease in circulating hepcidin.
In our studies, we did not have an additional control group of women without malaria who received antimalarial treatment and then underwent measurements of iron absorption and/or utilization. Thus, we cannot entirely rule out a potential effect of the antimalarial agents per se on iron metabolism, independent of their effect on P. falciparum. We administered prophylactic ciprofloxacillin and albendazole during the 6-wk study period to avoid potential confounding of the results by common local infections and associated inflammation. This was successful in that none of the subjects developed an infection during the study that would have led to their exclusion. Although ciprofloxacillin showed some in vitro activity against the blood stage of Plasmodium spp. (63, 64), it was not effective against Plasmodium infections (65, 66). Therefore, the negative blood smears in several of the subjects on day 15 (before antimalarial treatment) were likely explained either by self-clearance, which is thought to commonly occur in endemic areas (67, 68), or by the lack of sensitivity of blood smears to detect subpatent (eg, liver stage) parasitemia (69).
Afebrile parasitemia is very common in endemic areas and often has a protracted course (19, 23). Because it reduces iron absorption, it may be an important contributor to IDA in young women with poor diets but high iron requirements because of menstrual blood losses and repeated pregnancies. In this context, the benefits of intermittent treatment of malaria on anemia rates (70, 71) may be due not only to a reduction in acute malarial anemia, but also to the periodic clearance of asymptomatic parasitemia and a resulting improvement in dietary iron absorption.
Acknowledgments
We thank the subjects for their participation in the study and the nursing and laboratory staff at the Hopital du Zone Natitingou (Natitingou, Benin). We also thank Traore Mahamadou (Centre Suisse de Recherches Scientifiques, Cote d'Ivoire) and Siem Klaver (Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands) for laboratory assistance.
The authors’ responsibilities were as follows—CIC, IME, CZ, RD, JH, LS, RFH, and MBZ: designed the research; CIC, EA, IME, and CZ: conducted the research; CIC, CZ, TT, HT, and EW: analyzed the data; and CIC, IME, RFH, and MBZ: wrote the manuscript and had primary responsibility for the final content of the manuscript. All authors read and approved the final version of the manuscript. None of the authors had a conflict of interest with regard to this manuscript.
REFERENCES
- 1.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370:511–20 [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann MB, Chaouki N, Hurrell RF. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: a longitudinal cohort study in Moroccan children. Am J Clin Nutr 2005;81:115–21 [DOI] [PubMed] [Google Scholar]
- 3.Newton CRJC, Warn PA, Winstanley PA, et al. Severe anaemia in children living in a malaria endemic area of Kenya. Trop Med Int Health 1997;2:165–78 [DOI] [PubMed] [Google Scholar]
- 4.Menendez C, Schellenberg D, Quinto L, et al. The effects of short-term iron supplementation on iron status in infants in malaria-endemic areas. Am J Trop Med Hyg 2004;71:434–40 [PubMed] [Google Scholar]
- 5.WHO, FAO Guidelines on food fortification with micronutrients. Geneva, Switzerland: WHO/FAO, 2006 [Google Scholar]
- 6.Rohner F, Zimmermann MB, Amon RJ, et al. In a randomized controlled trial of iron fortification, anthelmintic treatment and intermittent preventive treatment of malaria for anemia control in ivorian children, only anthelmintic treatment shows modest benefit. J Nutr 2010;140:635–41 [DOI] [PubMed] [Google Scholar]
- 7.Wegmuller R, Camara F, Zimmermann MB, Adou P, Hurrell RF. Salt dual-fortified with iodine and micronized ground ferric pyrophosphate affects iron status but not hemoglobin in children in Cote d'Ivoire. J Nutr 2006;136:1814–20 [DOI] [PubMed] [Google Scholar]
- 8.Andang'o PEA, Osendarp SJM, Ayah R, et al. Efficacy of iron-fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet 2007;369:1799–806 [DOI] [PubMed] [Google Scholar]
- 9.WHO The World Health Report 1999: making a difference. Geneva, Switzerland: World Health Organization, 1999 [Google Scholar]
- 10.Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res 1998;18:555–9 [DOI] [PubMed] [Google Scholar]
- 11.Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Malarial anemia: of mice and men. Blood 2007;110:18–28 [DOI] [PubMed] [Google Scholar]
- 12.Wenisch C, Linnau KF, Looaresuwan S, Rumpold H. Plasma levels of the interleukin-6 cytokine family in persons with severe Plasmodium falciparum malaria. J Infect Dis 1999;179:747–50 [DOI] [PubMed] [Google Scholar]
- 13.Lyke KE, Burges R, Cissoko Y, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 2004;72:5630–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta 2009;1790:682–93 [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P, Peng HF, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA 2005;102:1906–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty CP, Cox SE, Fulford AJ, et al. Iron incorporation and post-malaria anaemia. PLoS ONE 2008;3:e2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 2007;13:1096–101 [DOI] [PubMed] [Google Scholar]
- 19.de Mast Q, Syafruddin D, Keijmel S, et al. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica 2010;95:1068–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eke RA, Chigbu LN. W. N. High prevalence of asymptomatic plasmodium infection in a suburb of Aba Town, Nigeria. Ann Afr Med 2006;5:42–5 [Google Scholar]
- 21.Agan TU, Ekabua JE, Iklaki CU, Oyo-Ita A, Ibanga I. Prevalence of asymptomatic malaria parasitaemia. Asian Pac J Trop Med 2010;3:51–4 [Google Scholar]
- 22.Onyenekwe CC, Meludu SC, Dioka CE, Salimonu LS. Prevalence of asymptomatic malaria parasitaemia amongst pregnant women. Indian J Malariol 2002;39:60–5 [PubMed] [Google Scholar]
- 23.Imrie H, Fowkes FJI, Michon P, Tavul L, Reeder JC, Day KP. Low prevalence of an acute phase response in asymptomatic children from a malaria-endemic area of Papua New Guinea. Am J Trop Med Hyg 2007;76:280–4 [PubMed] [Google Scholar]
- 24.Carneiro IA, Smith T, Lusingu JPA, Malima R, Utzinger J, Drakeley CJ. Modeling the relationship between the population prevalence of Plasmodium falciparum malaria and anemia. Am J Trop Med Hyg 2006;75:82–9 [DOI] [PubMed] [Google Scholar]
- 25.MARA/ARMA Collaboration 2001. Malaria seasonality model. Available from: www.mara.org.za. (cited May 2009)
- 26.MARA/ARMA Collaboration 2002. Malaria prevalence model. Available from: www.mara.org.za. (cited May 2009)
- 27.Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable-isotope technique for measuring iron-absorption in infants. Br J Nutr 1994;71:411–24 [DOI] [PubMed] [Google Scholar]
- 28.Walczyk T, Davidsson L, Zavaleta N, Hurrell RF. Stable isotope labels as a tool to determine the iron absorption by Peruvian school children from a breakfast meal. Fresenius J Anal Chem 1997;359:445–9 [Google Scholar]
- 29.Zimmermann MB, Fucharoen S, Winichagoon P, et al. Iron metabolism in heterozygotes for hemoglobin E (HbE), alpha-thalassemia 1, or beta-thalassemia and in compound heterozygotes for HbE/beta-thalassemia. Am J Clin Nutr 2008;88:1026–31 [DOI] [PubMed] [Google Scholar]
- 30.Roe MA, Heath ALM, Oyston SL, et al. Iron absorption in male C282Y heterozygotes. Am J Clin Nutr 2005;81:814–21 [DOI] [PubMed] [Google Scholar]
- 31.Makower RU. Extraction and determination of phytic acid in beans (Phaseolus-vulgaris). Cereal Chem 1970;47:288–96 [Google Scholar]
- 32.Van Veldhoven PP, Mannaerts GP. Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem 1987;161:45–8 [DOI] [PubMed] [Google Scholar]
- 33.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144–58 [Google Scholar]
- 34.Davidsson L, Ziegler E, Zeder C, Walczyk T, Hurrell R. Sodium iron EDTA [NaFe(III)EDTA] as a food fortificant: erythrocyte incorporation of iron and apparent absorption of zinc, copper, calcium, and magnesium from a complementary food based on wheat and soy in healthy infants. Am J Clin Nutr 2005;81:104–9 [DOI] [PubMed] [Google Scholar]
- 35.Dainty JR, Roe MA, Teucher B, Eagles J, Fairweather-Tait SJ. Quantification of unlabelled non-haem iron absorption in human subjects: a pilot study. Br J Nutr 2003;90:503–6 [DOI] [PubMed] [Google Scholar]
- 36.WHO/UNICEF/UNU, Iron deficiency anemia assessment, prevention and control. Geneva, Switzerland: World Health Organization, 2001 [Google Scholar]
- 37.Zimmermann MB. Methods to assess iron and iodine status. Br J Nutr 2008;99:S2–9 [DOI] [PubMed] [Google Scholar]
- 38.Swinkels DW, Girelli D, Laarakkers C, et al. Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS ONE 2008;3:e2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroot JJC, Hendriks JCM, Laarakkers CMM, et al. (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: implications for clinical studies. Anal Biochem 2009;389:124–9 [DOI] [PubMed] [Google Scholar]
- 40.Geigy Scientific Tables Hematology and human genetics. Basel, Switzerland: Ciba-Geigy Ltd, 1979 [Google Scholar]
- 41.Ballot DE, Macphail AP, Bothwell TH, Gillooly M, Mayet FG. Fortification of curry powder with NaFe(111)EDTA in an iron-deficient population—report of a controlled iron-fortification trial. Am J Clin Nutr 1989;49:162–9 [DOI] [PubMed] [Google Scholar]
- 42.Garby L, Areekul S. Iron supplementation in Thai fish-sauce. Ann Trop Med Parasitol 1974;68:467–76 [DOI] [PubMed] [Google Scholar]
- 43.Davidsson L, Dimitriou T, Boy E, Walczyk T, Hurrell RF. Iron bioavailability from iron-fortified Guatemalan meals based on corn tortillas and black bean paste. Am J Clin Nutr 2002;75:535–9 [DOI] [PubMed] [Google Scholar]
- 44.Macphail AP, Bothwell TH, Torrance JD, et al. Factors affecting the absorption of iron from Fe(III)EDTA. Br J Nutr 1981;45:215–27 [DOI] [PubMed] [Google Scholar]
- 45.Layrisse M, Martineztorres C. Fe(III)-EDTA complex as iron fortification. Am J Clin Nutr 1977;30:1166–74 [DOI] [PubMed] [Google Scholar]
- 46.Derman DP, Bothwell TH, Torrance JD, et al. Iron-Absorption from Ferritin and Ferric Hydroxide. Scand J Haematol 1982;29:18–24 [DOI] [PubMed] [Google Scholar]
- 47.Derman DP, Bothwell TH, Torrance JD, et al. Iron-absorption from maize (Zea mays) and sorghum (Sorghum vulgare) beer. Br J Nutr 1980;43:271–9 [DOI] [PubMed] [Google Scholar]
- 48.de Mast Q, Nadjm B, Reyburn H, et al. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis 2009;199:253–62 [DOI] [PubMed] [Google Scholar]
- 49.Phillips RE, Looareesuwan S, Warrell DA, et al. The importance of anemia in cerebral and uncomplicated falciparum-malaria—a role of complications, dyserythropoiesis and iron sequestration. Q J Med 1986;58:305–23 [PubMed] [Google Scholar]
- 50.Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr 2009;90:1280–7 [DOI] [PubMed] [Google Scholar]
- 51.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 2005;106:1864–6 [DOI] [PubMed] [Google Scholar]
- 52.Wrighting DM, Andrews NC. Interleukin induces hepcidin expression through STAT3. Blood 2006;108:3204–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood 2006;108:3730–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 2007;117:1926–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard CT, McKakpo US, Quakyi IA, et al. Relationship of hepcidin with parasitemia and anemia among patients with uncomplicated Plasmodium falciparum malaria in Ghana. Am J Trop Med Hyg 2007;77:623–6 [PubMed] [Google Scholar]
- 56.Hviid L. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop 2005;95:270–5 [DOI] [PubMed] [Google Scholar]
- 57.de Mast Q, van Dongen-Lases EC, Swinkels DW, et al. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol 2009;145:657–64 [DOI] [PubMed] [Google Scholar]
- 58.Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor-cell development. J Cell Physiol 1995;162:134–8 [DOI] [PubMed] [Google Scholar]
- 59.Dallalio G, Law E, Means RT. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood 2006;107:2702–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spivak JL. The blood in systemic disorders. Lancet 2000;355:1707–12 [DOI] [PubMed] [Google Scholar]
- 61.Wickramasinghe SN, Abdalla SH. Blood and bone marrow changes in malaria. Baillieres Best Pract Res Clin Haematol 2000;13:277–99 [DOI] [PubMed] [Google Scholar]
- 62.Nweneka CV, Doherty CP, Cox S, Prentice A. Iron delocalisation in the pathogenesis of malarial anaemia. Trans R Soc Trop Med Hyg 2009;104:175–84 [DOI] [PubMed] [Google Scholar]
- 63.Mahmoudi N, Ciceron L, Franetich JF, et al. In vitro activities of 25 quinolones and fluoroquinolones against liver and blood stage Plasmodium spp. Antimicrob Agents Chemother 2003;47:2636–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodman CD, Su V, McFadden GI. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum. Mol Biochem Parasitol 2007;152:181–91 [DOI] [PubMed] [Google Scholar]
- 65.Watt G, Shanks GD, Edstein MD, Pavanand K, Webster HK, Wechgritaya S. Ciprofloxacin treatment of drug-resistant falciparum malaria. J Infect Dis 1991;164:602–4 [DOI] [PubMed] [Google Scholar]
- 66.Stromberg A, Bjorkman A. Ciprofloxacin does not achieve radical cure of Plasmodium falciparum infection in Sierra Leone. Trans R Soc Trop Med Hyg 1992;86:373–373 [DOI] [PubMed] [Google Scholar]
- 67.Takala S, Branch O, Escalante AA, Kariuki S, Wootton J, Lal AA. Evidence for intragenic recombination in Plasmodium falciparum: identification of a novel allele family in block 2 of merozoite surface protein-1: Asembo Bay Area Cohort Project XIV. Mol Biochem Parasitol 2002;125:163–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patarroyo ME, Amador R, Clavijo P, et al. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature 1988;332:158–61 [DOI] [PubMed] [Google Scholar]
- 69.Bottius E, Guanzirolli A, Trape JF, Rogier C, Konate L, Druilhe P. Malaria: even more chronic in nature than previously thought; Evidence for subpatent parasitaemia detectable by the polymerase chain reaction. Trans R Soc Trop Med Hyg 1996;90:15–9 [DOI] [PubMed] [Google Scholar]
- 70.Greenwood B. Review: intermittent preventive treatment—a new approach to the prevention of malaria in children in areas with seasonal malaria transmission. Trop Med Int Health 2006;11:983–91 [DOI] [PubMed] [Google Scholar]
- 71.Odhiambo FO, Hamel MJ, Williamson J, et al. Intermittent preventive treatment in infants for the prevention of malaria in rural western Kenya: a randomized, double-blind placebo-controlled trial. PLoS ONE 2010;5:e10016. [DOI] [PMC free article] [PubMed] [Google Scholar]