Abstract
Background: Serum 25-hydroxyvitamin D [25(OH)D] concentrations can be affected by several environmental and individual factors. It is not clear to what extent genetic influences play a role in determining vitamin D status. Thus far, studies on the heritability of vitamin D have provided conflicting results.
Objective: We estimated the heritability of vitamin D concentrations and the effect of season on heritability estimates.
Design: We measured serum 25(OH)D concentrations in 510 middle-aged, male twins (310 monozygotic and 200 dizygotic twins) selected from the Vietnam Era Twin Registry. Generalized estimating equations were used to test the association between 25(OH)D and other study factors. Structural equation modeling was used to estimate the heritability of 25(OH)D.
Results: The twins’ mean (±SD) age was 55 ± 2.8 y. The mean (±SD) 25(OH)D concentration was 38.4 ± 23.3 ng/mL with a substantial seasonal variation (a 6.1-ng/mL lower value during the winter than during the summer, P = 0.003). Approximately 70% of the variation in 25(OH)D concentrations during the winter was explained by genetic factors. However, in the summer, 25(OH)D concentrations were not heritable. During the summer, 53% of the variation in 25(OH)D concentrations was due to shared environmental factors, and 47% of the variation in 25(OH)D concentrations was due to unique environmental factors.
Conclusions: Serum 25(OH)D concentrations are highly heritable during the winter season only. In the summer, environmental conditions (eg, sun exposure) prevail over genetic backgrounds in determining serum 25(OH)D concentrations. This trial was registered at clinicaltrials.gov as NCT00017836.
INTRODUCTION
Concern about vitamin D status has intensified because of the recognition that low vitamin D concentrations are highly prevalent across various segments of the population (1–11) and growing epidemiologic evidence that low vitamin D concentrations are associated with several chronic disease states including cardiovascular disease (10, 12–15). There is no consensus about the optimal concentration of 25-hydroxyvitamin D [25(OH)D], although some investigators suggested that vitamin D deficiency should be defined as a 25(OH)D concentration <20 ng/mL and insufficiency as a 25(OH)D concentration of 21–29 ng/mL (16). In the most recent National Health and Nutrition Examination Survey (NHANES 2001–2004) it was estimated that ≈77% of the US population had suboptimal concentrations of 25(OH)D (<30 ng/mL) (17), with a worsening since NHANES III (1988–1994), in which 55% of the US population was estimated to have suboptimal concentrations of vitamin D (7). Increases in body weight, decreases in milk consumption, and more widespread sunscreen use were suggested explanations for this decline.
The most important source of vitamin D is endogenous skin synthesis because of sunlight exposure; diet also contributes to vitamin D prohormone status but to a more limited extent. The large variations observed in the concentrations of vitamin D worldwide may be due to common environmental factors such as latitude, season, pollution, customs or cultural issues, diet, and fortified-food policies. Furthermore, individual sociocultural and behavioral factors such as clothing, use of sunscreen, time spent outdoors, sunbathing habits, use of vitamin supplements, and skin pigmentation may affect concentrations of vitamin D (16, 18, 19). Nonetheless, in addition to these individual and environmental factors, genetic predisposition could play a role in the susceptibility to vitamin D deficiency. Clarification of the relative contribution of genetic compared with environmental factors, and whether such influences vary by season, is important in the design of potential interventional trials. Limited studies on the heritability of vitamin D have provided conflicting results on this issue (20–23). Therefore, the purpose of this study was to estimate the influence of heritable and environmental factors on vitamin D concentrations in middle-aged, male twins living in different locations in the United States.
SUBJECTS AND METHODS
Study population
The patients whose serum was used for these analyses were recruited in the Emory Twin Study (ETS). The ETS included 2 pooled twin samples recruited in 2 companion studies, the Twins Heart Study (THS) and the Stress and Vascular Evaluation in Twins (SAVEIT). The purpose of these studies was to elucidate the role of psychologic, behavioral, and biological risk factors for subclinical cardiovascular disease by using twins. Both projects recruited middle-aged male monozygotic and dizygotic twin pairs from the Vietnam Era Twin (VET) Registry, which is one of the largest twin registries in the United States (24), and both studies used identical procedures, measurements, and protocols. The THS enrolled 180 twin pairs between 2002 and 2006 (25). The SAVEIT included 82 twin pairs recruited between 2005 and 2008. Twins included in the ETS were randomly selected from the VET Registry among twins born between 1946 and 1956. A random sample of twin pairs discordant for major depression was included in the THS, and a random sample of twin pairs discordant for posttraumatic stress disorder was included in the SAVEIT. All twins were examined in pairs at the Emory University General Clinical Research Center, and all data collection occurred during a 24-h admission under controlled conditions. The twins resided in a range of latitudes in the United States between 21° and 49° N, except for one subject who was living at 61° N. In accordance with the NHANES III study, we stratified the sample into 2 “seasonal” groups on the basis of the month of blood collection: November–March (winter) or April–October (summer) (11). One hundred twin pairs and 3 singletons underwent 25(OH)D measurements in the winter months, and 151 twin pairs and 5 singletons underwent 25(OH)D measurements in the summer months. Both studies were approved by the Emory Institutional Review Board, the research procedures used were in accordance with the ethical standards of Emory University, and all subjects signed an informed consent form to participate in the study.
Measurements
All measurements were performed in the morning after an overnight fast, and both twins in a pair were tested at the same time. Medical histories were obtained, and physical examinations were conducted for all twins. Measured weight and height were used to calculate the body mass index (BMI) as weight in kilograms divided by the height in square meters. The waist-hip ratio was defined as the ratio of the circumference of the waist to that of the hip. Systolic blood pressure and diastolic blood pressure were measured with a mercury sphygmomanometer in the right arm after 10 min of rest with the subject in a sitting position. The average of 2 measurements obtained 5 min apart was used in the analyses. Hypertension was defined as blood pressure >140/90 mm Hg or the current use of blood pressure–lowering medications. Diabetes was considered present if the patients received treatment with insulin or oral hypoglycemic drugs or had a fasting glucose concentration >126 mg/dL. Cigarette smoking was classified into 2 categories as current compared with never or past smokers. The Framingham risk score, which is a commonly used index to assess risk of coronary artery disease, was calculated by using information about the presence and severity of coronary artery disease risk factors, which incorporated age, LDL cholesterol, HDL cholesterol, blood pressure, a diagnosis of diabetes, and smoking. Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity with values ranging from 3.43 (minimal) to 13.04 (intense) (26). The glomerular filtration rate was estimated by using a creatinine-based formula recently published by the Chronic Kidney Disease Epidemiology Collaboration group (27).
Venous blood samples were drawn for the measurement of glucose, lipid profile, and 25(OH)D concentrations after an overnight fast. Glucose concentrations were measured with the Beckman CX-7 chemistry autoanalyzer (Beckman Counter Diagnosis, Fullerton, CA). The Emory Lipid Research Laboratory, which is a participant in the Centers for Disease Control/National Heart, Lung and Blood Institute Lipid Standardization Program, performed all analyses from freshly isolated plasma in EDTA-coated tubes. Total cholesterol and triglycerides concentrations were measured by enzymatic methods (Beckman Coulter Diagnostics). Direct HDL and direct LDL cholesterol were obtained by using homogeneous assays (Equal Diagnostics, Exton, PA). Serum 25(OH)D concentrations were measured by using an enzyme-linked immunosorbent assay (IDS Inc, Fountain Hills, AZ). The accuracy of measurements of 25(OH)D concentrations were ensured by participation in the vitamin D external quality assessment scheme. All biochemical assays for each twin pair were processed in the same analytic run. The values of serum 25(OH)D concentrations were log-transformed to improve the distribution.
Statistical analyses
In initial descriptive analyses, we compared mean values and proportions of study factors between monozygotic and dizygotic twins. Associations between serum 25(OH)D concentrations and other covariates were assessed by using generalized estimating equations, which took into account the relatedness between co-twins. A 2-tailed P <0.05 was considered significant. These analyses were performed with the statistical software package STATA 10 (StataCorp, College Station, TX).
To estimate the relative contributions of genetic and environmental influences on serum 25(OH)D concentrations, structural equation models were constructed with the software package Mx (28). The twin design allowed separation of the observed phenotypic variance into underlying additive genetic variance (A), common environmental variance shared by a twin pair (C), and environmental variance specific to individuals (E). The assumptions under these models were that monozygotic twins share 100% of their genes, whereas dizygotic twins share 50% of their genes. Shared environmental effects were assumed to be 100% for both monozygotic and dizygotic twins because they were reared together, whereas unique environmental effects were not shared between the siblings for either monozygotic or dizygotic twins. A greater similarity of phenotypes in monozygotic twins compared with in dizygotic twins, as indicated by a higher correlation in monozygotic than dizygotic twins, suggested a genetic effect. The significance of genetic (A) and common environmental (C) influences were tested by removing them sequentially in specific submodels (AE and CE) and comparing them with the full model (ACE). Standard likelihood-ratio tests between models were used to assess the importance of each variance component on the fit of the model. Another statistic, Akaike's information criterion, was used to determine the optimal fitting model, in which a lower Akaike's information criterion indicated a more parsimonious and, thus, better-fitting model.
Because there is substantial individual seasonal variation in serum 25(OH) D concentrations, we hypothesized that the genetic influences on serum 25(OH)D concentrations were differentially affected by the season in which the samples were collected. Accordingly, results by season are presented separately. The gene-season interaction was formally tested by comparing the model in which the genetic variance was constrained to be the same in the winter and summer groups with the model in which the genetic variance was not constrained. All analyses were repeated after adjusting for BMI and the Framingham risk score. However, the estimates were virtually identical, and therefore, these results are not reported.
RESULTS
Subject demographics
Of 524 twins (262 pairs), 510 twins had measurements of serum 25(OH)D concentrations available, including 251 pairs (153 monozygotic and 98 dizygotic) and 8 singletons. The clinical characteristics of our twins according to tertiles of 25(OH)D concentrations are shown in Table 1. Overall, the mean ± SD 25(OH)D concentration was 38.4 ± 23.3 ng/mL (median: 32 ng/mL; range: 6–201 ng/mL). Two hundred thirty-nine (47%) subjects had serum 25(OH)D concentrations <30 ng/mL and 92 (18%) subjects had serum 25(OH)D concentrations <20 ng/mL. The mean age of enrolled subjects was 55 y, 129 (29.3%) subjects were current smokers, the mean physical activity score of subjects was 7.3, 59 (11.6%) subjects had diabetes mellitus, and 239 (46.8%) subjects had hypertension. There were no differences between monozygotic and dizygotic twins for any of the demographic and behavioral characteristics and cardiovascular risk factors, except for the mean Framingham risk score, which was lower in dizygotic than in monozygotic twins (5.68 ± 2.7 compared with 6.2 ± 2.3, respectively; P = 0.02). Subjects with lower serum 25(OH)D concentrations had larger BMI and waist-hip ratios, higher Framingham risk scores, and included more diabetic patients.
TABLE 1.
Demographic characteristics and cardiovascular risk factors by 25-hydroxyvitamin D [25(OH)D] tertiles in 510 twins1
| 25(OH)D tertile | ||||
| 19.9 ± 5.2 ng/mL (n = 182) | 33.1 ± 3.9 ng/mL (n = 164) | 64.2 ± 23.8 ng/mL (n = 164) | P2 | |
| Demographic characteristics | ||||
| Age (y) | 55.2 ± 2.73 | 54.8 ± 3.0 | 55.3 ± 2.8 | 0.9 |
| White race [n (%)] | 168 (92.3) | 158 (96.3) | 156 (95.1) | 0.7 |
| Latitude (°N) | 39 ± 5.4 | 39.3 ± 5.5 | 38.6 ± 4.8 | 0.7 |
| Cardiovascular risk factors | ||||
| Smoking [n (%)] | 54 (29.7) | 41 (25) | 34 (20.9) | 0.1 |
| Physical activity score | 7.1 ± 1.7 | 7.3 ± 1.4 | 7.5 ± 1.5 | 0.07 |
| BMI (kg/m2) | 29.8 ± 5.2 | 30.2 ± 5.0 | 28.5 ± 4.4 | 0.03 |
| Waist-hip ratio | 0.96 ± 0.1 | 0.95 ± 0.1 | 0.94 ± 0.1 | 0.004 |
| Systolic blood pressure (mm Hg) | 129.7 ± 15.5 | 130 ± 15.9 | 129.8 ± 15.9 | 0.4 |
| Diastolic blood pressure (mm Hg) | 81.5 ± 10.5 | 80.7 ± 10.3 | 81.6 ± 10.4 | 0.9 |
| Total cholesterol concentration (mg/dL) | 182.2 ± 38 | 189.5 ± 40.7 | 186.2 ± 36.3 | 0.3 |
| Triglyceride concentration (mg/dL) | 178.7 ± 92.4 | 190.3 ± 101.1 | 171.9 ± 94.4 | 0.6 |
| HDL concentration (mg/dL) | 37.6 ± 10.7 | 38.1 ± 9.2 | 40.9 ± 12.2 | 0.1 |
| LDL concentration (mg/dL) | 121.1 ± 35.5 | 123.6 ± 36.1 | 120.2 ± 32.2 | 0.9 |
| Glucose concentration (mg/dL) | 104.8 ± 29.4 | 102 ± 19.5 | 101.5 ± 12.9 | 0.2 |
| Glomerular filtration rate (mL · minminus1 · 1.73 mminus2) | 89.6 ± 14.3 | 88.6 ± 14.9 | 87.4 ± 11.6 | 0.07 |
| Framingham risk score | 6.4 ± 2.2 | 5.9 ± 2.1 | 5.6 ± 2.3 | 0.01 |
| Medical history [n (%)] | ||||
| Coronary heart disease | 22 (12.1) | 41 (25) | 34 (20.9) | 0.7 |
| Hypertension | 93 (51.7) | 15 (9.3) | 13 (7.9) | 0.09 |
| Diabetes | 31 (17) | 15 (9.3) | 13 (7.9) | 0.02 |
| Concurrent medications [n (%)] | ||||
| Antihypertensive medications | 52 (28.6) | 39 (23.8) | 37 (22.6) | 0.1 |
| Statins | 47 (25.8) | 39 (23.8) | 35 (21.3) | 0.4 |
| Aspirin | 51 (28) | 33 (20) | 46 (28) | 0.9 |
| β-Blockers | 13 (7.1) | 9 (5.5) | 14 (8.5) | 0.1 |
For all characteristics shown in the table, there were no differences between twin pairs tested during the winter and twin pairs tested during the summer.
Derived from the generalized estimating equations that take into account the relatedness between co-twins.
Mean ± SD (all such values).
Vitamin D status by season
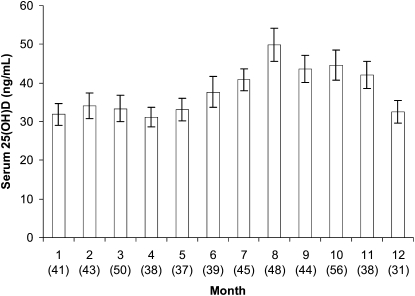
The distribution of 25(OH)D concentrations by months of the year is illustrated in Figure 1. When we stratified the sample into 2 seasonal groups, as previously described by Looker et al (11), higher 25(OH)D concentrations were measured during the summer season (April–October) than during the winter season (November–March). Mean serum 25(OH)D concentrations during the summer and winter seasons were 40.8 ± 24.4 ng/mL and 34.7 ± 21 ng/mL, respectively (P = 0.003).
FIGURE 1.
Mean (±SE) distribution of serum 25-hydroxyvitamin D [25(OH)D] concentrations by months of the year [January (= 1)–December (= 12); n = 510]. Bars represent values before log transformation. Numbers in parentheses indicate how many twins underwent measurements of 25(OH)D concentrations during that month.
Influence of environmental and genetic factors on vitamin D status
Given the significant seasonal variation in 25(OH)D concentrations in our study population, heritability was estimated separately in the winter and summer seasons (Table 2). A strong genetic influence (70%) in total variance of serum 25(OH)D concentrations was observed for subjects with serum 25 (OH)D concentrations measured during the winter but not summer seasons. During the summer, environmental factors shared by the 2 twins (53%) and environmental factors unique to each twin (47%) explained all of the variance of 25(OH)D concentrations. Furthermore, constraining the estimates for the genetic-variance component to be equal led to a significant decrease in the goodness of fit of the model (χ2 = 7.26 with 1 df). Thus, the season modified the heritability of serum 25(OH)D concentrations (P = 0.007 for the gene-season interaction). These results did not change after adjustment for other covariates including BMI and the Framingham risk score (data not shown).
TABLE 2.
Twin correlation for vitamin D and percentage of variance in vitamin D explained by additive genetic factors (A) and common (C) and unique (E) environmental factors during winter and summer seasons1
| Season | 25(OH)D concentration2 | Twin correlation3 | Variance components4 | |||
| MZ | DZ | A | C | E | ||
| ng/mL | ||||||
| Winter (November–March) | 34.7 ± 21.0 | 0.69 [122] | 0.29 [81] | 70 (31, 80) | 0 (0, 34) | 30 (20, 46) |
| Summer (April–October) | 40.8 ± 24.4 | 0.50 [188] | 0.58 [119] | 0 (0, 35) | 53 (21, 64) | 47 (36, 59) |
25(OH)D, 25-hydroxyvitamin D; MZ, monozygotic; DZ, dizygotic.
Values are means ± SDs.
n in brackets indicates the number of twins (including twin pairs and singletons).
Values are variable estimates (95% CIs) calculated by using structural equation models.
DISCUSSION
In this predominantly white, male twin sample, we observed a large heritability in the variance of serum 25(OH)D concentrations during the winter months but not in the summer months. During the summer, there was no contribution of genetic factors to individual differences in serum 25(OH)D concentrations, and similarities between twins were mostly attributable to a shared environment. The remainder of the variability in individual serum 25(OH)D concentrations during the summer was due to environmental exposures that were unique to each twin. A twin study is considered the most definitive way to assess the relative effects of genetic factors and environment on traits and diseases because it allows estimation of genetic effects as well as common and unshared unique environments (29).
Previous studies addressed the genetic influence and common and unshared unique environmental components in total variance of 25(OH)D concentrations and showed discordant results. Some studies showed a high heritability in one sex but not the other (21), and other studies reported contrasting results as to the effect of season. For example, Snellman et al (23) evaluated genetic and environmental influences on 25(OH)D concentrations in 102 white, twin pairs of both sexes living in Uppsala, Sweden. The investigators showed that genes contributed to 48% of the variance in 25(OH)D concentrations during the summer season, but during the winter season, shared and unique environmental factors predominated (23). However, there are substantial differences between the published studies. Although we enrolled predominantly adult, white, male twins (94.5%) living in North America, Snellman et al (23) evaluated adult, white twins of both sexes living in Sweden, and Arguelles et al (21) evaluated Chinese, adolescent twins of both sexes. Sex may indeed be very important because there appears to be a smaller genetic effect on serum 25(OH)D concentrations in women (20, 21).
Although speculative, there are several potential ways in which genetic factors could influence vitamin D concentrations; among others, an individual's skin ability to generate vitamin D3, which includes the availability of the substrate 7-dehydrocholesterol, the ability to convert 7-dehydrocholesterol to pre–vitamin D3 and then to vitamin D3, and the catabolism of formed pre–vitamin D3 into inactive vitamin D photoproducts. As reported in a review by Ebert et al (30), little is known of the polymorphisms in cholesterol metabolism that may affect the vitamin D3 metabolic cascade. Aging may also have some influence on the production of vitamin D3 in the skin because of decreased 7-dehydrocholesterol substrate availability (31). The important confounding issue of skin pigmentation, as a determinant of vitamin D production, was mostly avoided in our study because >90% of the patients were white.
A second potential genetic factor that may influence 25(OH)D concentrations involves vitamin D binding protein (DBP), which is a member of the albumin and α-fetoprotein gene family (32, 33). Recently, Sinotte et al (34) evaluated 741 premenopausal white women and showed that circulating 25(OH)D concentrations were strongly associated with DBP-1 and DPB-2 polymorphisms. Engelman et al (35) showed an association between polymorphism in the DBP and concentrations of 25(OH)D. In addition, Hunter et al (20) showed that ≈60% of the variance in DBP in women could be explained by genetic factors. The third pathway that is potentially influenced by genes is the hydroxylation of vitamin D3 to 25(OH)D by the enzyme 25-hydroxylase, especially because 25-hydroxylase has been found in several tissues (36, 37). Wang et al (38), who used a large cohort from merged databases, recently confirmed that the genes that encode for the 3 factors we discussed, and a fourth gene that encodes for 24-hydroxylase, are strong determinants of 25(OH)D insufficiency [defined as a 25(OH)D concentration <30 or <20 ng/mL).
Finally, human and animal studies have shown that vitamin D can be stored in fat cells and released under an unclear set of stimuli (39, 40). Epidemiologic studies have confirmed that vitamin D concentrations are typically lower in obese individuals as a result of sequestration in the adipose tissue (41).
Among the environmental factors that potentially affect vitamin D concentrations are season, latitude, cultural dress patterns, outdoor physical activity, and diet. Latitude is an important determinant of 25(OH)D status in whites. A recent meta-analysis of 394 studies showed a significant decline in 25(OH)D concentrations with increasing latitude in healthy, white subjects but not in other ethnicities (42). In contrast, we did not find differences in serum 25(OH)D concentrations by latitude despite the fact that our twins were selected from latitudes ranging from 21° N to 49° N, except for one subject who was living at 61° N.
The observed interaction between season and genes emphasized the importance of the environment in the expression of an individual's genetic predisposition to the generation of vitamin D. In the current study, we observed a strong genetic influence during the winter season but not during the summer season, with a significant gene-season interaction. During the winter, the relative lack of sun exposure may make vitamin D concentrations more dependent on the synthesis or mobilization of vitamin D from other sources through a number of possible heritable pathways as previously discussed. In contrast, during the summer, environmental ultraviolet-B radiation becomes a predominant source of vitamin D and may supersede a genetic predisposition. The strong genetic influence during the winter and not during the summer suggested that genes are more important when ultraviolet-B radiation is limited.
There are several limitations to this study. First, the cross-sectional design limited our ability to make causal inferences between risk factors and 25(OH)D concentrations. Second, our patients were recruited from a twin registry of military veterans and included only middle-aged, predominantly white men; thus, one should not generalize our results to women, younger individuals, and different races or ethnic groups.
In conclusion, during the winter season when 25(OH)D concentrations are at their lowest, we observed that serum concentrations of 25(OH)D were under considerable genetic influence. In contrast, during the summer, environmental factors were the prevailing mechanisms whereby 25(OH)D concentrations were influenced. The identification of genetic and nongenetic influences on 25(OH)D concentrations is important to consider when comparing the vitamin D status of individuals from different genetic backgrounds and when attempting to define optimal vitamin D concentrations for health outcomes. Furthermore, this information may become very useful when designing interventions studies with vitamin D to establish optimal targets of 25(OH)D concentrations in populations with different genetic backgrounds.
Acknowledgments
The authors’ responsibilities were as follows—CK, SS, and PR: analysis of data and manuscript drafting and final approval; MK: laboratory analyses and manuscript review; VT: manuscript drafting and final approval and advice on relevant statistical analyses; EV: database management and data analyses; VV: primary investigator on National Institutes of Health studies that formed the basis of these analyses, data collection and analysis, manuscript drafting and final approval, and provision of significant advice. None of the authors disclosed a conflict of interest.
REFERENCES
- 1.McMurtry CT, Young SE, Downs RW, Adler RA. Mild vitamin D deficiency and secondary hyperparathyroidism in nursing home patients receiving adequate dietary vitamin D. J Am Geriatr Soc 1992;40:343–7 [DOI] [PubMed] [Google Scholar]
- 2.Stein MS, Scherer SC, Walton SL, et al. Risk factors for secondary hyperparathyroidism in a nursing home population. Clin Endocrinol (Oxf) 1996;44:375–83 [DOI] [PubMed] [Google Scholar]
- 3.van der Wielen RP, Lowik MR, van den Berg H, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet 1995;346:207–10 [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005;90:3215–24 [DOI] [PubMed] [Google Scholar]
- 5.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr 1998;67:1232–6 [DOI] [PubMed] [Google Scholar]
- 6.Yanoff LB, Parikh SJ, Spitalnik A, et al. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf) 2006;64:523–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr 2008;88:1519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen R, Melgaard C, Skovgaard LT, et al. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur J Clin Nutr 2005;59:533–41 [DOI] [PubMed] [Google Scholar]
- 9.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med 2002;112:659–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–20 [DOI] [PubMed] [Google Scholar]
- 11.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 2002;30:771–7 [DOI] [PubMed] [Google Scholar]
- 12.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–9 [DOI] [PubMed] [Google Scholar]
- 13.Pilz S, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008;93:3927–35 [DOI] [PubMed] [Google Scholar]
- 14.Judd SE, Nanes MS, Ziegler TR, Wilson PWF, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr 2008;87:136–41 [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 17.Ginde AA, Liu MC, Camargo CAJ. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med 2009;169:626–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev 2008;66:S182–94 [DOI] [PubMed] [Google Scholar]
- 19.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr 2005;135:310–6 [DOI] [PubMed] [Google Scholar]
- 20.Hunter D, De Lange M, Snieder H, et al. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res 2001;16:371–8 [DOI] [PubMed] [Google Scholar]
- 21.Arguelles LM, Langman CB, Ariza AJ, et al. Heritability and environmental factors affecting vitamin D status in rural Chinese adolescent twins. J Clin Endocrinol Metab 2009;94:3273–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shea MK, Benjamin EJ, Dupuis J, et al. Genetic and non-genetic correlates of vitamin K and D. Eur J Clin Nutr 2009;63:458–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snellman G, Melhus H, Gedeborg R, et al. Seasonal genetic influence on serum 25-hydroxyvitamin D levels: a twin study. PLoS ONE 2009;4:e7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res 2002;5:476–81 [DOI] [PubMed] [Google Scholar]
- 25.Vaccarino V, Brennan ML, Miller AH, et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry 2008;64:476–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson MT, Ainsworth BE, Wu HC, Jacobs DRJ, Leon AS. Ability of the atherosclerosis risk in communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol 1995;24:685–93 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. 6th ed.Richard, VA: Virginia Commonwealth University Medical School, 2003 [Google Scholar]
- 29.MacGregor AJ, Snieder H, Schork NJ, Spector TD. Twins. Novel uses to study complex traits and genetic diseases. Trends Genet 2000;16:131–4 [DOI] [PubMed] [Google Scholar]
- 30.Ebert R, Schutze N, Adamski J, Jacob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol 2006;248:149–59 [DOI] [PubMed] [Google Scholar]
- 31.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 1985;76:1536–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreassen TK. The role of plasma-binding proteins in the cellular uptake of lipophilic vitamins and steroids. Horm Metab Res 2006;38:279–90 [DOI] [PubMed] [Google Scholar]
- 33.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta 2006;372:33–42 [DOI] [PubMed] [Google Scholar]
- 34.Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr 2009;89:634–40 [DOI] [PubMed] [Google Scholar]
- 35.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab 2008;93:3381–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res 2004;19:680–8 [DOI] [PubMed] [Google Scholar]
- 37.Cheng JB, Levine NH, Bell NH, Mangelsdorf DJ, Russel DW. Genetic evidence that the human CYP2R1 is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 2004;101:7711–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenstreich SJ, Rich C, Volwiller W. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest 1971;50:679–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blum M, Dolnikowski G, Seyoum E, et al. Vitamin D(3) in fat tissue. Endocrine 2008;33:90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3 [DOI] [PubMed] [Google Scholar]
- 42.Hagenau T, Vest R, Gissel TN, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int 2009;20:133–40 [DOI] [PubMed] [Google Scholar]