Abstract
Background: Blood-based indicators of biotin status in humans were shown to be useful tools in several clinical situations, including pregnancy. We previously validated the activity of the biotin-dependent enzyme propionyl-coenzyme A carboxylase (PCC) in lymphocytes as a sensitive and specific blood-based indicator of marginal degrees of biotin deficiency. However, the measurement of PCC activity in population studies presents substantial analytic challenges. 3-Hydroxyisovaleryl carnitine (3HIA-carnitine) increases in response to the decreased activity of the biotin-dependent enzyme methylcrotonyl-coenzyme A carboxylase and might reflect biotin status.
Objective: We sought to determine whether the plasma concentration of 3HIA-carnitine increases significantly in marginal biotin deficiency.
Design: We experimentally induced marginal, asymptomatic biotin deficiency in 10 healthy adults (8 women) by having the subjects consume undenatured egg white for 28 d; biotin status was then repleted. Plasma concentrations of 3HIA-carnitine were measured on days 0, 14, 28, 35, and 50 by liquid chromatography–mass spectroscopy.
Results: The mean plasma 3HIA-carnitine concentration increased with depletion (P < 0.0001) and decreased with repletion (P < 0.0001). Plasma 3HIA-carnitine concentrations were greater than the upper limit of normal concentrations in 7 of 10 subjects by day 14 and in 9 of 10 subjects by day 28 and decreased to within normal limits in 9 of 10 subjects by day 50.
Conclusions: These studies provide evidence that 3HIA-carnitine is an early and sensitive indicator of marginal biotin deficiency. The ease of sample collection, small sample volume requirement, and stability of 3HIA-carnitine during storage suggest that plasma 3HIA-carnitine concentration is likely to be a useful indicator of marginal biotin deficiency for larger population studies.
INTRODUCTION
Biotin is a water-soluble vitamin that is generally classified in the B group. Mounting evidence indicates that marginal biotin deficiency is not rare, which is contrary to previous assumptions. Studies from our group and others have provided evidence that marginal biotin deficiency occurs in such disparate clinical circumstances as pregnancy (1, 2), protein energy malnutrition (3), and long-term therapy with certain anticonvulsants (4–8). Accordingly, early and sensitive indicators of biotin status are likely to be useful (9).
Biotin is a covalently bound prosthetic group for 5 mammalian carboxylases. The 5 carboxylases are propionyl-coenzyme A (CoA) carboxylase [PCC; European Commission number (EC) 6.4.1.3], methylcrotonyl-CoA carboxylase (MCC; EC 6.4.1.4), pyruvate carboxylase (EC 6.4.1.1), and 2 isoforms of acetyl-CoA carboxylase (EC 6.4.1.2). Each carboxylase catalyzes an essential step in intermediary metabolism.
In isolated genetic deficiency of MCC (10) and in deficiency of multiple carboxylases because of holocarboxylase-synthetase deficiency or biotinidase deficiency (11, 12), the plasma concentration of 3-hydroxyisovaleryl carnitine (3HIA-carnitine) increases, likely as a consequence of the following metabolic sequence. The reduced activity of MCC impairs the catalysis of an essential step in the mitochondrial catabolism of the branched-chain amino acid leucine. This metabolic impairment causes the accumulation of its substrate 3-methylcrotonyl-CoA and metabolism via an alternate pathway; enoyl-CoA hydratase converts 3-methylcrotonyl-CoA to 3HIA-CoA (13). One of the 2 mechanisms for further metabolism of this potentially toxic metabolite is the transfer of the 3HIA moiety to carnitine most likely by the enzyme carnitine acetyltransferase forming 3HIA-carnitine (14). Acyl carnitines are moved across the mitochondrial membrane by carnitine-acylcarnitine translocase, leave the cell, and ultimately appear in plasma and urine (15).
Because nutritional biotin deficiency also causes MCC deficiency and increased urinary excretion of 3-hydroxyisovaleric acid (3-HIA) (16, 17), we speculated that the plasma concentration of 3HIA-carnitine might increase in response to biotin deficiency. In the current article, we report an assessment of the validity of the plasma concentration of 3HIA-carnitine as an indicator of marginal biotin deficiency measured by using a liquid chromatography–tandem mass spectroscopy (LC-MS/MS) method that was recently developed by our laboratory (18).
SUBJECTS AND METHODS
The human aspects of this research were approved by the Institutional Review Board of the University of Arkansas for Medical Sciences. Written informed consent was obtained from each subject as part of the ongoing consent process. Specific details of this study, which was conducted in October 1999–July 2000, including biotin loading and washout, and study depletion and repletion phases have been published previously (19). Ten subjects (8 women) completed the study. Subjects ranged in age from 25 to 78 y and were deemed healthy by physical examinations and health histories. Potential subjects were excluded if they were consuming a medication known to affect biotin status and were prescreened for conditions (ie, pregnancy) that could interfere with biotin metabolism or excretion (eg, chronic renal disease).
Loading and washout protocol
We observed that marginally deficient individuals can spontaneously appear in normal populations, such as pregnant women (1, 2, 20). We also documented that marginal deficiency can develop in individuals who chronically receive anticonvulsant therapy (4–8); accordingly, we speculate that exposure to other environmental factors might lead to marginal deficiency in otherwise normal individuals. Consequently, we instituted a loading-and-washout protocol before inducing deficiency to ensure biotin sufficiency on day 0 of the biotin depletion phase (19).
Subjects consumed a 300-μg biotin supplement (10 times the recommended daily allowance) (21) daily for 7 d (loading phase) starting on approximately day −14 (2 wk before initiating the low-biotin diet that contained undenatured avidin in egg white). This biotin supplement was discontinued at day −7; thereafter, subjects consumed a multivitamin supplement without biotin from days −7 to 28.
Biotin depletion phase
Subjects were admitted on day −1 to the University of Arkansas for Medical Sciences Clinical Research Center (CRC). The biotin depletion regimen consisted of a dietitian-planned, low-biotin diet; in addition, an egg-white beverage was consumed. Consumption of this diet commenced on day 0 and continued for 28 d. Subjects spent evenings and nights in the CRC but were free to leave the CRC on weekdays. Lunches with egg-white drinks were provided for these meals.
Biotin repletion phase
On day 28, subjects were randomly assigned to an immediate supplement group (n = 5) and a late supplement group (n = 5). The immediate supplement group resumed a self-selected, mixed general diet on day 28 and consumed a 30-μg biotin supplement daily from days 28 to 50. The late supplement group resumed a self-selected mixed general diet at day 28 but consumed the biotin supplement only from days 35 to 50. We sought preliminary insight into the effect of resuming a normal diet alone compared with starting the biotin supplement immediately; after 1 wk, we started supplementing both groups to substantially replete all subjects before being discharged from the study.
Blood sample collection
Peripheral venous blood was collected in a heparinized syringe on days 0, 14, 28, 35, and 50 as previously described (22). Peripheral blood mononuclear cells were obtained from whole blood by density gradient centrifugation and stored at −80°C as previously described (22).
Determination of lymphocyte PCC activity
PCC activity in peripheral blood lymphocytes is the best established indicator of marginal biotin deficiency in humans (23). In rats, lymphocyte PCC activity decreases in parallel with hepatic biotin and hepatic PCC activity (24), and in mice, hepatic PCC activity decreases in parallel with hepatic MCC activity in the biotin-deficient mouse dam and biotin-deficient mouse fetus (25, 26). PCC activities were measured by 14CO2 incorporation as previously described (22). Activities were normalized by lymphocyte protein content and measured in triplicate for each sample.
Determination of urinary 3-HIA concentrations
Urinary 3-HIA concentrations were measured by gas chromatography–tandem mass spectroscopy as described previously (16). In the current study, excretion is presented as normalized by urinary concentration of creatinine rather than as excretion rates because the determination of urinary indicators in untimed urine samples is more practical in population studies. We previously observed that excretion normalized by creatinine is approximately as sensitive as the excretion rate (16), and excretion rate data are available (from DMM) upon request.
Determination of plasma 3HIA-carnitine concentrations
Plasma 3HIA-carnitine was extracted from 100 μL plasma by solid-phase extraction and measured by LC-MS/MS as previously described (18). Briefly, 3HIA-carnitine was extracted from plasma with Strata X-CW solid-phase extraction cartridges (Phenomenex, Torrance, CA). Separations were performed with an Agilent Series 1200 quaternary liquid chromatography system (Agilent Technologies, Santa Clara, CA). Quantitation was performed with an authentic deuterated internal standard (D3-3HIA-carnitine, >98%; Cambridge Isotope Laboratories, Andover, MA) with an Applied Biosystems API-4000 Q TRAP tandem mass spectrometer (Applied Biosystems, Carlsbad, CA). Appropriate analytic calibration and quality-control standards were prepared and run with study plasma samples. Plasma concentrations of 3HIA-carnitine were reported in micromoles per liter.
Statistical analyses
Differences in mean lymphocyte PCC activities, urinary 3-HIA excretion, and plasma 3HIA-carnitine concentrations during the depletion phase of the study (days 0, 14, and 28) were tested for significance by one-factor analysis of variance with repeated measures. When significance was detected (P < 0.05), Dunnett's post hoc test was used to test for the significance of increases relative to day 0. Differences in group mean plasma 3HIA-carnitine concentrations during the repletion phase of the study (days 28, 35, and 50) were tested for significance by 2-factor analysis of variance with repeated measures and Dunnett's post hoc test relative to day 28.
To mathematically model relations between various indicators of biotin deficiency, mixed-effects regression models were used. The logarithmically transformed plasma 3HIA-carnitine concentration was modeled as a function of transformed lymphocyte PCC by using a mixed-effects regression model. The specific model, termed a random coefficient model, assumed that the regression model for each subject was a random deviate from some overall regression model that described the population. This same model was used to examine the correlation between urinary excretion of 3-HIA and plasma 3HIA-carnitine concentrations.
All tests were performed with KaleidaGraph software (version 4.1.1; Synergy Software, Reading, PA) or the SAS PROC MIXED procedure (SAS, version 9.2; SAS Institute, Cary, NC).
Normal ranges
The term normal range was used to denote values from biotin-sufficient normal subjects in whom biotin sufficiency was established by loading and washout as previously described. Because population distributions were not normal, upper and lower limits were chosen as 10th and 90th percentiles.
The normal range for PCC activities was estimated from previously published values from 18 subjects (10 women) whose biotin sufficiency was assured by biotin supplementation and washout at the initiation of 2 studies of biotin deficiency induced experimentally in healthy adults (23). The normal range for the urinary excretion of 3-HIA was calculated from 54 normal subjects (23); this population included 18 subjects, who were assured to be biotin sufficient by loading and washout as previously described, plus 36 healthy individuals who did not supplement their dietary biotin with other sources. The mean 3-HIA excretion of the 36 subjects was not significantly different from that of the 18 biotin-sufficient subjects.
No prior data were available for the plasma concentration of 3HIA-carnitine. Consequently, the range for plasma 3HIA-carnitine concentrations was chosen as the full range of values at day 0 (ie, biotin sufficiency) of the subjects in this study.
RESULTS
Evidence of successful induction of marginal biotin deficiency
Marginal biotin deficiency was successfully induced in the depletion phase of the study by the diet of low biotin plus egg white as judged by decreased PCC activities (Figure 1A) and increased urinary 3-HIA excretions (Figure 1B). By day 14, PCC activities were less than the lower limit of normal in 7 of 10 subjects (70% diagnostic sensitivity), and by day 28, PCC activities were less than the lower limit of normal for all subjects. At day 28, the group mean PCC activity was ≈30% of the biotin-sufficient value at day 0. The mean urinary 3-HIA excretion increased ≈3-fold and was above the upper limit of normal in 8 of 10 subjects by day 14 and in all subjects by day 28.
FIGURE 1.
Decreased lymphocyte propionyl-coenzyme A carboxylase (PCC) activities (A) and increased urinary excretions of 3-hydroxyisovaleric acid (3-HIA) (B) provide evidence for the successful induction of marginal biotin deficiency by egg-white consumption in 10 study subjects by day 28. Individual subject data are shown by individual symbols (eg, ▪, •, ▴, etc). The analytic variability calculated as the SD for a single subject at a single time point is approximately the same size as the symbols (data not presented). The shaded rectangle denotes the normal range (NR). Significance of changes were tested by one-factor ANOVA with repeated measures and Dunnett's post hoc test relative to day 0.
No symptoms of frank biotin deficiency were reported by any subject at any time in the study. No signs of frank biotin deficiency were observed by the study physician (DMM) or CRC nurses.
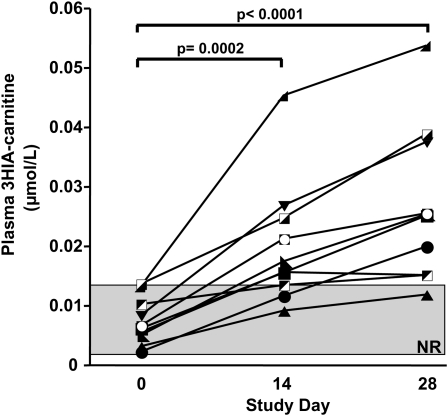
Plasma 3HIA-carnitine concentrations
During the depletion phase of the study, the mean plasma concentration of 3HIA-carnitine increased significantly (Figure 2). By day 14, individual concentrations were greater than the upper limit of normal in 7 of 10 subjects (70% diagnostic sensitivity). By day 28, the mean plasma concentration had increased 4-fold from day 0 and was greater than the upper limit of normal in 9 of 10 subjects.
FIGURE 2.
Effects of biotin depletion on plasma 3-hydroxyisovaleryl carnitine (3HIA-carnitine) concentrations in 10 study subjects. Individual subject data are shown by individual symbols (eg, ▪, •, ▴, etc). The shaded rectangle denotes the normal range (NR). Significance of changes in mean data were tested by one-factor ANOVA with repeated measures and Dunnett's post hoc test relative to day 0.
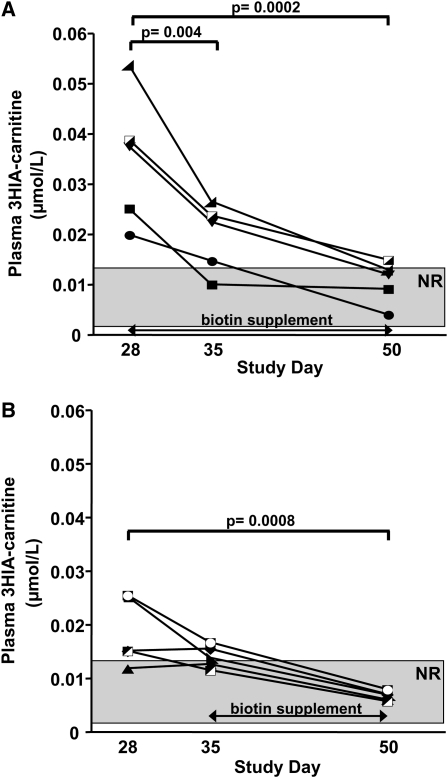
For the immediate supplementation group (Figure 3A), who started the biotin supplement on day 28, plasma concentrations of 3HIA-carnitine decreased strikingly after 1 wk. By day 50, plasma 3HIA-carnitine concentrations for 4 of 5 subjects had returned to the normal range.
FIGURE 3.
Effects of biotin repletion on plasma 3-hydroxyisovaleryl carnitine (3HIA-carnitine) in 5 subjects with immediate biotin supplementation (A) and 5 subjects with late biotin supplementation (B). Individual subject data are shown by individual symbols (eg, ▪, •, ▴, etc). The shaded rectangle denotes the normal range (NR). Significance of changes in group mean data were tested by one-factor ANOVA with repeated measures and Dunnett's post hoc test relative to day 28.
During the repletion phase of the study, plasma 3HIA-carnitine concentrations in the late supplementation group (Figure 3B) decreased in the week of resuming a mixed general diet without biotin supplementation, but the decrease did not reach significance (P = 0.10); after supplementing this group from days 35 to 50, the decrease in the mean plasma concentration of 3HIA-carnitine was significant. By day 50, individual concentrations were normal in all 5 subjects.
The randomization to immediate and late supplement groups produced subgroups that had significantly different plasma concentrations of 3HIA-carnitine on day 28 (P = 0.047). The immediate group started repletion with an ≈2-fold greater mean plasma concentration of 3HIA-carnitine.
Correlation analyses
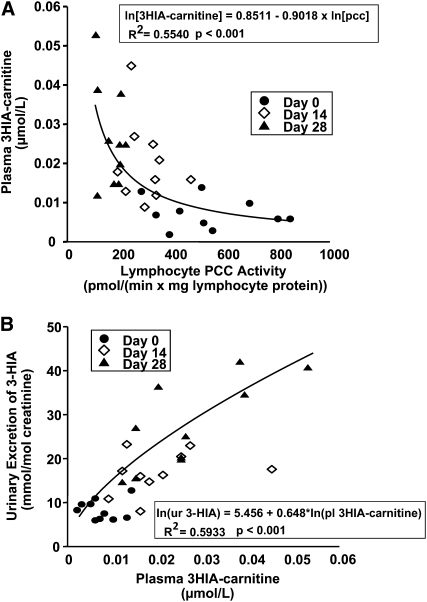
On the basis of studies in rats, lymphocyte PCC activity reflects hepatic PCC activity (24), and on the basis of murine studies (25, 26), hepatic MCC activity correlates modestly with hepatic PCC activity. To further investigate these relations in humans, we plotted plasma 3HIA-carnitine concentrations against lymphocyte PCC activity for all subjects at all time points. The observed curvilinear relation between plasma 3HIA-carnitine concentrations and lymphocyte PCC activity (Figure 4A) was reasonably well fit by log transformation of plasma 3HIA-carnitine concentrations and PCC activity. The regression model indicated that ≈55% of the variation in plasma 3HIA-carnitine concentrations could be explained by the equation given in the figure inset. We also examined the dependence of urinary excretion of 3-HIA on plasma concentrations of 3HIA-carnitine (Figure 4B). We observed a significant linear relation in which the plasma 3HIA-carnitine concentration predicted 59% of the urinary 3-HIA excretion.
FIGURE 4.
Correlation between plasma 3-hydroxyisovaleryl carnitine (3HIA-carnitine) concentrations and lymphocyte propionyl-coenzyme A carboxylase (PCC) activity (A) and between urinary excretion of 3-hydroxyisovaleric acid (3-HIA) and plasma 3HIA-carnitine concentrations (B) calculated by mixed regression modeling for 10 study subjects in the depletion phase of the study. Individual subject data are shown at 3 time points. The formulas for regression analyses are provided in each panel, and the goodness-of-fit is indicated by R2. pcc, propionyl-coenzyme A carboxylase; ur, urine; pl, plasma.
DISCUSSION
Properly diagnosing marginal biotin deficiency is potentially important because recent clinical studies have provided evidence that marginal biotin deficiency is more common than previously thought. For example, our studies provided evidence that marginal, asymptomatic biotin deficiency is a common occurrence in normal human pregnancy (1, 2, 20, 27) and is teratogenic in some mammals (27, 28). Moreover, in children and adults, marginal biotin deficiency appears to be a frequent consequence of long-term therapy with certain anticonvulsants (4–8). Smoking and malnutrition were also reported to cause impaired biotin status (3, 29, 30).
This study provided initial evidence that the plasma concentration of 3HIA-carnitine may be an early and sensitive indicator of marginal biotin deficiency. Diagnostic sensitivity was similar to that of lymphocyte PCC activity (currently the best indicator of marginal biotin status in humans) and urinary 3-HIA. The use of plasma 3HIA-carnitine concentrations in application to population studies is likely to be superior to that of lymphocyte PCC activity on the basis of the following methodologic considerations.
The fundamental principle of the lymphocyte PCC assay is the quantitation of the activity of PCC by 14C incorporation from 14C-bicarbonate into acid-precipitable methylmalonyl-CoA. The PCC must be enzymatically active after blood sample processing, cell isolation, and storage. Accordingly, the samples require special handling, the lymphocytes must be carefully isolated from peripheral blood, and the cell isolates must be stored frozen. Hence, the unavoidable unpredictability of the timing of sample availability for certain populations (eg, cord blood) is potentially problematic for the PCC assay. Moreover, care must be taken during the processing and isolation steps of sample preparation because any hemoglobin contamination from red blood cells in the lymphocyte pellet interferes with the normalization of the PCC activity by lymphocyte protein. Likewise, the clean separation of the 14C in the reaction product (14C-labeled methylmalonyl-CoA) from the 14C-bicarbonate substrate and maintenance of a stable specific activity of 14C-bicarbonate because of bicarbonate volatility are technically demanding.
In contrast, the separation of plasma and storage at −70° is technically straightforward. 3HIA-carnitine stored frozen in plasma or in blood on filter paper stored at room temperature is likely stable for months or years (31, 32), and quantitation is precise, accurate (18), and requires small plasma volumes (100 μL or 0.4% of total blood volume needed for the PCC assay) for analysis. Moreover, plasma 3HIA-carnitine is likely not as dependent on renal function as the urinary excretion of 3-HIA. This advantage is potentially important in clinical circumstances in which renal function may be altered (eg, pregnancy or renal disease) or in which urine collection is problematic (eg, infants).
The relations reported here between plasma 3HIA-carnitine and urinary 3-HIA concentrations and lymphocyte PCC activity are consistent with current understanding of the metabolic pathogenesis of biotin deficiency. Theoretically, the direct measurement of lymphocyte and hepatic MCC activity would provide a measurement of biotin status that is more proximate to the metabolic block that leads to increased synthesis and excretion of 3HIA-carnitine. However, in freshly isolated lymphocytes, the MCC activity is substantially lower than the PCC activity, and quantitation with our current assay is problematic.
Because acyl-CoA compounds are compartmentalized in the mitochondria, the increased concentration of 3-hydroxyisovaleryl-CoA leads to a disruption of the esterified CoA-to-free CoA ratio and, ultimately, to mitochondrial toxicity (10, 33). Detoxification of this metabolic end product is performed by 2 mechanisms (13). The first mechanism is the deacylation of the 3-hydroxyisovaleryl-CoA to form 3-HIA. The other mechanism is the transfer of the 3-hydroxyisovaleryl acyl moiety to carnitine by the enzyme carnitine palmitoyltransferase II forming 3HIA-carnitine (34). Both of these are able to cross the outer mitochondrial membrane and leave the cell to be transported in plasma and finally excreted in urine (20).
Hence, a direct relation between plasma 3HIA-carnitine and urinary 3-HIA concentrations is not surprising, even at marginal degrees of biotin deficiency. Moreover, a rapidly rising plasma 3HIA-carnitine concentration in response to decreased activity of lymphocyte PCC as an index of hepatic MCC activity is reasonable and consistent with the very high 3-HIA excretion observed when MCC activity approaches reductions seen in inborn errors (eg, 95%).
Indeed, in cases of MCC deficiency that are due to inborn errors such as isolated MCC deficiency (10), holocarboxylase synthetase deficiency (11), biotinidase deficiency (12), and biotin transporter deficiency (35), plasma 3HIA-carnitine concentrations and 3-HIA excretion rates are often increased 10–100-fold over the normal range (36). For inborn-error screening, the emphasis is appropriately placed on minimizing false-positive conclusions by choosing higher upper limits of normal. For example, the published upper limit of normal used for metabolic screening is 0.11 μmol 3HIA-carnitine/L (11, 36).
In contrast, the LC-MS/MS method used in the preset study to measure 3HIA-carnitine concentrations was developed to accurately and precisely measure plasma concentrations of 3HIA-carnitine that are normal or only modestly increased. The normal range of plasma 3HIA-carnitine concentrations reported in this study was assessed in healthy adults after biotin loading and washout; on day 0, plasma 3HIA-carnitine concentrations ranged from 0.002 to 0.008 μmol/L. Even by day 28, when the subjects were marginally biotin deficient, plasma 3HIA-carnitine concentrations ranged from 0.012 to 0.038 μmol/L; these values would not have been deemed abnormal by inborn-error screening criteria. The differences in the apparent normal range may also have arisen from the following additional factors: 1) the subjects were loaded and washed out to ensure biotin sufficiency; 2) healthy adults may not be metabolically similar to newborns, whose blood samples were drawn in close proximity to the birth process; and 3) there may be important differences in the analytic methods (particularly the use in this study of an authentic deuterated 3HIA-carnitine as the internal standard).
This study has several important limitations. The confidence in any generalization of conclusions concerning diagnostic sensitivity to larger populations is limited by the small total number of subjects studied. Likewise, the number of subjects for each of the normal ranges was small from a statistical-sampling standpoint. Moreover, the 3HIA-carnitine data from this study provided no evidence concerning diagnostic specificity in other clinical situations. Whether abnormally increased plasma concentrations of 3HIA-carnitine will be observed in other clinical situations in which biotin status is not impaired remains unknown. For example, both heterozygous MCC deficiency and genetic deficiencies of enzymes catalyzing steps in the catabolism of leucine distal to the MCC step might well cause increased plasma concentrations of 3HIA-carnitine. In this study, plasma 3HIA-carnitine concentrations were only measured in the fasted state; the effect of postprandial status must be assessed to judge utility for population studies. Moreover, all subjects were well nourished and had normal protein intake during the study, and therefore, they were likely carnitine sufficient. To our knowledge, the effect of mild to moderate carnitine deficiency on plasma concentrations of 3HIA-carnitine is not known.
In conclusion, the plasma 3HIA-carnitine concentration appears to be a sensitive, practical indicator of marginal biotin deficiency that has advantages relative to currently validated indexes of biotin status, especially PCC activity. The application of the LC-MS/MS method used in the present study to measure 3HIA-carnitine concentrations potentially allows for the assessment of marginal biotin deficiency in humans subjected to a variety of phenotypical factors that can cause marginal biotin deficiency. The determination of specificity and robustness awaits application in future, larger population studies.
Acknowledgments
We thank Kim Stuckey, Jeanne Poppelreiter, Melain Raguseo, Mike Ruckle, and Teresa Evans for study coordination and laboratory technical work; Teresa Evans for measurements of urinary excretions of 3-HIA; and Suzanne Owen and Lindsay Pack for technical assistance in the method development and measurement of 3HIA-carnitine concentrations.
The authors’ responsibilities were as follows—DMM, JHM, and TDH: designed the research; SLS, TDH, AB, NIM, CLH, and DMM: conducted the research; JHM: provided essential equipment for the research; SLS and HJS: analyzed data; SLS and DMM: wrote the manuscript; DMM: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Mock DM, Stadler DD. Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr 1997;16:252–7 [DOI] [PubMed] [Google Scholar]
- 2.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr 1997;127:710–6 [DOI] [PubMed] [Google Scholar]
- 3.Velázquez A, Martín-del-Campo C, Báez A, et al. Biotin deficiency in protein-energy malnutrition. Eur J Clin Nutr 1989;43:169–73 [PubMed] [Google Scholar]
- 4.Krause K-H, Berlit P, Bonjour J-P. Impaired biotin status in anticonvulsant therapy. Ann Neurol 1982;12:485–6 [DOI] [PubMed] [Google Scholar]
- 5.Krause K-H, Berlit P, Bonjour J-P. Vitamin status in patients on chronic anticonvulsant therapy. Int J Vitam Nutr Res 1982;52:375–85 [PubMed] [Google Scholar]
- 6.Krause K-H, Kochen W, Berlit P, Bonjour J-P. Excretion of organic acids associated with biotin deficiency in chronic anticonvulsant therapy. Int J Vitam Nutr Res 1984;54:217–22 [PubMed] [Google Scholar]
- 7.Mock DM, Dyken ME. Biotin catabolism is accelerated in adults receiving long-term therapy with anticonvulsants. Neurology 1997;49:1444–7 [DOI] [PubMed] [Google Scholar]
- 8.Mock DM, Mock NI, Lombard KA, Nelson RP. Disturbances in biotin metabolism in children undergoing long-term anticonvulsant therapy. J Pediatr Gastroenterol Nutr 1998;26:245–50 [DOI] [PubMed] [Google Scholar]
- 9.Said HM. Biotin bioavailability and estimated average requirement: why bother? Am J Clin Nutr 1999;69:352–3 [DOI] [PubMed] [Google Scholar]
- 10.Roschinger W, Millington DS, Gage DA, et al. 3-Hydroxyisovalerylcarnitine in patients with deficiency of 3-methylcrotonyl CoA carboxylase. Clin Chim Acta 1995;240:35–51 [DOI] [PubMed] [Google Scholar]
- 11.Van Hove JL, Josefsberg S, Freehauf C, et al. Management of a patient with holocarboxylase synthetase deficiency. Mol Genet Metab 2008;95:201–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf B. Disorders of biotin metabolism Scriver CR, Beaudet AL, Sly WS, Valle D, eds The metabolic and molecular basis of inherited disease. 8th ed.New York, NY: McGraw-Hill Inc, 2001:3151–77 [Google Scholar]
- 13.Sweetman L, Williams JC. Branched chain organic acidurias. Scriver CR, Beaudet AL, Sly WS, Valle D, The metabolic and molecular basis of inherited disease. 7th ed.New York, NY: McGraw-Hill Inc, 1995:1387–422 [Google Scholar]
- 14.Zammit VA, Ramsay RR, Bonomini M, Arduini A. Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev 2009;61:1353–62 [DOI] [PubMed] [Google Scholar]
- 15.Pande SV. A mitochondrial carnitine acylcarnitine translocase system. Proc Natl Acad Sci USA 1975;72:883–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased status in experimental biotin deficiency. Am J Clin Nutr 1997;65:951–8 [DOI] [PubMed] [Google Scholar]
- 17.Mock NI, Mock DM. Use of an improved method for determination of urinary 3-hydroxyisovaleric acid allows earlier detection of biotin deficiency in the rat. Clin Res 1989;37:955A (abstr). [Google Scholar]
- 18.Horvath TD, Stratton SL, Bogusiewicz A, Pack L, Moran J, Mock DM. Quantitative measurement of plasma 3-hydroxyisovaleryl carnitine by LC-MS/MS as a novel biomarker of biotin status in humans. Anal Chem 2010;82:4140–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am J Clin Nutr 2002;76:1061–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr 2002;75:295–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council Dietary reference intakes tables—the complete set Food and Nutrition Board Institute of Medicine, Dietary reference intakes. Washington, DC: The National Academies, 2000. Available from: www.iom.edu/Reports/2000/Dietary-Reference-Intakes-for-Thiamin-Riboflavin-Niacin-Vitamin-B6-Folate-Vitamin-B12-Pantothenic-Acid-Biotin-and-Choline.aspx (cited 10 September 2010) [Google Scholar]
- 22.Mock DM, Henrich CL, Carnell N, Mock NI, Swift L. Lymphocyte propionyl-CoA carboxylase and accumulation of odd-chain fatty acid in plasma and erythrocytes are useful indicators of marginal biotin deficiency. J Nutr Biochem 2002;13:462–70 [DOI] [PubMed] [Google Scholar]
- 23.Stratton SL, Bogusiewicz A, Mock MM, Mock NI, Wells AM, Mock DM. Lymphocyte propionyl-CoA carboxylase and its activation by biotin are sensitive indicators of marginal biotin deficiency in humans. Am J Clin Nutr 2006;84:384–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mock DM, Mock NI. Lymphocyte propionyl-CoA carboxylase activity is an early and sensitive indicator of biotin deficiency in rats, but urinary excretion of 3-hydroxyisopropionic acid is not. J Nutr 2002;132:1945–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr 2003;133:2519–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sealey W, Stratton SL, Hansen DK, Mock DM. Marginal maternal biotin deficiency in CD-1 mice reduces fetal mass of biotin-dependent carboxylases. J Nutr 2005;135:973–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in the mouse. J Nutr 2009;139:154–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zempleni J, Mock D. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med 2000;223:14–21 [DOI] [PubMed] [Google Scholar]
- 29.Velazquez A, Teran M, Baez A, Gutierrez J, Rodriguez R. Biotin supplementation affects lymphocyte carboxylases and plasma biotin in severe protein-energy malnutrition. Am J Clin Nutr 1995;61:385–91 [DOI] [PubMed] [Google Scholar]
- 30.Sealey WM, Teague AM, Stratton SL, Mock DM. Smoking accelerates biotin catabolism in women. Am J Clin Nutr 2004;80:932–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strnadova KA, Holub M, Muhl A, et al. Long-term stability of amino acids and acylcarnitines in dried blood spots. Clin Chem 2007;53:717–22 [DOI] [PubMed] [Google Scholar]
- 32.Shigematsu Y, Bykov IL, Liu YY, et al. Acylcarnitine profile in tissues and body fluids of biotin-deficient rats with and without L-carnitine supplementation. J Inherit Metab Dis 1994;17:678–90 [DOI] [PubMed] [Google Scholar]
- 33.Pasquali M, Monsen G, Richardson L, Alston M, Longo N. Biochemical findings in common inborn errors of metabolism. Am J Med Genet C Semin Med Genet 2006;142C:64–76 [DOI] [PubMed] [Google Scholar]
- 34.Costa CG, Struys EA, Bootsma A, et al. Quantitative analysis of plasma acylcarnitines using gas chromatography chemical ionization mass fragmentography. J Lipid Res 1997;38:173–82 [PubMed] [Google Scholar]
- 35.Mardach R, Zempleni J, Wolf B, et al. Biotin dependency due to a defect in biotin transport. J Clin Invest 2002;109:1617–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ficicioglu C, Payan I. 3-Methylcrotonyl-CoA carboxylase deficiency: metabolic decompensation in a noncompliant child detected through newborn screening. Pediatrics 2006;118:2555–6 [DOI] [PubMed] [Google Scholar]