Abstract
Background: Recently, a genetic variant (rs738409; C→G) of the PNPLA3 gene was identified to be associated with increased hepatic fat deposition, and the effect was more pronounced in Hispanics. Animal models have also shown that PNPLA3 expression can be regulated by dietary carbohydrate.
Objective: The aim of this study was to examine whether the influence of PNPLA3 genotype on hepatic fat is modulated by dietary factors in Hispanic children.
Design: PNPLA3 was genotyped in 153 Hispanic children (75% female, ages 8–18 y) by using the TaqMan method. Dietary intake was assessed by using three 24-h dietary recalls or diet records. Visceral adipose tissue (VAT), subcutaneous abdominal adipose tissue (SAAT), and hepatic fat fraction (HFF) were assessed in multiple abdominal slices by magnetic resonance imaging. Analysis of covariance was used to assess the diet × genotype interaction in liver fat, with the following a priori covariates: sex, age, energy, VAT, and SAAT.
Results: HFF was influenced by a significant interaction between genotype and diet (genotype × carbohydrate, P = 0.04; genotype × total sugar, P = 0.01). HFF was positively related to carbohydrate (r = 0.31, P = 0.04) and total sugar (r = 0.34, P = 0.02) intakes but only in the GG group, independent of covariates. Dietary variables were not related to HFF in the CC or CG group or to other fat depots in all genotype groups.
Conclusions: These findings suggest that Hispanic children carrying the GG genotype are susceptible to increased hepatic fat when dietary carbohydrate intake, specifically sugar, is high. Specific dietary interventions based on genetic predisposition in this population may lead to more effective therapeutic outcomes for fatty liver. This trial was registered at clinicaltrials.gov as NCT00697580, 195-1642394A1, and NCT00693511.
INTRODUCTION
Hispanics are particularly susceptible to the accumulation of fat in the liver, in part because of genetic predisposition. Recent reports suggest that nearly 4 of 10 obese Hispanic children and adolescents have nonalcoholic fatty liver disease (NAFLD)—a condition that can lead to cirrhosis, liver disease, and eventually liver cancer (1). A recent genome-wide association study in adults identified a novel genetic factor that explains some of the striking ethnic differences in liver fat accumulation (2). An amino acid substitution (I148M; C→G; rs738409) in the patatin-like phospholipase domain containing 3 (PNPLA3) gene was associated with a >2-fold higher liver fat content in adults, with the strongest effect observed in Hispanics, in whom the frequency of the variant was much higher (49%) than in whites (23%) or African Americans (17%). Importantly, these associations were not confounded by genetic admixture, which supports the consistent effects observed across populations. We recently validated the prevalence and effect of this genotype on liver fat accumulation in Hispanic children and adolescents (3). Genotype distributions were significantly different between Hispanics and African Americans, which is consistent with previous reports (4). For example, the liver fat fraction in Hispanic subjects with the GG genotype was 1.68 and 2.36 times higher than that in those with the GC or CC genotype, respectively, and this effect was observed even in the youngest children (age 8–10 y).
The function of the PNPLA3 gene is not entirely known but has been reported to have lipase-like activity and to promote triglyceride hydrolysis in the liver (5). A recent animal study has also shown that expression levels of hepatic PNPLA3 mRNA is low during fasting and increases ≈90 fold in response to carbohydrate feeding (6). This effect occurs as a secondary effect of insulin-mediated up-regulation of sterol regulatory element binding protein 1 (SREBP-1) and liver X receptor, which are major transcription factors responsible for fat metabolism in the liver (6). Whereas this effect would presumably not differ across genotypes, recent studies have shown that the G allele, which encodes a methionine substitution at position 148, abolishes PNPLA3 activity. As a consequence, GG subjects could be more susceptible to the effects of dietary sugar because transcriptional up-regulation of PNPLA3 would still result in a protein with severely reduced function and therefore reduced hydrolysis of hepatic triglycerides (6). Thus, the purpose of this study was to examine whether the effects of PNPLA3 genotype on liver fat in Hispanic children might be exacerbated under conditions of a high carbohydrate/sugar intake. On the basis of our previous observations, we hypothesized that sugar intake will be more related to liver fat accumulation in subjects with the GG genotype than in those with the GC and CC genotype.
SUBJECTS AND METHODS
Participants
For this study we pooled subjects from a variety of studies using essentially identical protocols and measures conducted by the University of Southern California Childhood Obesity Research Center over the past 6 y. In brief, subjects were recruited from schools, community centers, health clinics, health fairs via word of mouth, flyers/brochures, and in-person contact. These subjects include 153 Hispanic children and adolescents (ages 8–18 y) for whom complete genotype, visceral, subcutaneous, and liver fat, and dietary data were available. All subjects were of Hispanic ancestry (all 4 grandparents of Hispanic origin as determined by parental self-report). Subjects were ineligible if they were taking medications known to affect body composition, had syndromes or diseases known to affect body composition or fat distribution, had any major illness since birth, or had participated in structured exercise, nutrition, or a weight-loss program within the 6 mo before recruitment. Data from some of these subjects was previously reported (7–9), but this study is the first to examine the relation between diet and fat depots among PNPLA3 genotypes. The Institutional Review Board of the University of Southern California, Health Sciences Campus, approved this study. Informed written consent and assent were obtained from both parents and child before testing commenced.
Adiposity measures
Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, by using a beam medical scale and wall-mounted stadiometer; body mass index (BMI) and BMI percentiles for age and sex were determined by using EpiInfo 2000 (version 1.1, 2001; Centers for Disease Control and Prevention, Atlanta, GA) (10). Total fat mass and total lean mass were measured by dual-energy X-ray absorptiometry by using a Hologic QDR 4500W (Hologic, Bedford, MA). Abdominal fat distribution was measured by magnetic resonance imaging (MRI) on a Siemens (Malvern, PA) 1.5-Tesla magnet. Slices were acquired by using a 420-mm field of view (FOV) and FOV phase of 75%. Three abdominal scans were performed consecutively, and the total acquisition time was 24 s per total abdominal scan. Each scan obtained 19 axial images of the abdomen with a thickness of 10 mm. After image acquisition, subcutaneous abdominal adipose tissue (SAAT) and visceral adipose tissue (VAT) were segmented by using image analysis software (SliceOmatic Tomovision, Montreal, Canada) at the Image Reading Center (New York, NY). SAAT and VAT volumes were calculated from these images as previously described (11). Hepatic fat fraction (HFF) was assessed during the same MRI test by using a modification of the Dixon 3-point technique. Quantitative corrections for the influence of T2 decay on the fat fraction estimates are taken into account by the third dual-echo sequence, where T2 is estimated on a pixel-by-pixel basis. Because the fat fraction was estimated from low flip-angle images (20°), the effect of T1 relaxation on the quantification was minimized.
Genotyping
A fasting blood sample was obtained after an overnight fast (nothing to eat or drink after 2000) for DNA isolation. Genotyping of SNP rs738409 in PNPLA3 was performed on all subjects at the same time by using the Applied Biosystems Inc (ABI) TaqMan system (12, 13) with an assay available through ABI's “Assays on Demand” database. Our overall genotype call rate was 97%, and we obtained 100% genotype concordance of 4 control DNA samples that were included on each DNA plate. Genotype frequencies did not deviate from that expected under Hardy-Weinberg equilibrium (P = 0.92).
Dietary intake
Dietary intake was assessed by either three 24-h dietary recalls (n = 30) by using the multiple-pass technique or by 3-d diet records (2 weekdays, 1 weekend day; n = 123). No difference in energy or macronutrients was observed between subjects who had dietary recalls and those with diet records. All recalls or records were administered or clarified by a bilingual dietary technician in person or via phone. Nutrition data were analyzed by using the Nutrition Data System for Research (NDS-R version 5.0_35), a software program developed by the University of Minnesota. The NDS-R program calculated key dietary variables for this analysis, including total sugars, dietary fiber, added sugar, glycemic index, and glycemic load (by using both the standard glucose and the white bread reference). Added sugars were defined as only those sugars/syrups added to foods during food preparation or commercial food processing (eg, high-fructose corn syrup) but not naturally occurring sugars such as lactose and fructose (14). The dietary data were carefully screened for plausibility through a multistep process. Data were first screened by evaluating the participants' comments; 4 subjects were excluded because they reported an illness. The dietary data were also examined for plausibility of caloric intake by assessing the distribution of the residuals of the linear regression of caloric intake by body weight at baseline. None of the participants had a residual that was >2 SDs from the mean; thus, 153 participants were included in the present analyses.
Statistical procedures
Data were examined for normality, and transformations were made if data were found to be significantly different from normal. The following outcome variables were nonnormally distributed and log-transformed before analysis: total dual-energy X-ray absorptiometry fat, VAT, SAAT, and HFF. However, we present nontransformed values in the tables and figures for ease of interpretation. For the preliminary analyses, analysis of variance with post hoc pairwise comparisons with Bonferroni adjustments and chi-square tests (for sex only) were used to assess differences in physical and adiposity measures and dietary variables between genotypes. Several subjects had extremely high amounts of liver fat (>20%), and analyses were run with and without these outliers. A linear regression was used to assess whether there was a diet × genotype interaction on the various fat depots (VAT, SAAT, and HFF) as continuous variables. We first computed the diet × genotype (GG, CG, and CC) interaction for all dietary variables of interest (energy, macronutrients, sugar, fiber, glycemic index, and glycemic load) by simply multiplying the genotype group by the dietary variable. The dependent variable was the fat depot, and the independent factor was the diet × genotype interaction. In all models, the following a priori covariates were included: sex, age, energy, BMI, the diet variable of interest, VAT (when SAAT and HFF were dependent variables), and SAAT (when VAT was a dependent variable). If there was a significant diet × gene interaction, partial correlations were performed to assess the relation between the diet variable and the adiposity measures. Scatter plots were then produced to display these significant relations. All analyses were performed by using SPSS version 16.0 (SPSS, Chicago, IL), and the significance level was set at P < 0.05.
RESULTS
The physical and adiposity measures between genotype groups are shown in Table 1. Aside from increased hepatic fat in the GG group that we reported recently (3), no significant differences were observed between genotype groups for any of the other variables. Dietary variables between genotypes are shown in Table 2. The GG group consumed 20% less protein (g/d) than did the CG group (P = 0.03). The CG group also consumed 16%, 16%, and 18% less total, insoluble, and soluble fiber (g/1000 kcal), respectively, than did the CC group (P < 0.05). A trend for the GG group to consume 11% and 14% less energy than the CC and CG groups was observed. Carbohydrate and sugar intake was similar between genotype groups.
TABLE 1.
Physical and metabolic characteristics of subjects by genotype (n = 153)1
| CC (n = 38) | CG (n = 70) | GG (n = 45) | P value2 | |
| Sex (n) | 0.42 | |||
| Male | 6 | 22 | 11 | |
| Female | 32 | 48 | 34 | |
| Age (y) | 13.3 ± 3.03 | 14.1 ± 2.9 | 14.0 ± 2.9 | 0.32 |
| Weight (kg) | 75.9 ± 33.8 | 77.9 ± 28.7 | 81.5 ± 31.8 | 0.74 |
| BMI (kg/m2) | 30.7 ± 8.6 | 31.4 ± 7.5 | 32.5 ± 8.6 | 0.61 |
| BMI percentile | 91.7 ± 14.7 | 94.7 ± 9.4 | 95.7 ± 6.4 | 0.50 |
| BMI z score | 1.8 ± 0.8 | 2.0 ± 0.6 | 2.0 ± 0.6 | 0.22 |
| Total fat mass (kg) | 30.3 ± 15.1 | 30.4 ± 11.6 | 31.8 ± 14.9 | 0.87 |
| Total lean mass (kg)4 | 45.0 ± 15.1 | 44.7 ± 14.7 | 45.5 ± 15.3 | 0.98 |
| VAT (L) | 1.6 ± 1.0 | 1.7 ± 1.3 | 1.8 ± 1.0 | 0.89 |
| SAAT (L) | 9.1 ± 5.2 | 10.0 ± 6.2 | 10.3 ± 6.8 | 0.63 |
| Hepatic fat (%) | 4.5 ± 4.1a | 5.9 ± 5.8a | 9.7 ± 8.10b | <0.001 |
VAT, visceral adipose tissue; SAAT, subcutaneous abdominal adipose tissue. Values with different superscript letters are significantly different, P < 0.001 (Bonferroni multiple comparisons).
ANOVAs with Bonferonni adjustments and chi-square tests (for sex only) were used to determine significant differences between genotype groups. The P values represent the overall significance between genotype groups.
Mean ± SD (all such values).
Does not include bone mineral content.
TABLE 2.
Dietary characteristics of subjects by genotype (n = 153)1
| Nutrients | CC (n = 38) | CG (n = 70) | GG (n = 45) | P value2 |
| Energy (kcal) | 1801.2 ± 575.4 | 1850.6 ± 544.8 | 1599.4 ± 463.9 | 0.06 |
| Protein (g/d) | 69.5 ± 22.6a,b | 74.8 ± 25.5a | 62.3 ± 20.1b | 0.03 |
| Protein (% of energy) | 15.6 ± 3.0 | 16.4 ± 3.6 | 15.8 ± 3.3 | 0.45 |
| Fat (g/d) | 67.0 ± 27.9 | 69.6 ± 26.3 | 58.3 ± 21.7 | 0.09 |
| Fat (% of energy) | 31.8 ± 6.0 | 32.5 ± 6.1 | 31.3 ± 6.7 | 0.57 |
| Carbohydrates (g/d) | 236.6 ± 76.4 | 235.6 ± 74.2 | 211.4 ± 71.2 | 0.21 |
| Carbohydrates (% of energy) | 53.0 ± 7.1 | 51.3 ± 6.8 | 53.3 ± 8.5 | 0.29 |
| Total sugar (g/d) | 112.0 ± 48.0 | 104.3 ± 42.8 | 96.1 ± 44.8 | 0.28 |
| Total sugar (% of energy) | 24.7 ± 6.6 | 22.7 ± 6.7 | 23.7 ± 7.5 | 0.34 |
| Added sugar (g/d) | 73.8 ± 44.6 | 67.5 ± 38.9 | 69.5 ± 47.5 | 0.75 |
| Added sugar (% of energy) | 15.9 ± 7.4 | 14.6 ± 6.8 | 16.5 ± 8.9 | 0.41 |
| Dietary fiber (g/1000 kcal) | 9.1 ± 3.5a | 7.6 ± 2.4b | 8.1 ± 3.4a,b | 0.03 |
| Insoluble fiber (g/1000 kcal) | 6.2 ± 2.5a | 5.1 ± 1.9b | 5.5 ± 2.7a,b | 0.05 |
| Soluble fiber (g/1000 kcal) | 2.8 ± 1.1a | 2.3 ± 0.7b | 2.4 ± 1.0a,b | 0.02 |
| Glycemic index3 | 59.2 ± 3.7 | 59.0 ± 3.9 | 59.7 ± 4.7 | 0.45 |
| Glycemic load3 | 131.6 ± 45.8 | 131.5 ± 41.9 | 119.2 ± 45.5 | 0.33 |
All values are means ± SDs. Values with different superscript letters are significantly different, P < 0.05 (Bonferroni multiple comparisons).
ANOVAs with Bonferonni adjustments were used to determine significant differences between genotype groups. The P values represent the overall significance between genotype groups.
Calculated by using glucose as the reference.
A significant carbohydrate (% of energy/d) × genotype interaction on HFF (P = 0.04) was observed, independent of sex, age, BMI, and VAT. The relation between hepatic fat and total carbohydrate for each genotype is shown in Figure 1. Hepatic fat was positively related to carbohydrate (r = 0.38, P = 0.02) only in the GG group, independent of covariates. Carbohydrate intake was not related to hepatic fat in the CC or CG group. The results were essentially the same when carbohydrate was expressed as g/d and energy intake was included as a covariate. The results were also similar after exclusion of subjects who were outliers for hepatic fat or carbohydrate intake.
FIGURE 1.
Relation between liver fat and carbohydrate (CHO) intakes for each of the PNPLA3 genotypes. There was a significant CHO × genotype interaction on hepatic fat fraction (P = 0.04), independent of sex, age, BMI, and visceral adipose tissue. Liver fat was positively related to CHO (r = 0.38, P = 0.02) only in the GG group, independent of sex, age, BMI, and visceral adipose tissue. CHO intake was not related to liver fat in the CC or CG group.
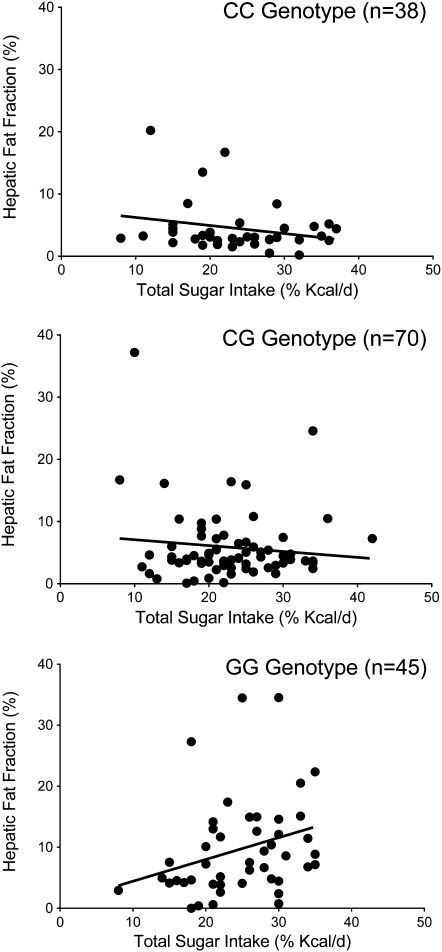
A significant total sugar (% of energy/d) × genotype interaction on HFF was observed (P = 0.01), independent of the covariates listed above. The relation between hepatic fat and total sugar for each genotype is shown in Figure 2. Hepatic fat was positively related to total sugar intake (r = 0.33, P = 0.04) only in the GG group, independent of covariates and unrelated to liver fat in the CC or CG group. A trend for an added sugar (% of energy/d) × genotype interaction on HFF was also observed (P = 0.06). However, when relation within each genotype group was examined, hepatic fat was positively related to added sugar in the GG group, but the trend was attenuated (r = 0.25, P = 0.12) and negatively related in the CC group (r = −0.28, P = 0.12). The results were essentially the same when total and added sugar intake was expressed as g/d and energy intake was included as a covariate. In addition, exclusion of outliners for hepatic fat and dietary sugar did not change the results. No other diet × genotype interactions were significant for hepatic fat or for any of the other adiposity measures (ie, total fat, VAT, or SAAT), and no dietary variable was related to adiposity measures overall, when not split by genotype group.
FIGURE 2.
Relation between liver fat and total sugar intake for each of the PNPLA3 genotypes. There was a significant total sugar × genotype interaction on hepatic fat fraction (P = 0.01), independent of sex, age, BMI, and visceral adipose tissue. Liver fat was positively related to total sugar intake (r = 0.33, P = 0.04) only in the GG group, independent of sex, age, BMI, and visceral adipose tissue. Total sugar intake was not related to liver fat in the CC or CG group.
DISCUSSION
To our knowledge, this was the first study to assess the effect of interactions between dietary intake and PNPLA3 gene variants on hepatic fat accumulation, particularly in a Hispanic pediatric population. We showed a positive association between carbohydrate intake, specifically total and added sugars, and hepatic fat in the GG group only. No other diet × genotype interactions were present, nor were any dietary variables related to hepatic fat overall. These novel findings highlight the importance of examining this data by genotype and suggest that obese Hispanic children with the GG genotype have an increased capacity for fat storage and decreased hepatic lipid mobilization in the context of diets high in sugar.
In the past decade, the decline in dietary carbohydrate quality has been identified as a key factor leading to increased adiposity and metabolic disorders among children and adults. In numerous studies conducted in Hispanic youth (10–17 y of age) by our group, we showed that increased sugar intake is associated with increased adiposity and decreased insulin secretion and β cell function (15, 16). We have consistently shown that sugar intake is relatively high in Hispanic youth living in Los Angeles (>100 g/d) and accounts for nearly 50% of the overall daily carbohydrate intake and 25% of the energy intake. Whereas a link between high sugar intake and excessive liver fat in Hispanic children is not unexpected, our observation that this relation was only found in participants with the GG genotype is of particular interest and has important implications for strategies to treat NAFLD.
Human and animal studies have consistently shown that the PNPLA3 gene is highly responsive to changes in energy balance (17), ie, it is down-regulated by fasting and dramatically up-regulated by refeeding (18, 19). One study found that mice fed a high-fat diet had a 23-fold increase in the expression level of PNPLA3, which was reversed under fasting conditions (19). Another recent animal study showed that hepatic PNPLA3 mRNA levels increased ≈90 fold in response to carbohydrate feeding through insulin-mediated stimulation of transcriptional regulatory networks involving SREBP-1 and liver X receptor (6). Carbohydrate, specifically fructose, is a key player in liver fat deposition because it activates SREBP-1, which stimulates hepatic de novo lipogenesis and inhibits hepatic fatty acid oxidation (20, 21). Therefore, our results are consistent with the notion that the reduced capacity of subjects with the GG genotype to hydrolyze triglycerides in the liver would be exacerbated in the context of high dietary sugar because carbohydrate-mediated up-regulation of PNPLA3 with a methionine substitution at position 148 would still lead to an enzyme with severely reduced activity.
Although, this study reports on the relation between nutrient intake and hepatic fat among genotype groups, we also assessed the relation between hepatic fat and different types of sugar (ie, glucose, fructose, sucrose, lactose, and maltose) and food and beverage servings per day (ie, meat, dairy products, fruit, vegetable, fat, grains, refined grains/breads, whole-wheat grains/breads, sugar-sweetened beverages, and sweets). Whereas total carbohydrate and total and added sugar intake were positively related to hepatic fat among GG carriers, specific types of sugar or food or beverages (servings/d) were not. One explanation for these findings may be that the intake of carbohydrate and total and added sugar made up a larger percentage of daily calories (≈50% and 25%) compared with specific types of sugars and/or food and beverage groups, which were present in much smaller proportions. Thus, detection of significant nutrigenetic relations with these specific dietary variables and hepatic fat accumulation would be more difficult and require larger sample sizes. In addition, determination of the fructose intake, specifically that coming from high-fructose corn syrup, is very difficult to estimate with the use of dietary recalls/records and available nutrition software programs. Nonetheless, our results suggest that high intakes of any type of dietary sugar lead to excessive liver fat accumulation in carriers of the GG genotype.
Several limitations of our study need to be noted. Because this study was cross-sectional, a cause and effect relation between dietary intake and hepatic fat between the genotypes cannot be made. An efficacy intervention trial testing the effects of reducing carbohydrates, specifically sugars, in subjects with different variants of the PNPLA3 gene may thus help to determine causality. The current study is also limited by the use of either dietary recalls or records, which rely solely on the participants' self-report and are often prone to errors. However, several steps were taken to ensure the accuracy of dietary data, such as using well-trained diet technicians to clarify records or collect recalls, screening for participants' comments, and assessing the plausibility of caloric intake by body weight. In addition, the literature suggests that overweight children underreport dietary intake to a greater extent than do lean children (22). However, because most of our subjects were overweight (n = 133, or 87%), we would expect a similar degree of underreporting across the whole sample. A review study by McPherson et al (23) on dietary assessment methods among school-age children, including Hispanic children, showed that multiple recalls and records agreed more with the validation standards than did the FFQ or dietary screens. Several studies also showed that dietary recalls and records had excellent agreement on both food and nutrition information (24, 25). Another potential limitation was that most of our study subjects were female (n = 114, or 75%), which we addressed by controlling for sex in all analyses. Additional studies in larger samples that include more males, leaner subjects and/or other ethnicities are warranted to further understand the relation between PNPLA3, dietary nutrients, and liver fat accumulation.
These findings have potentially important clinical implications. Given that our data show that 38% of obese Hispanic children and adolescents have a liver fat content >5.5% by MRI, which likely indicates NAFLD (3), it is crucial to understand the causes of hepatic triglyceride accumulation and how to prevent or reverse it in children. Numerous studies have shown that reducing weight by ≥5%, primarily through caloric restriction and exercise, has beneficial effects on NAFLD, as evidenced by a concomitant 30–60% reduction of liver fat (26–28). Thus, aggressive strategies aimed at weight loss would perhaps be the obvious approach for addressing NAFLD. However, in our ongoing experience with interventions in Hispanic children and adolescents, this weight loss goal is difficult to achieve (11, 29) and even more difficult to maintain for extended periods of time. There is therefore a great need to identify novel and more sustainable strategies that can address this problem based on the mechanism of liver fat accumulation, especially in individuals with a genetic predisposition.
In conclusion, these findings suggest that personalized dietary interventions based on reductions in dietary sugar intake in genetically predisposed individuals with the GG genotype may lead to more effective therapeutic outcomes for fatty liver. Interventions focused on reducing sugar intake could have immediate potential for clinical translation and long-term sustainability, particularly for genetically predisposed individuals in the Hispanic population.
Acknowledgments
We thank Mark Punyanita, Director of the Image Reading Center in New York, who processed and read all of our images; Sherryl Esplana, project coordinator and responsible for organizing the MRI files; and the study participants and their families for their involvement.
The authors' responsibilities were as follows—JND: was responsible for the study design, statistical analysis, and manuscript preparation; K-AL: assisted with the data evaluation and manuscript preparation; RWW and SV: assisted with data processing, analysis, and manuscript preparation; DS-M and MJW: assisted with evaluation and interpretation of the data and manuscript preparation; HA (Director of the Molecular Genetics Laboratory, where the genetic analyses were performed): assisted with the study design, statistical analyses, interpretation of the results, and manuscript preparation; MIG (Director of the Childhood Obesity Research Center): assisted with the study design, statistical analyses, interpretation of the results, and manuscript preparation; and JND, DS-M, MJW, and MIG: were principal investigators on studies whose subjects were used in this analysis. None of the authors had any financial or personal conflicts of interest.
REFERENCES
- 1.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 2010;5:145–71 [DOI] [PubMed] [Google Scholar]
- 2.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goran MI, Walker R, Le KA, et al. Effects of PNPLA3 on liver fat and metabolic profile in hispanic children and adolescents. Diabetes (Epub ahead of print 17 September 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–93 [DOI] [PubMed] [Google Scholar]
- 5.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820–32 [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, He S, Li JZ, et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci USA 2010;107:7892–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goran MI, Bergman R, Avilla Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history of type 2 diabetes. J Clin Endocrinol Metab 2004;89:207–12 [DOI] [PubMed] [Google Scholar]
- 8.Cruz ML, Weigensberg MJ, Huang T, Ball GDC, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab 2004;89:108–13 [DOI] [PubMed] [Google Scholar]
- 9.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care 2005;28:2519–24 [DOI] [PubMed] [Google Scholar]
- 10.CDC. Behavioral Risk Factor Surveillance System Obesity and diabetes prevalence among U.S. adults, by selected characteristics. Atlanta, GA: Centers for Disease Control and Prevention, 2001 [Google Scholar]
- 11.Davis JN, Kelly LA, Lane CJ, et al. Randomized control trial to reduce obesity related diseases in overweight Latino adolescents. Obesity (Silver Spring) 2009;17:1542–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ. SNP genotyping by the 5′-nuclease reaction. Methods Mol Biol 2003;212:129–47 [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 1999;14:143–9 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services, US Department of Agriculture Dietary guidelines for Americans. 2005. Available from: www.healthierus.gov/dietaryguidelines (cited 2 January 2007)
- 15.Davis JN, Ventura E, Weigensberg M, et al. The relation of sugar intake to beta-cell function in overweight Latino children. Am J Clin Nutr 2005;82:1004–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JN, Alexander KE, Ventura EE, et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am J Clin Nutr 2007;86:1331–8 [DOI] [PubMed] [Google Scholar]
- 17.Liu YM, Moldes M, Bastard JP, et al. Adiponutrin: a new gene regulated by energy balance in human adipose tissue. J Clin Endocrinol Metab 2004;89:2684–9 [DOI] [PubMed] [Google Scholar]
- 18.Johansson LE, Hoffstedt J, Parikh H, et al. Variation in the adiponutrin gene influences its expression and associates with obesity. Diabetes 2006;55:826–33 [DOI] [PubMed] [Google Scholar]
- 19.Hoekstra M, Li Z, Kruijt JK, Van Eck M, Van Berkel TJ, Kuiper J. The expression level of non-alcoholic fatty liver disease-related gene PNPLA3 in hepatocytes is highly influenced by hepatic lipid status. J Hepatol 2010;52:244–51 [DOI] [PubMed] [Google Scholar]
- 20.Le KA, Ith M, Kreis R, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 2009;89:1760–5 [DOI] [PubMed] [Google Scholar]
- 21.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005;54:1907–13 [DOI] [PubMed] [Google Scholar]
- 22.Rennie KL, Jebb SA, Wright A, Coward WA. Secular trends in under-reporting in young people. Br J Nutr 2005;93:241–7 [DOI] [PubMed] [Google Scholar]
- 23.McPherson R, Hoelscher D, Alexander M, Scanlon K, Serdula M. Dietary assessment methods among school-aged children: validity and reliability. Prev Med 2000;31:S11–33 [Google Scholar]
- 24.Scagliusi FB, Ferriolli E, Pfrimer K, et al. Underreporting of energy intake in Brazilian women varies according to dietary assessment: a cross-sectional study using doubly labeled water. J Am Diet Assoc 2008;108:2031–40 [DOI] [PubMed] [Google Scholar]
- 25.Krantzler NJ, Mullen BJ, Schutz HG, Grivetti LE, Holden CA, Meiselman HL. Validity of telephoned diet recalls and records for assessment of individual food intake. Am J Clin Nutr 1982;36:1234–42 [DOI] [PubMed] [Google Scholar]
- 26.Vitola BE, Deivanayagam S, Stein RI, et al. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity (Silver Spring) 2009;17:1744–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki A, Lindor K, St Saver J, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 2005;43:1060–6 [DOI] [PubMed] [Google Scholar]
- 28.Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 2009;58:1281–8 [DOI] [PubMed] [Google Scholar]
- 29.Davis JN, Ventura EE, Shaibi GQ, et al. Interventions for improving metabolic risk in overweight Latino youth. Int J Pediatr Obes 2010;5:451–5 [DOI] [PMC free article] [PubMed] [Google Scholar]