Abstract
Background. Dialysis patients and patients with chronic kidney disease (CKD) experience a substantial risk for abnormal autonomic function and abnormal heart rate variability (HRV). It remains unknown whether HRV changes across sleep stages in patients with different severity of CKD or dialysis dependency. We hypothesized that high-frequency (HF) HRV (vagal tone) will be attenuated from wakefulness to non-rapid eye movement (NREM) and then to rapid eye movement (REM) sleep in dialysis patients as compared to patients with CKD.
Methods. In-home polysomnography was performed in 95 patients with stages 4–5 CKD or end-stage renal disease (ESRD) on haemodialysis (HD) or peritoneal dialysis (PD). HRV was measured using fast Fourier transform of interbeat intervals during wakefulness and sleep. Low-frequency (LF) and HF intervals were generated. Natural logarithm HF (LNHF) and the logarithm LF/HF ratio (sympathovagal tone) were analysed by multivariable quantile regression and generalized estimating equations.
Results. Of the 95 patients, 63.2% (n = 60) was male, 35.8% (n = 34) was African American and 20.4% (n = 19) was diabetic. Average age was 51.6 ± 15.1 (range 19–82). HRV variables were significantly associated with diabetic status, higher periodic limb movement indices and lower bicarbonate levels. Patients with advanced CKD did not differ from dialysis patients in their inability to increase vagal tone during sleep. During wakefulness, female gender (P = 0.05) was associated with the increases in the vagal tone.
Conclusions. Patients with CKD/ESRD exhibit dysregulation of the autonomic nervous system tone manifesting as a failure to increase HRV during wakefulness and sleep. Different patient characteristics are associated with changes in HRV at different sleep stages.
Keywords: chronic kidney disease, haemodialysis, heart rate variability, peritoneal dialysis, polysomnography
Introduction
In healthy subjects, the normal heart rate is generated by action potentials in the cells of the sinoatrial node at a fairly constant frequency. This frequency is modulated by many factors that add variability to the heart signal at different frequencies throughout the day [1,2]. Low-frequency variability (LF; 0.04–0.15 Hz) reflects changes in cardiac sympathetic (and possibly parasympathetic) nerve activity [3,4], while high frequencies (HF; 0.15–0.4 Hz) are primarily modulated by cardiac nerve parasympathetic activity [5].
Sleep is intimately involved in the autonomic regulation of arterial pressure and heart rate. Past studies in healthy participants have shown that non-rapid eye movement (NREM) sleep is characterized by vagal (parasympathetic) predominance leading to reductions in arterial pressure and heart rate, while rapid eye movement (REM) sleep is associated with increased sympathetic activity [6,7] resulting in relative tachycardia and hypertension. Hence, cardiac vagal tone increases from wakefulness to NREM sleep [8,9], while sympathetic activity increases significantly during REM sleep [8] in the general population. These changes result in a high beat-to-beat variation in heart rate, which is considered a marker of a functionally efficient autonomic control. Abnormal fluctuations in the continuous cycling of NREM and REM sleep phases may be associated with marked changes in normal patterns of heart rate variability (HRV), with potential adverse consequences including hypertension, myocardial infarction and sudden cardiac death [7].
Despite the association of abnormal patterns of HRV with an increased risk of sudden cardiac death in cardiac disorders [ischaemia, chronic heart failure (CHF), valvular disease], stroke and diabetes [10–13], determinants of such patterns have not been established in patients with renal impairment. Previous studies that have directly assessed autonomic outflow (microneurography) have documented an abnormal sympathetic overactivation in awake subjects with renal dysfunction and end-stage renal disease (ESRD) [14,15]. It remains unknown whether vagal or sympathetic influences predominate across sleep stages in patients with chronic kidney disease (CKD) or ESRD with dialysis dependency. Furthermore, the impact of abnormal sleep patterns [16] and sleep disorders on HRV remains relatively underexplored in patients with renal dysfunction. Based on the underlying risk of sudden death in ESRD [17], incidence of cardiomyopathy in ESRD and dysautonomia [18], and influence of dialysis on HRV [19], we hypothesized that dialysis patients would have significantly less high-frequency HRV (reflecting vagal output) compared to CKD patients in wakefulness. Furthermore, we hypothesized that the expected increase in the HF component during the transition from wakefulness to NREM sleep will be attenuated in dialysis patients as compared to those with CKD stages 4 and 5. Finally, we examined the extent to which demographic characteristics, laboratory values, co-morbidity, use of medications and polysomnography (PSG) outcome variables were associated with decrements in HRV in our population of CKD and dialysis patients.
Materials and methods
Study population
Patients were enrolled from local dialysis units, outpatient nephrology clinics and the Thomas E. Starzl Transplant Institute in Western Pennsylvania between April 2004 and November 2006. Patients were eligible to participate if they were older than 18 years, had an estimated GFR <30 mL/min/1.73 m2 or were on maintenance dialysis (either haemodialysis, HD or peritoneal dialysis, PD). Patients were excluded from the study, if they had: craniofacial abnormalities, actively treated sleep apnoea, active malignancy, active infection (e.g. pneumonia), active coronary artery disease (CAD) (e.g. unstable angina, myocardial infarction within the last 6 months), advanced cirrhosis, active alcohol abuse, dementia or treatment-refractory psychiatric disease leading to inability to provide informed consent. All inclusion/exclusion criteria were confirmed by interview and ascertained by reviewing the patients' medical records. The study was approved by the University of Pittsburgh Investigation Review Board (IRB protocol numbers: 312047, 501068, 604042), and all participants provided informed consent.
Data collection and validation
Baseline data collection included a brief standardized health interview and a self-completed questionnaire, assessment of current use of beta-blockers and other medication use (anti-depressants, sleep-inducing agents, opioids), blood pressure and anthropometric measurements. The self-completed questionnaire (administered before or at the time of the baseline examination) provided information on age, gender, race and body mass index (BMI). For this report, race was categorized as African American or European American. Depression, diabetes and cardiovascular disease (CVD) were categorized as separate confounders. Laboratory data were collected from patient’s medical records, and the most recent values available (in the last 3 months) to the time of study were used. Such data included serum levels of haemoglobin, albumin, calcium, phosphorus and bicarbonate. Furthermore, serum levels of creatinine were collected for the CKD population in order to estimate the GFR, and the most recent Kt/V was obtained (an indicator of dialysis adequacy) for both dialysis groups from their dialysis records.
Selection of covariates
The selection of covariates was guided by factors found to be associated with changes on HRV in the general population and included: (i) Demographics: age, gender and race [20–22]; (ii) Anthropometrics: obesity [23]; (iii) Disease and dialysis treatment-related factors: a number of studies have examined clinical (mode of dialysis, delivered dose of dialysis) and laboratory (haemoglobin, serum albumin, serum creatinine, serum phosphorus and calcium, serum bicarbonate) factors [24]; (iv) Psychiatric disorders: Depression has also been inconsistently reported to be associated with an overall reduction in total HRV [25,26]; (v) Comorbidity: Diabetes has been linked to decreased HRV, while CVD morbidity and mortality have also been linked to decreased HRV [27,28]; (vi) Use of medications (beta-blockers, anti-depressants, sleep-inducing agents and opioids): Numerous large-scale randomized trials have shown that prolonged beta-blocker administration in chronic heart failure patients significantly improved their long-term prognosis, presumably due to a decrease in sympathetic activity and in HRV [29–32]. Antidepressant agents can cause electrophysiological changes of cardiac function leading to ventricular arrhythmias and sudden death. However, antidepressants have also protective effects on the heart through their capacity to modulate cardiac autonomic mediated physiological responses [33]; (vii) PSG outcome variables included [periodic limb movements index (PLMI), apnoea–hypopnoea index (AHI), arousal index (AI), total sleep time (TST), sleep efficiency, stages of sleep (stage 1, 2, 3 and 4, REM)]: Sleep apnoea may directly or indirectly (through sleep arousal) increase HRV [34]. In previous studies of patients without renal dysfunction, periodic limb movements have been associated with an excessive sleep fragmentation and abnormal HRV [35,36].
Sleep assessment and HRV measurement
One night of in-home PSG recording was performed using an ambulatory Compumedics Siesta monitor (Charlotte, North Carolina). Trained PSG technologists scored sleep records using standard sleep stage scoring criteria for each 20-s epoch, and standard definitions were used to identify apnoeas and hypopnoeas as previously described [37]. Each study was separated into stages of NREM and REM sleep. Four contiguous minutes of each study representing wakefulness, NREM sleep and REM sleep were analysed.
Frequency domain measures of HRV were calculated using fast Fourier transform-based power spectral analysis of interbeat intervals during sleep (Mindware®, Ghanna, OH) [38]. Each participant’s EKG record was visually inspected in 2-min epochs to identify ectopic beats and arrhythmias. If over nine arrhythmias or a 10-sec pause was noted in an epoch, the epoch was excluded. The entire record was excluded if there were nine or more arrhythmic beats in over 50% of the record. Power was calculated for the low-frequency (LF) band (0.04–0.15 Hz Eq) and the high-frequency (HF) band (0.15–0.40 Hz Eq). Analyses for this report focus on the HF band, as a marker of vagal (parasympathetic) activity, and the LF/HF ratio, as an indicator of sympathovagal tone. Both HRV variables were transformed prior to analyses (natural logarithm) due to skewness of the retrieved data and are presented as natural logarithm of HF (LNHF) and natural logarithm of LF/HF ratio (log LF/HF).
Definitions and assessments
Adequate HD was defined as a thrice-weekly in-centre HD with single-pool Kt/V (spKt/V) >1.4 or urea reduction ratio (URR) >65% for the last 3 months prior to enrolment. Adequacy of PD was assessed by the patient’s weekly Kt/V. Kidney function in CKD patients was estimated by the Modification of Diet in Renal Disease (MDRD) equation [39]. Diabetes was defined by self-reporting and by current use of insulin or oral hypoglycaemic agents. CVD was defined as an affirmative response to physician diagnosed heart failure, previous myocardial infarction or heart attack, or previous coronary artery bypass or angioplasty. PLMI, AHI and AI were defined as the number of periodic leg movements, the number of apnoeas–hypopnoeas and the number of arousals per hour of sleep, respectively. Sleep efficiency (SE) was defined as the percentage of time in bed spent sleeping, while parameters of sleep architecture represented the percentage of TST spent in stage 1, stage 2, stages 3–4 and REM sleep.
Depression was assessed using the Patient Health Questionnaire (PHQ) [40], which is an established questionnaire that covers symptoms of major depressive disorder, as well as subsyndromal variants.
Statistical analysis
Non-parametric statistics (e.g. medians and interquartile ranges) were used to describe the continuous variables. For categorical variables, numbers of patients and their percentages are reported. Equality of the summary measures or percentages of patients for a particular risk factor were tested by chi-square test for categorical variables and Mann–Whitney U-test for continuous variables. The corresponding P-values are reported.
The association between HRV variables and covariates was analysed both with methods for repeated measures data as well as with methods for non-repeated outcomes. The first approach acknowledges the correlation among HRV variables obtained in a particular patient at the different stages (wakefulness, NREM and REM sleep stage) when assessing the influence of predictors on the HRV response. The second approach analyses the HRV outcome variables separately at each stage of sleep. Due to the non-normal distribution of the outcome variables, we utilized generalized estimating equations (GEE) [41,42] and quantile regression for the repeated and non-repeated measures analyses, respectively [43]. Univariate quantile regression modelling was used in order to select covariates (P-value ≤ 0.05) for multi-variable GEE and quantile regression models. Quantile regression allowed us to look for differential associations at the extremes (extremely low or extremely high) and the typical HRV responses. For the purpose of comparing the effects of predictors on different ranges of the outcome variable, we designated the first, middle and upper three deciles as low, middle and upper, respectively. All analyses were performed in R v2.9.1 and SPSS v15.0.
Results
Study population
Overall, 95 patients were assessed, and their characteristics by treatment status are shown in Table 1. Twenty-seven patients (28.4%) had stages 4 and 5 CKD, and 68 (71.6%) were dialysis dependent (ESRD). Fifty-six individuals with ESRD (82.3%) were receiving a thrice-weekly in-centre HD, and 12 (17.7%) were using PD. The median age of the whole population was 51 (25th and 75th percentile: 42, 63), 64.2% (n = 60) were male, 64.2% (n = 61) were European American and 20.4% (n = 19) were diabetic. There were more men in the non-dialysis-dependent CKD group compared to the dialysis group (70.4% vs. 60.3%). A statistically significant difference was observed for the serum levels of phosphorus (P = 0.05), TST (P = 0.01) and SE (P = 0.01) between advanced CKD and dialysis patients. For the HD population, the average spKt/V was 1.63 (±0.3). For the PD population, the average weekly Kt/V was 1.98 (±0.6). For the CKD group, the average MDRD-measured estimated glomerular filtration rate (eGFR) [39] was 16.5 (±3.9) mL/min/1.73 m2.
Table 1.
Overall and by treatment group study population characteristics
| All (n = 95) | CKD (n = 27) | Dialysis (n = 68) | P-value | |
|---|---|---|---|---|
| Age | 51 (42, 63)) | 47 (40, 59) | 52 (43, 66.5) | 0.25 |
| Males | 60 (64.2%) | 19 (70.4%) | 41 (60.3%) | 0.36 |
| Whites | 61 (64.2%) | 21 (77.8%) | 40 (58.8%) | 0.08 |
| BMI | 27.3 (24, 31) | 28.4 (25.7, 32.4) | 27.3 (23.3, 30.5) | 0.15 |
| Diabetes | 19 (20.4%) | 5 (18.5%) | 14 (21.2%) | 0.77 |
| CVD | 22 (23.7%) | 4 (14.8%) | 18 (27.3%) | 0.2 |
| Depression | 11 (11.8%) | 4 (14.8%) | 7 (10.6%) | 0.57 |
| Haemoglobin (g/dL)a | 11.6 (11.1, 12.6) | 11.5 (11.0, 12.5) | 11.7 (11.2, 12.9) | 0.21 |
| Albumin (g/dL) | 3.9 (3.6, 4.1) | 3.9 (3.6, 4.1) | 3.9 (3.6, 4.2) | 0.87 |
| Bicarbonate (mEq/L)b | 23.3 (21.3, 25.5) | 22.6 (20.8, 25.5) | 23.5 (21.3, 25.6) | 0.41 |
| Calcium (mg/dL)c | 9.1 (8.7, 9.4) | 9.1 (8.6, 9.6) | 9.1 (8.8, 9.4) | 0.95 |
| Phosphorus (mg/dL)c | 4.8 (4.2, 6.1) | 4.5 (3.9, 5.2) | 4.9 (4.3, 6.2) | 0.05 |
| PLMI | 3.1 (0.9, 6.6) | 3.5 (1.8, 5.9) | 2.7 (0.6, 6.9) | 0.23 |
| AHI | 11 (4.0, 29.7) | 7.7 (3.0, 22.4) | 12.8 (5.2, 32.0) | 0.15 |
| AI | 8.4 (3.0, 25.9) | 6.3 (1.8, 18.7) | 9.9 (3.8, 27.9) | 0.20 |
| TST (mins) | 337.3 (259.3–401) | 382 (328.3–442.7) | 321.5 (207.7–395.7) | 0.01 |
| Sleep efficiency (%) | 76.7 (62.3–85.3) | 82.6 (74.05–85.7) | 72.1 (59.4–84.1) | 0.01 |
| Stage 1 (%TST) | 10.8 (16.6–6.3) | 12.3 (8.7–19.2) | 9.3 (6.1–16.3) | 0.1 |
| Stage 2 (%TST) | 55.5 (46.6–63.1) | 54.6 (45.5–60.3) | 56.1 (48.3–64.6) | 0.78 |
| Stage 3 and 4 (%TST) | 9.9 (3.6–19.8) | 10.5 (5.6–19.1) | 9.7 (2.5–21.4) | 0.34 |
| REM (%TST) | 19.5 (14.2–25.1) | 21.5 (18.1–26.1) | 18.2 (11.9–24.1) | 0.1 |
| Beta-blockers | 46 (49%) | 12 (44%) | 34 (51%) | 0.58 |
| Anti-depressantsd | 15 (16.5%) | 6 (23.1%) | 9 (13.8%) | 0.29 |
| Sleep-inducing medicationse | 14 (14.7%) | 2 (7.4%) | 12 (17.6%) | 0.2 |
| Opiates | 1 (5.3%) | 1 (3.7%) | 4 (5.9%) | 0.66 |
n, number of participants; CKD, chronic kidney disease; BMI, body mass index; CVD, cardiovascular disease; PLMI, periodic limb movement index; AHI, apnoea–hypopnoea index; AI, arousal index.
Haemoglobin and albumin in g/dL may be converted to g/L by multiplying by 10.
Bicarbonate in mEq/L may be converted to mmol/L by multiplying by 1.0.
Calcium and phosphorus in mg/dL may be converted to mmol/L by multiplying by 0.25 and 0.323, respectively.
Anti-depressants include selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclic anti-depressants and other anti-depressants.
Sleep-inducing medications include benzodiazepines and antihistamines.
Changes of HRV components in wakefulness and throughout sleep
In Figure 1 (and Supplementary Videos 1 and 2), the HRV outcome variables are shown for all three stages. There appears to be a positive correlation between these outcome variables and no obvious relationship to dialysis dependency (quantified in the next section). Medians and interquartile ranges of the measured HRV components (LF/HF and HF) for both study groups in wakefulness, NREM and REM sleep are summarized in Table 2. There were no statistically significant differences of the HRV components among the two groups in wakefulness, NREM and REM sleep stage (Table 2).
Fig. 1.
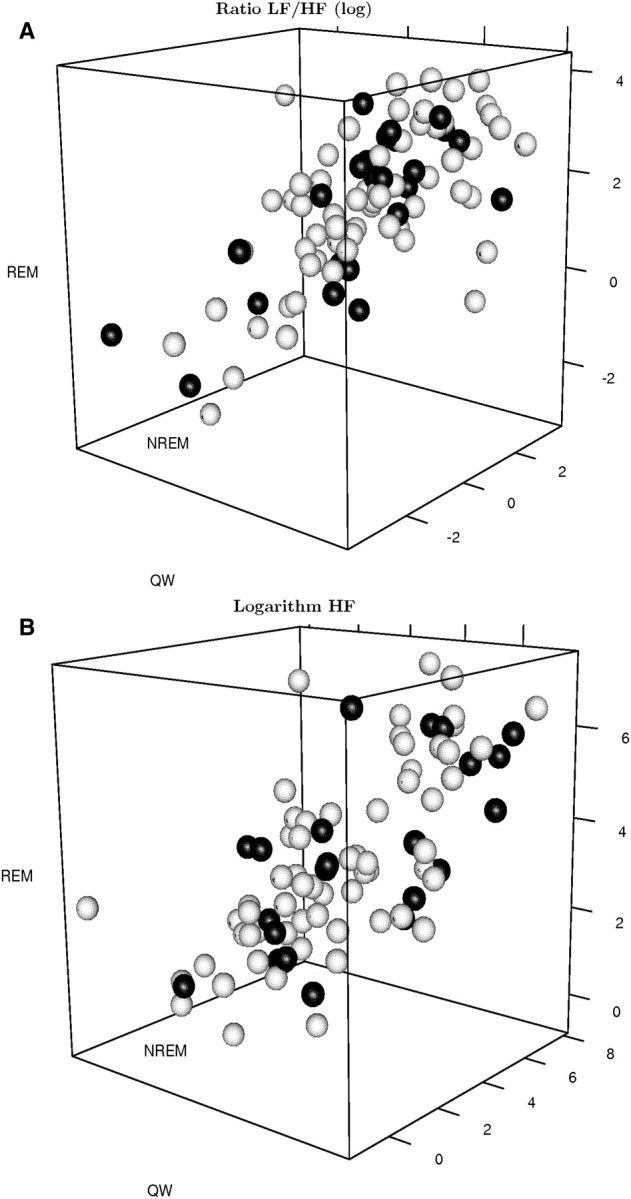
A. Three-dimensional scatter plot of the logarithm of the LF/HF ratio of the HRV in wakefulness, NREM and REM sleep stage. Responses of patients with chronic kidney disease are coloured black, while responses of dialysis patients are coloured white. B. Three-dimensional scatter plots of the logarithm of the HF (LNHF) component of the HRV in wakefulness, NREM and REM sleep stage. Responses of patients with chronic kidney disease are coloured black, while responses of dialysis patients are coloured white.
Table 2.
HRV outcomes for both study groups during wakefulness, NREM and REM sleep stages
| All | CKD | Dialysis | Normative values [45] | P-value | |
|---|---|---|---|---|---|
| W LF/HF ratio | 3.4 (1.5, 5.9) | 3.1 (2.1, 5.6) | 3.4 (1.1, 6.9) | 1.5–2.0 | 0.96 |
| W HF (nu) | 30.3 (9.4, 143.4) | 60.1 (13.3, 214.5) | 26.6 (8.7, 119.5) | 29 (±3) | 0.13 |
| NREM LF/HF ratio | 2.2 (0.8, 6.9) | 2.3 (0.9, 6.7) | 2.1 (0.8, 7.4) | 1.5–2.0 | 0.95 |
| NREM HF (nu) | 22.6 (8.5, 109.9) | 21.3 (8.1, 175.1) | 24.6 (8.5, 104.1) | 29 (±3) | 0.79 |
| REM LF/HF ratio | 4.7 (1.6, 13) | 7.0 (1.2, 13) | 4.2 (2.0, 13) | 1.5–2.0 | 0.88 |
| REM HF (nu) | 24.2 (7.0, 115.2) | 24.2 (7.0, 115.2) | 20.8 (7.0, 115.2) | 29 (±3) | 0.92 |
HF is expressed in normalized units (nu). Normative values are listed in the guidelines from the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [45]. Results are presented as medians and 25th and 75th interquartiles (in parenthesis).
CKD, chronic kidney disease; W, wakefulness; HF, high frequency; LF, low frequency; LNHF, natural logarithm of HF; NREM, non-rapid eye movements; REM, rapid eye movements.
Predictors of overall HRV variables during sleep
We used GEE models adjusted for age, gender, diabetes, dialysis dependency, CVD, haemoglobin, bicarbonate, AHI and PLMI as well as use of beta-blocking agents. Out of the original 95 patients, 85 had complete information for covariates and HRV outcome variables in all three stages. In GEE regression models, presence of diabetes, higher bicarbonate levels and lower PLMI scores were associated with higher sympathovagal balance (Table 3). Diabetes and bicarbonate scores had opposite effects on vagal tone (Table 4). These findings persisted after adjusting for the interaction between stage and dialysis dependency (not shown).
Table 3.
General estimation equation results for the association between baseline characteristics and Ratio of LF/HF in the study population
| Coefficient | Standard error | P-value | |
|---|---|---|---|
| Intercept | 2.30 | 1.50 | 0.09 |
| Age | −0.01 | 0.01 | 0.23 |
| Female gender | −0.37 | 0.24 | 0.11 |
| BMI | −0.01 | 0.03 | 0.83 |
| Diabetes | −0.96 | 0.37 | 0.01 |
| Cardiovascular disease | −0.02 | 0.33 | 0.96 |
| Haemoglobin | 0.13 | 0.09 | 0.11 |
| Bicarbonate | −0.07 | 0.04 | 0.04 |
| Dialysis dependency | 0.12 | 0.24 | 0.60 |
| PLMI | 0.04 | 0.02 | 0.01 |
| AHI | 0.00 | 0.00 | 0.91 |
| Beta-blockers | −0.03 | 0.24 | 0.89 |
BMI, body mass index; PLMI, periodic limb movement index; AHI, apnoea–hypopnoea index.
Table 4.
General estimation equations results for the association between baseline characteristics and LNHF in the study population
| Coefficient | Standard error | P-value | |
|---|---|---|---|
| Intercept | 1.18 | 2.87 | 0.66 |
| Age | −0.01 | 0.01 | 0.67 |
| Female gender | 0.37 | 0.37 | 0.30 |
| BMI | 0.04 | 0.04 | 0.35 |
| Diabetes | −1.46 | 0.44 | <0.001 |
| Cardiovascular disease | −0.47 | 0.52 | 0.33 |
| Haemoglobin | −0.07 | 0.16 | 0.69 |
| Bicarbonate | 0.10 | 0.06 | 0.04 |
| Dialysis dependency | −0.14 | 0.40 | 0.71 |
| PLMI | −0.03 | 0.03 | 0.36 |
| AHI | 0.00 | 0.01 | 0.48 |
| Beta-blockers | −0.05 | 0.41 | 0.89 |
BMI, body mass index; PLMI, periodic limb movement index; AHI, apnoea–hypopnoea index.
HRV changes during wakefulness and sleep
There was no statistically significant interaction between stage and dialysis dependency in GEE regressions for either HRV outcome variable (not shown). Compared to wakefulness, we observed a statistically significant reduction in the sympathovagal balance during NREM sleep (P = 0.017) and an increase in REM sleep (P = 0.013). There was no statistically significant change in the vagal tone among the three stages (not shown). All GEE models estimated a positive correlation between the two HRV outcome variables and the three stages (Table 5) confirming the graphical assessments (Figure 1 and Supplementary Videos 1 and 2).
Table 5.
Pairwise correlations between HRV outcome variables
| Outcome variable | NREM | REM | |
|---|---|---|---|
| Ratio (log) | Wakefulness | 0.42 (0.08)a | 0.33 (0.11)a |
| NREM | 0.47 (0.09)a | ||
| LNHF | Wakefulness | 0.55 (0.08)a | 0.46 (0.11)a |
| NREM | 0.78 (0.07)a |
HRV, heart rate variability; NREM, non-rapid eye movement; REM, rapid eye movement; LNHF; natural logarithm of HF.
Correlations reported as general estimation equations estimate (standard error).
Predictors of HRV variables at specific stages
In order to assess the significance of dialysis treatment and other selected covariates across the range of the HRV components at the three stages, we examined the deciles from 10% to 90% adjusting for the same covariates as in the GEE models. The effects of diabetes and PLMI in quantile regression models are shown in Figure 2A and B, and are discussed further below. In each figure, the influence of one specific covariate (diabetes or PLMI) on the two HRV variables studied during the three stages is shown with the interrupted line. An association is considered statistically significant for a particular quantile of the response if the associated confidence interval (grey-shaded area) does not include zero (solid horizontal line).
Fig. 2.
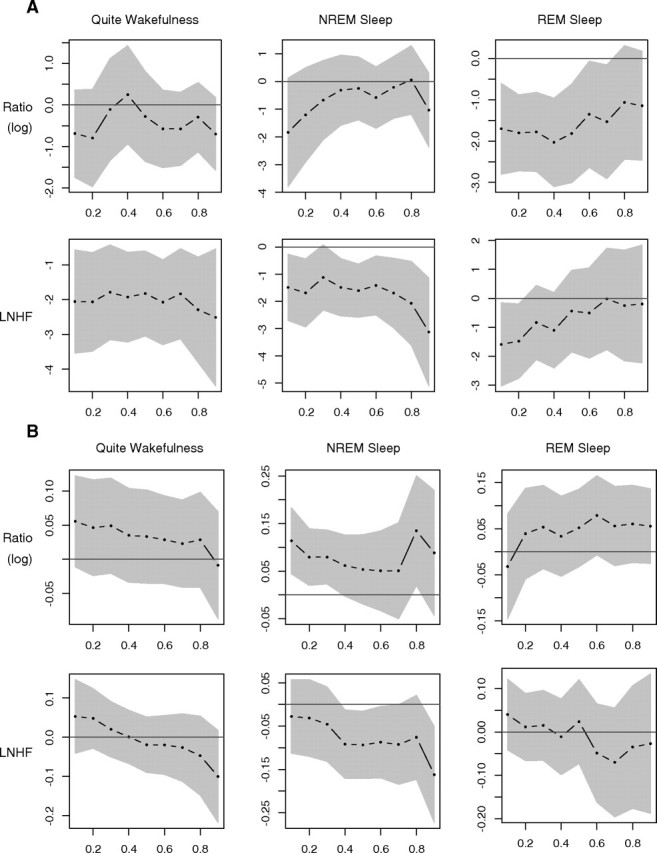
A. Panel of the multivariable quantile regression coefficient plots of the HRV outcomes during wakefulness, NREM and REM sleep stage for diabetes. Quantile regression was carried out for all deciles between the 10th and the 90th (x-axis). In each plot of this panel, the coefficients obtained by the quantile regression are plotted in the y-axis. The black dotted line represents the point estimates of the coefficient of each covariate of the model. The grey-shaded area depicts a 95% pointwise confidence band. The results of quantile regression are considered statistically significant if this band excludes zero (solid black horizontal line). B. Panel of the multivariable quantile regression coefficients plots of the HRV outcomes during wakefulness, NREM and REM sleep stage for PLMI. Quantile regression was carried out for all deciles between the 10th and the 90th (x-axis). In each plot of this panel, the coefficients obtained by the quantile regression are plotted in the y-axis. The black dotted line represents the point estimates of the coefficient of each covariate of the model. The grey-shaded area depicts a 95% pointwise confidence band. The results of quantile regression are considered statistically significant if this band excludes zero (solid black horizontal line).
Diabetes (Figure 2A)
During wakefulness and NREM sleep, presence of diabetes was associated with statistically significant reductions in vagal tone but not in the sympathovagal balance. The reduction in vagal tone during wakefulness was consistent throughout the range of vagal tone but was much higher for patients with vagal tone scores in the upper quantiles. During REM sleep, presence of diabetes was associated with reductions in sympathovagal balance that weakened in patients with responses in the upper two deciles. The effect of diabetes on vagal tone weakened with the magnitude of the vagal tone variable.
PLMI (Figure 2B)
PLMI was associated with higher sympathovagal balance responses during NREM sleep and lower vagal tone responses but only at the extremely high values (over the normal, middle to upper quantiles).
Female gender
Vagal tone was higher in women (vs. men) during wakefulness but not during NREM or REM sleep. The association between vagal tone and gender was quantitatively more important in the middle deciles of the response, but there was no effect for patients with low or higher responses.
Bicarbonate
Higher bicarbonate levels were associated with consistent decreases in the sympathovagal balance and higher vagal tone components, but the results were not consistently statistically significant.
None of the other covariates entered in the quantile regression models (BMI, presence of CVD and enrolment age) expressed consistent association patterns with HRV variables during the sleep stages.
Discussion
These findings confirm that patients with ESRD have increased sympathetic tone in wakefulness and that this finding extends to patients with advanced CKD (stages 4–5). Furthermore, in this study of HRV in individuals with advanced CKD or ESRD dependent on dialysis, we did not observe the physiologic pattern of an increase in parasympathetic tone upon transitioning from wakefulness to NREM sleep. While abnormal HRV has been well described in patients with renal impairment, the disruption of normal physiologic patterns of increased HRV during sleep is a novel finding that may shed further light on the risk of cardiovascular events and sudden cardiac death in this population.
Previous studies have demonstrated abnormal HRV responses in patients undergoing HD [6,44] as well as transplant recipients [11] with findings broadly similar to the ones we report here, i.e. a reduction in the vagally modulated (HF) component of the HRV as well as the balance between sympathetic and parasympathetic effects (LF/HF ratio). However, these investigations did not control for the effects of different sleep stages. In contrast, our analysis controlled for sleep stage, allowing detailed analysis of autonomic regulation as reflected by power spectral analysis within different sleep stages. By adjusting for the modulating effect of sleep stage on HRV, we demonstrated that an abnormal nocturnal HRV is a feature of both advanced CKD and ESRD.
Nevertheless, abnormal HRV response is not uniformly observed in patients with renal impairment as some would be classified within the normal range according to the present guidelines [45]. However, more recent studies [46] point out the need to reach a new consensus towards a normative range for HRV indices [47]. In this report, we highlight several important predictors of abnormal HRV responses, namely diabetes, PLMI, female gender and serum bicarbonate levels. Diabetes can cause severe autonomic dysfunction, while cardiac (parasympathetic) autonomic activity is blunted even before clinical symptoms of diabetic neuropathy become evident [48]. In our population, diabetes was associated with a marked reduction in vagal tone during wakefulness and NREM sleep followed by a partial increase during transition to REM sleep. Even during REM sleep, diabetics exhibited an abnormal (reduced) sympathovagal balance likely reflecting cardiac autonomic neuropathy [49]. In our patient population, a high PLMI score was associated with predominance of sympathetic over vagal tone (high LF/HF ratio) and a drop in HRV. In previous reports, periodic limb movements have been associated with increased cardiovascular morbidity and mortality in patients with ESRD [50–52]. It is well known that patients with CKD also exhibit a sustained overactivity of the sympathetic nervous system, which is likely caused by neurohormonal mechanisms arising in the failing kidney, increased levels of angiotensin II and possibly asymmetrical dimethylarginine. This overactivity is related to hypertension and cardiovascular events, and is a predictor of mortality [53]. Our data suggest that a high PLMI score may be a clinically useful marker for sympathetic activity predominance. Screening patients with CKD or ESRD for PLMI could then be used to refer patients for a more direct assessment of their sympathetic activity, e.g. by measurements of norepinephrine, microneurography in sympathetic muscle nerve fibres, and power spectral analysis of heart rate and blood pressure variability [53]. A higher bicarbonate level appears to exert a protective role and is associated with a modest linear improvement in HRV variables suggesting that meticulous attention to acidosis could partially normalize the patterns of HRV variability in the CKD/ESRD population.
The findings of this manuscript should be interpreted in light of the following limitations. First, this is a cross-sectional study and therefore unable to distinguish causal direction of the link between decreased HRV and advancing CKD. A longitudinal follow-up is needed to better characterize the natural history of HRV in patients with progressive CKD. Second, the lack of a healthy control group may certainly be perceived as a limitation of our work. Finally, chronic health conditions have been defined by self-report or self-report and medication use (i.e. insulin or oral hypoglycaemics). However, previous work has demonstrated that patients with ESRD are relatively good at reporting conditions such as diabetes and CVD [10].
In conclusion, autonomic dysregulation during normal wake/sleep was indentified among patients with CKD and ESRD. This dysregulation was manifested both during wakefulness and as a failure to increase the parasympathetic aspect of the HRV in the transition from wakefulness to NREM and REM sleep. Sympathetic overactivity has been identified in numerous clinical conditions with many deleterious pathological consequences on the vasculature (e.g. vascular hypertrophy), metabolism (e.g. insulin resistance) and cardiac function (e.g. increased incidence of arrhythmia) [54]. Abnormal patterns of HRV during sleep stage transitions are a logical candidate marker of sympathetic overactivity and possibly higher risk of cardiac-related deaths in CKD and ESRD.
Supplementary Material
Acknowledgments
This work was supported by Fresenius National Kidney Foundation Young Investigator Grant, Paul Teschan Research Grant, NIH DK66006 and DK77785 (Mark L Unruh). This publication was supported by funds received from the NIH/NCRR/CTSA grant UL1 RR024153 and by the Pittsburgh Mind-Body Center (PMBC; NIH grants HL076852/076858). J.R.H. is supported by NIH grant R01HL084113. C.T.C. holds the R Fraser Elliott Chair in Home Dialysis. We thank the patients, staff and physicians who participated in this study.
Conflict of interest statement. None declared.
Supplementary data
Supplementary data is available online at http://ndt.oxfordjournals.org.
References
- 1.Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. 2004;8:R367–R384. doi: 10.1186/cc2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2003;285:R927–R931. doi: 10.1152/ajpregu.00452.2003. [DOI] [PubMed] [Google Scholar]
- 3.Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283:R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- 4.Malpas SC. Neural influences on cardiovascular variability: possibilities and pitfalls. Am J Physiol Heart Circ Physiol. 2002;282:H6–H20. doi: 10.1152/ajpheart.2002.282.1.H6. [DOI] [PubMed] [Google Scholar]
- 5.Hedman AE, Hartikainen JE, Tahvanainen KU, et al. The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic ‘tone’. Acta Physiol Scand. 1995;155:267–273. doi: 10.1111/j.1748-1716.1995.tb09973.x. [DOI] [PubMed] [Google Scholar]
- 6.Rubinger D, Revis N, Pollak A, et al. Predictors of haemodynamic instability and heart rate variability during haemodialysis. Nephrol Dial Transplant. 2004;19:2053–2060. doi: 10.1093/ndt/gfh306. [DOI] [PubMed] [Google Scholar]
- 7.Legramante JM, Galante A. Sleep and hypertension: a challenge for the autonomic regulation of the cardiovascular system. Circulation. 2005;112:786–788. doi: 10.1161/CIRCULATIONAHA.105.555714. [DOI] [PubMed] [Google Scholar]
- 8.Versace F, Mozzato M, De Min Tona G, et al. Heart rate variability during sleep as a function of the sleep cycle. Biol Psychol. 2003;63:149–162. doi: 10.1016/s0301-0511(03)00052-8. [DOI] [PubMed] [Google Scholar]
- 9.Vanoli E, Adamson PB, Ba L, et al. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91:1918–1922. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 10.Cavanaugh KL, Merkin SS, Plantinga LC, et al. Accuracy of patients’ reports of comorbid disease and their association with mortality in ESRD. Am J Kidney Dis. 2008;52:118–127. doi: 10.1053/j.ajkd.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirvela M, Salmela K, Toivonen L, et al. Heart rate variability in diabetic and non-diabetic renal transplant patients. Acta Anaesthesiol Scand. 1996;40:804–808. doi: 10.1111/j.1399-6576.1996.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 12.Ly J, Chan CT. Impact of augmenting dialysis frequency and duration on cardiovascular function. Asaio J. 2006;52:e11–e14. doi: 10.1097/01.mat.0000249504.07371.5e. [DOI] [PubMed] [Google Scholar]
- 13.Chan CT, Hanly P, Gabor J, et al. Impact of nocturnal hemodialysis on the variability of heart rate and duration of hypoxemia during sleep. Kidney Int. 2004;65:661–665. doi: 10.1111/j.1523-1755.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 14.Grassi G, Seravalle G, Arenare F, et al. Behaviour of regional adrenergic outflow in mild-to-moderate renal failure. J Hypertens. 2009;27:562–566. doi: 10.1097/hjh.0b013e32831fb371. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Campese VM, Nobakht N, et al. Differential distribution of muscle and skin sympathetic nerve activity in patients with end-stage renal disease. J Appl Physiol. 2008;105:1873–1876. doi: 10.1152/japplphysiol.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker KP, Bliwise DL, Bailey JL, et al. Polysomnographic measures of nocturnal sleep in patients on chronic, intermittent daytime haemodialysis vs those with chronic kidney disease. Nephrol Dial Transplant. 2005;20:1422–1428. doi: 10.1093/ndt/gfh816. [DOI] [PubMed] [Google Scholar]
- 17.Herzog CA. Can we prevent sudden cardiac death in dialysis patients? Clin J Am Soc Nephrol. 2007;2:410–412. doi: 10.2215/CJN.01130307. [DOI] [PubMed] [Google Scholar]
- 18.Karayaylali I, San M, Kudaiberdieva G, et al. Heart rate variability, left ventricular functions, and cardiac autonomic neuropathy in patients undergoing chronic hemodialysis. Ren Fail. 2003;25:845–853. doi: 10.1081/jdi-120024299. [DOI] [PubMed] [Google Scholar]
- 19.Genovesi S, Bracchi O, Fabbrini P, et al. Differences in heart rate variability during haemodialysis and haemofiltration. Nephrol Dial Transplant. 2007;22:2256–2262. doi: 10.1093/ndt/gfm125. [DOI] [PubMed] [Google Scholar]
- 20.Umetani K, Singer DH, McCraty R, et al. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 21.Urbina EM, Bao W, Pickoff AS, et al. Ethnic (black-white) contrasts in heart rate variability during cardiovascular reactivity testing in male adolescents with high and low blood pressure: the Bogalusa Heart Study. Am J Hypertens. 1998;11:196–202. doi: 10.1016/s0895-7061(97)00314-2. [DOI] [PubMed] [Google Scholar]
- 22.Sevre K, Lefrandt JD, Nordby G, et al. Autonomic function in hypertensive and normotensive subjects: the importance of gender. Hypertension. 2001;37:1351–1356. doi: 10.1161/01.hyp.37.6.1351. [DOI] [PubMed] [Google Scholar]
- 23.Kim JA, Park YG, Cho KH, et al. Heart rate variability and obesity indices: emphasis on the response to noise and standing. J Am Board Fam Pract. 2005;18:97–103. doi: 10.3122/jabfm.18.2.97. [DOI] [PubMed] [Google Scholar]
- 24.Furuland H, Linde T, Englund A, et al. Heart rate variability is decreased in chronic kidney disease but may improve with hemoglobin normalization. J Nephrol. 2008;21:45–52. [PubMed] [Google Scholar]
- 25.GGBaJ T. Heart Rate Variability: Stress and Psychiatric Conditions. In: AJC Marek Malik., editor. Dynamic Electrocardiography. New York: Blackwell; 2003. pp. 56–63. [Google Scholar]
- 26.Nahshoni E, Aravot D, Aizenberg D, et al. Heart rate variability in patients with major depression. Psychosomatics. 2004;45:129–134. doi: 10.1176/appi.psy.45.2.129. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder EB, Chambless LE, Liao D, et al. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28:668–674. doi: 10.2337/diacare.28.3.668. [DOI] [PubMed] [Google Scholar]
- 28.Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med. 1999;50:249–261. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- 29.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 30.Willenheimer R, van Veldhuisen DJ, Silke B, et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426–2435. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- 31.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 32.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 33.Sala M, Lazzaretti M, De Vidovich G, et al. Electrophysiological changes of cardiac function during antidepressant treatment. Ther Adv Cardiovasc Dis. 2009;3:29–43. doi: 10.1177/1753944708096282. [DOI] [PubMed] [Google Scholar]
- 34.Szollosi I, Krum H, Kaye D, et al. Sleep apnea in heart failure increases heart rate variability and sympathetic dominance. Sleep. 2007;30:1509–1514. doi: 10.1093/sleep/30.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavoie S, de Bilbao F, Haba-Rubio J, et al. Influence of sleep stage and wakefulness on spectral EEG activity and heart rate variations around periodic leg movements. Clin Neurophysiol. 2004;115:2236–2246. doi: 10.1016/j.clinph.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Ferri R, Zucconi M, Rundo F, et al. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–448. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70:410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Technologies M. Heart Rate variability Analysis Software. Model 60–1100–00 ed.
- 39.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 40.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 41.Hanley JA, Negassa A, Edwardes MD, et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 42.Hu FB, Goldberg J, Hedeker D, et al. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol. 1998;147:694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- 43. Koenker R. Quantile regression. In Press CU ed: 2005.
- 44.Liu M, Takahashi H, Morita Y, et al. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. 2003;18:563–569. doi: 10.1093/ndt/18.3.563. [DOI] [PubMed] [Google Scholar]
- 45.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 46.Pinna GD, Maestri R, Torunski A, et al. Heart rate variability measures: a fresh look at reliability. Clin Sci (Lond) 2007;113:131–140. doi: 10.1042/CS20070055. [DOI] [PubMed] [Google Scholar]
- 47.Sandercock G. Normative values, reliability and sample size estimates in heart rate variability. Clin Sci (Lond) 2007;113:129–130. doi: 10.1042/CS20070137. [DOI] [PubMed] [Google Scholar]
- 48.Rajendra Acharya U, Paul Joseph K, Kannathal N, et al. Heart rate variability: a review. Med Biol Eng Comput. 2006;44:1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 49.Fujimoto Y, Fukuki M, Hoshio A, et al. Decreased heart rate variability in patients with diabetes mellitus and ischemic heart disease. Jpn Circ J. 1996;60:925–932. doi: 10.1253/jcj.60.925. [DOI] [PubMed] [Google Scholar]
- 50.Portaluppi F, Cortelli P, Buonaura GC, et al. Do restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) play a role in nocturnal hypertension and increased cardiovascular risk of renally impaired patients? Chronobiol Int. 2009;26:1206–1221. doi: 10.3109/07420520903245276. [DOI] [PubMed] [Google Scholar]
- 51.Hanly P. Sleep disorders and end-stage renal disease. Curr Opin Pulm Med. 2008;14:543–550. doi: 10.1097/MCP.0b013e3283130f96. [DOI] [PubMed] [Google Scholar]
- 52.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ksiazek A, Zaluska W. Sympathetic overactivity in uremia. J Ren Nutr. 2008;18:118–121. doi: 10.1053/j.jrn.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 54.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci. 2009;148:5–15. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


