Abstract
IL-7 is pivotal for B cell development. Proteins of the Bcl-2 family are essential regulators of lymphocyte survival. Particularly, the pro-apoptotic BH3-only members Bim and Puma mediate lymphocyte apoptosis provoked by cytokine deprivation. Herein, we addressed whether the absence of Bim or Puma within the hematopoietic compartment could bypass the requirement for IL-7-driven B cell development in adult mice. We found that deficiency of Bim, but not Puma, partially rescued B cell development in the absence of IL-7. The numbers of both sIgM− and sIgM+ B cells were markedly increased in the bone marrow of recipients lacking IL-7 upon reconstitution with Bim-deficient hematopoietic progenitors, compared with their control or Puma-deficient counterparts. The augmentation of B cell lymphopoiesis in the absence of Bim was reflected in the mature peripheral compartment by an increase in both the number of immature and mature B cells in the spleen and in the circulating IgM levels. Bim-deficient B cells were also increased in IL-7-sufficient recipients suggesting that peripheral B cells homeostasis is governed by a Bim-dependent and IL-7-independent mechanism. Our data highlight the role of Bim as a key regulator of cell survival during B lymphocyte development in vivo.
Keywords: Bim, B lymphopoiesis, IL-7, Puma
Introduction
Apoptosis is a fundamental process for maintaining cellular homeostasis of the immune system. During their lifespan, hematopoietic cells, including B lymphocytes, constantly receive environmental signals that influence cell survival and death decisions (1). IL-7, a common cytokine receptor gamma chain (γc)-dependent cytokine produced by stromal cells, is essential for B cell development (2). IL-7 binds a dimeric receptor composed of the specific IL-7 receptor (IL-7R) α chain and the γc chain (3). Upon ligand binding to the IL-7 receptor complex, multiple survival, mitogenic and maturation signals are triggered that orchestrate normal B cell differentiation (4). IL-7-driven signaling cascades are tightly connected to the mitochondrial (also called ‘intrinsic’ or ‘Bcl-2-regulated’) apoptosis pathway that dictates the decision of a cell to survive or die (5).
Members of the Bcl-2 protein family are critical apoptosis regulators that can be divided into three main subgroups based on their structural and functional properties (5). The anti-apoptotic members (e.g. Bcl-2 and Mcl-1) contain up to four Bcl-2 homology (BH) regions and are essential for cell survival by direct and/or indirect inhibition of pro-apoptotic counterparts. Pro-apoptotic members are clustered in two groups: Bax-like and BH3-only members. Bax–Bak-like proteins contain BH1, BH2, BH3 regions and share extensive structural homology with their pro-survival relatives, although they mediate mitochondrial outer membrane permeabilization, mitochondrial release of apoptogenic factors (e.g. cytochrome c) and subsequent caspase-mediated cell destruction. The BH3-only proteins, including Bim/Bod, Bid, Bik/Blk/Nbk, Bad, Bmf, Harakiri/DP5, Puma/Bbc3 and Noxa, share with each other and the Bcl-2 family the BH3 domain. This group of pro-apoptotic proteins lies upstream in this apoptotic cascade and is activated in response to several intracellular and extracellular forms of stress insults (e.g. cytokine deprivation) that initiate apoptosis. While Bax and Bak are unequivocally required to induce apoptosis, the role of the individual BH3-only proteins in regulating lymphocyte apoptosis remains unresolved (1, 5).
Growth factor receptor signaling controls cell survival (at least in part) through transcriptional and post-translational regulation of both pro- and anti-apoptotic members of the Bcl-2 family (5). Growth factor receptor-mediated signals augment the expression of the anti-apoptotic proteins Bcl-2 and Mcl-1 and reduce the expression of the pro-apoptotic BH3-only proteins Bim and Puma. Conversely, growth factor or cytokine deprivation engages opposing signaling cascades, including the de novo synthesis of the BH3-only proteins Puma and Bim, which have both been shown to modulate apoptosis under these conditions (6–10). The overt lymphadenopathy observed in the absence of Bim in mice identified this protein as an essential mediator of apoptosis under cytokine and growth factor deprivation (11, 12). Bim-deficient mice have augmented numbers of immature and mature B cells as well as Ig-secreting plasma cells (13). However, loss of Bim was reported to be insufficient to rescue B cell development but to secure survival of B cell precursors without further B cell maturation in the absence of IL-7 (14, 15). It was concluded that IL-7/IL-7R-driven signals appear to promote, apart from cell survival, essential additional maturation signals essential for B cell ontogeny (16). It is noteworthy that the aforementioned studies were performed using mice lacking Bim in all somatic cells, which may encompass apoptotic defects in cells and tissues of non-hematopoietic origin (17–19), including stromal cells known to be critical for B cell development. Moreover, IL-7 sustains the viability of activated Bim-deficient T cells (10) indicating that IL-7/IL-7R signaling might activate additional, previously unappreciated, Bim-independent pathways securing lymphocyte survival that warrant further investigation. The BH3-only protein Puma appears as an interesting candidate for apoptosis signaling provoked by IL-7 deprivation since its loss has been shown to enhance resistance of several hematopoietic cell subsets to growth factor withdrawal and combined loss of Bim and Puma in mice led to the accumulation of B cell numbers beyond those observed in mice lacking Bim alone (8, 10, 13, 20). However, whether Puma is also involved in the apoptosis cascade provoked by absence of IL-7 is unknown.
To dissect the roles of Puma and Bim in IL-7-driven adult B cell development, we addressed how the deficiency in these BH3-only proteins in the hematopoietic compartment influences B cell ontogeny in the presence or absence of IL-7. Our data show that lack of Bim, but not Puma, permits to some extent adult B cell development and maturation in the absence of IL-7.
Materials and methods
Mice
Rag2.IL-7 double-knockout (DKO) and RAG2.γc DKO mice on a C57BL/6 background were used as recipients and were described previously (21, 22). WT (C57BL/6), Puma KO and Bim KO mice were used as a source of hematopoietic stem cells (Lin−Sca+cKit+, LSK cells). The generation and genotyping of the Puma KO (generated on an inbred C57BL/6 background using C57BL/6-derived ES cells) and Bim KO (generated on a mixed C57BL/6 × 129SV background using 129SV-derived ES cells but backcrossed onto the C57BL/6 background for >15 generations) have been described previously (12, 23). All mice were kept in specific pathogen-free conditions at the Institute Pasteur or at the Innsbruck Medical University and all animal experiments were approved by the committee on animal experimentation at the Institute Pasteur (Paris, France) and were approved by the French Ministry of Agriculture.
Hematopoietic stem cell sorting and hematopoietic reconstitution assays
Hematopoietic stem cells (Lin−Sca+cKit+, LSK cells) were isolated as described previously (16). Briefly, bone marrow cells were stained with PE-conjugated monoclonal antibodies for CD3 (145-2C11), CD4 (RM4-5), CD8alpha (53-6.7), B220 (RA3-6B2), NK1.1 (PK136), CD11b (M1/70), Gr-1 (RB6-8C5) and Ter-119. Depletion of Lin+ cells was performed by magnetic-activated cell separation using anti-PE antibody coated MicroBeads (Miltenyi Biotec) according to the manufacturer's instructions. After depletion, the Lin− fraction was stained with anti-Sca-1-FITC (E13-161.7), anti-c-Kit-allophycocyanin (2B8) antibodies. Dead cells were stained with 4′,6-diamidino-2-phenylindole and excluded by electronic gating. Cell sorting was performed on a cell sorter (FACSAria, BD Biosciences).
Recipient mice were exposed to a single dose of irradiation (600 rad). Mice were reconstituted intravenously using purified hematopoietic stem cells from WT, Puma KO and Bim KO mice. Analysis was performed 9–11 weeks following reconstitution.
Antibodies and flow cytometry
FITC-conjugated anti-Sca-1(E13-161.7), FITC-conjugated anti-CD21(7G6), PE-conjugated anti-Ter-119 (Ly-76), PE-conjugated anti-CD4 (RM4-5), PerCP–cyanine 5.5-conjugated anti-NK1.1 (PK136), allophycocyanin–cyanine 7-conjugated anti-CD19 (1D3), allophycocyanin-conjugated anti-B220 (RA3-6B2), allophycocyanin–cyanine 7-conjugated anti-CD11b (M1/70), PerCP–cyanine 5.5-conjugated anti-CD45.2 (104), biotin-conjugated anti-CD23 (B3B4) and streptavidin–PE–cyanine 7 antibodies were purchased from Becton Dickinson PharMingen. FITC-conjugated anti-CD49b (DX5), PE-conjugated anti-CD122 (TM-β1), PerCP–cyanine 5.5-conjugated anti-CD3 (145-2C11), PE-cyanine 7-conjugated anti-GR-1 (Ly-6G RB6.8C5) and allophycocyanin-conjugated anti-c-Kit (2B8) antibodies were purchased from eBiosciences. FITC-conjugated anti-IgD and PE-conjugated anti-IgM antibodies were purchased from Southern Biotechnologies Associate. Stained cells were analyzed in an FACSCanto (Becton Dickinson PharMingen) and data processed with FlowJo software (Treestar).
Quantification of serum Ig titers
Total IgG and IgM serum titers were measured by ELISA. Flexible U-bottom 96-well plates were coated with 1 mg ml−1 unlabeled goat anti-mouse Ig(H + L) antibodies in sodium carbonate buffer pH 9.6 (Sigma) overnight at 4°C. The plates were blocked with 200 ml PBS with 1% FCS, 0.5% Tween 20 and 0.6% skim milk powder and then washed three times in PBS containing 0.1% Tween 20, PBS and H2O before the addition of 1/3 serial dilutions of mouse serum in blocking solution. Sera were prepared by eye bleeding of mice without the addition of heparin. Blood was left to clot for 4 h at 4°C and centrifuged at 13 000 r.p.m. The supernatants were removed and starting dilutions of 1/10 were used for ELISA. Plates were incubated for 2 h at room temperature and washed three times as described above. Goat anti-mouse IgG1 or goat anti-mouse IgM antibodies conjugated to HRP (Southern Biotech) diluted to 1 mg ml−1 in blocking solution were added to the wells and incubated for 4 h at room temperature before further washing. Bound HRP was detected by the addition of 100 ml of TMB liquid substrate (Sigma). Reactions were stopped after 15 min by addition of 100 ml 2M H2SO4 and the absorbances at 405 nm were measured using a Kinetic Microplate Reader (Molecular Devices).
In vitro assays
Total splenocytes were labeled with 5 μM CFSE (Molecular Probes, Oregon, USA). CFSE-labeled splenocytes were cultured for 5 days at 37°C in 5% CO2 atmosphere, in RPMI with 10% FCS in the presence of 20 μg ml−1 LPS (Sigma) and 50 ng ml−1 IL-4 (Peprotech) as previously described (24).
Statistical analysis
The Mann–Whitney t-test was used to perform statistical analysis of significance. P < 0.05 was considered significant.
Results
Defects in adult B cell development caused by the absence of IL-7 can be partially overcome by lack of Bim but not Puma
To address the roles of the BH3-only proteins Puma and Bim during IL-7-driven B cell development, two groups of sub-lethally irradiated immunodeficient recipients, RAG2.γc DKO and RAG2.IL-7 DKO mice, were reconstituted with WT, Puma KO, and Bim KO hematopoietic stem cell precursors (Lin−Sca+cKit+ or LSK cells; see Methods). RAG2.γc DKO mice were used as a control group to RAG2.IL-7 DKO mice because both recipients have a similar block in B cell development (pro-B cell stage) due to the lack of IL-7 signaling (25). Secondly, both recipients are devoid of Peyer's patches and have reduced numbers of peripheral lymph nodes (2, 26) and therefore questions on peripheral B cell homeostasis can be more appropriately compared. Mice were analyzed 9–11 weeks following reconstitution. Unreconstituted hosts were devoid of mature B and T cells, and in the case of RAG2.γc DKO, NK cells were also absent (21, 22). The number of LSK cells was comparable between all genotypes analyzed (data not shown). NK cells that developed in RAG2.γc DKO recipients after reconstitution were donor derived and their numbers were similar for all genotypes analyzed (data not shown), consistent with a similar number of injected hematopoietic progenitors. Further, the number of myeloid and erythroid cells was identical in all genotypes (data not shown), suggesting that NK, myeloid and erythroid cell development was grossly unaffected by lack of Puma or Bim. For simplicity, we will henceforth refer to RAG2.γc DKO and RAG2.IL-7 DKO-recipient mice as IL-7+ and IL-7− recipients, respectively.
Firstly, we analyzed the requirements for IL-7 during post-natal B lymphopoiesis. The progression from the pro-B/pre-B cell (B220+CD19+IgM−) stage to the immature/mature-B cell (B220+CD19+IgM+) stages was assessed in the bone marrow of recipient mice. Since both recipients were deficient in RAG2, any B lymphoid cells that developed beyond the pro-B stage in these recipients were necessarily of donor origin. IL-7− recipients reconstituted with WT and Puma KO stem cells were generally devoid of B220+CD19+ cells. Interestingly, however, a clear population of B220+CD19+ cells emerged in Bim KO stem cell-reconstituted IL-7− recipients; and this population included both sIgM− B cell progenitors as well as sIgM+ B cells. The presence of sIgM− B cell progenitors provided evidence for ongoing B lymphopoiesis (Fig. 1A). In IL-7+ recipients, B cells of all three genotypes clearly developed although a degree of variability in the percentages of B cells was obtained upon reconstitution. A general increase in the frequency of B220+CD19+ cells in Bim KO stem cell-reconstituted recipients was noted (Fig. 2A). The total bone marrow cellularity was augmented in IL-7+ recipients compared with IL-7− counterparts without significant differences among different donor genotypes (Figs 1Bi and 2Bi). Nevertheless, the number of total B-lineage cells (B220+CD19+) was significantly augmented in IL-7− recipients reconstituted with Bim KO stem cells compared with WT or Puma KO stem cells (average fold difference Bim/WT = 5.4), with a marked increased in both sIgM− B cell progenitors (pro-B/pre-B cells: B220+CD19+sIgM−) and sIgM+ B cells (immature/mature: B220+CD19+sIgM+) (average fold difference Bim/WT = 8.8 and 4.1, respectively). Numbers of total B-lineage cells, sIgM− B cell progenitors and sIgM+ B cells in Puma KO stem cell-reconstituted mice were not significantly different from those in WT stem cell-reconstituted mice (Fig. 1B ii–iv).
Fig. 1.
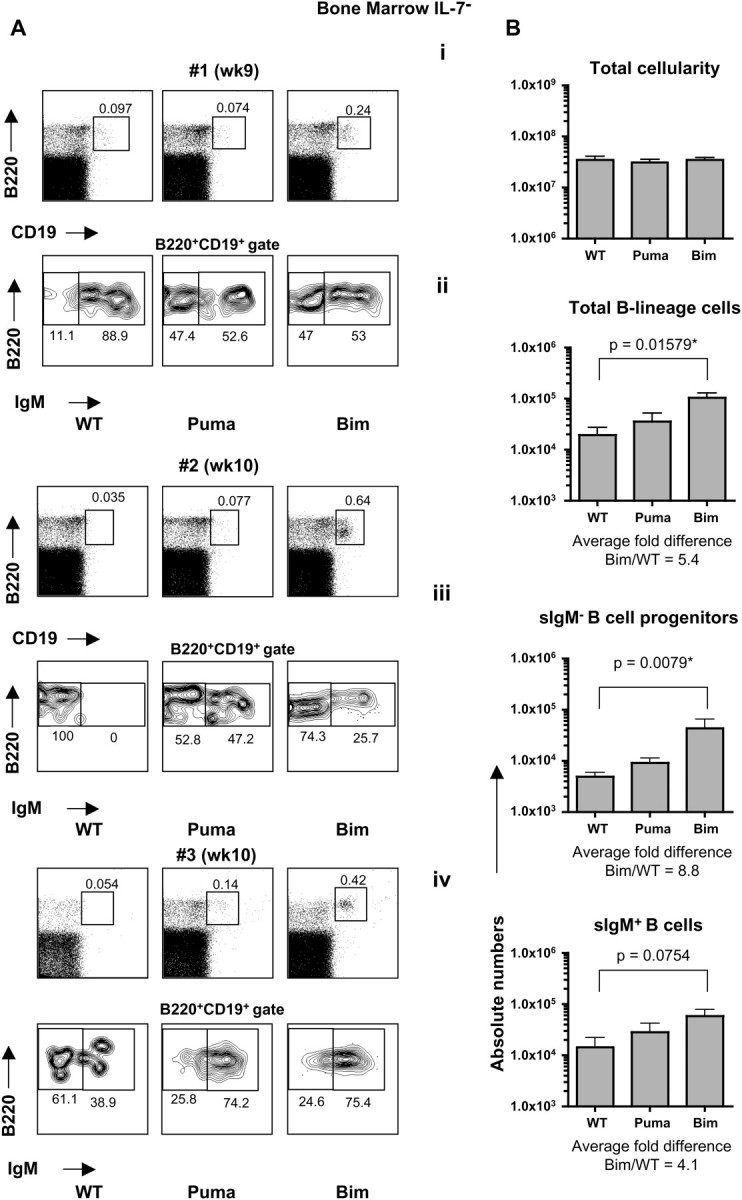
Lack of Bim, but not lack of Puma, in hematopoietic stem cells permits B cell development in the absence of IL-7. IL-7− (RAG2.IL-7 DKO)-recipient mice were analyzed 9–11 weeks following reconstitution with WT, Puma KO or Bim KO LSK cells. (A) Flow cytometric analysis of bone marrow-derived single-cell suspensions. Dot plots represent three different time points from two independent experiments. (B) Quantification of total cellularity (i), numbers of total B-lineage cells (B220+CD19+) (ii), sIgM− B cell progenitors (B220+CD19+IgM−) (iii) and sIgM+ B cells (B220+CD19+IgM+) (iv) in the bone marrow. Error bars represent the means ± SEMs of two independent experiments (n = 5–7 mice/genotype).
Fig. 2.
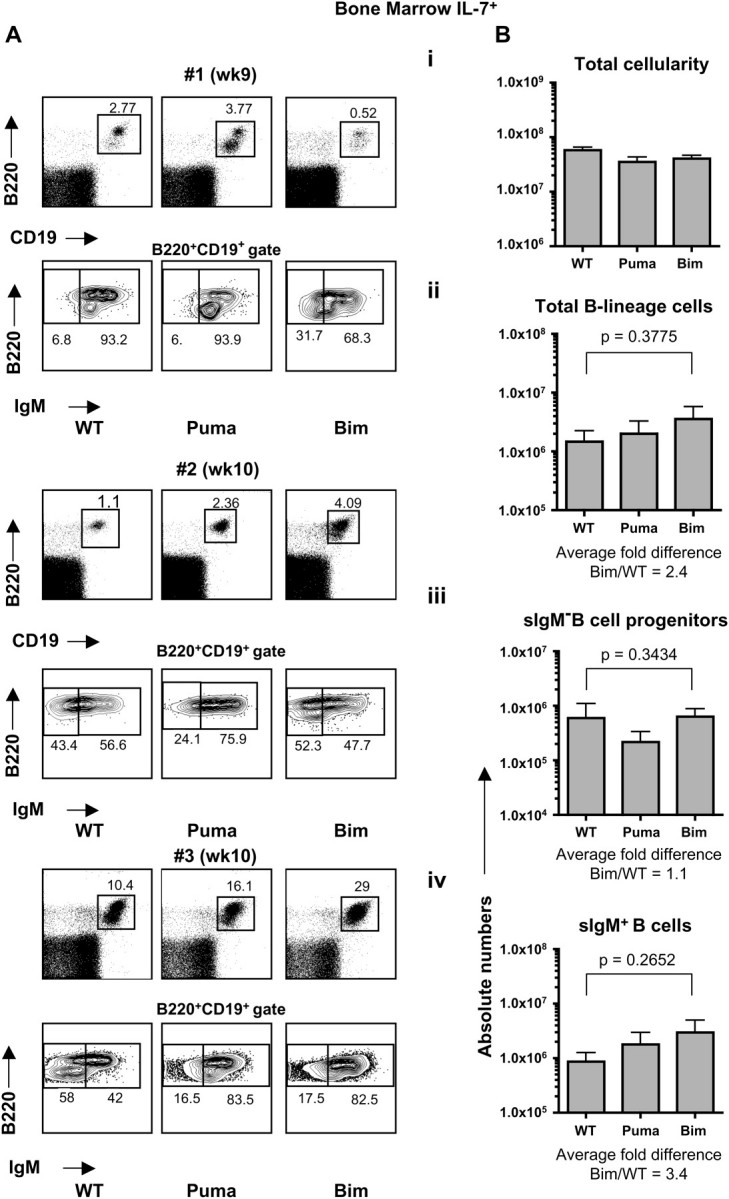
B cell development in IL-7-competent recipient mice. IL-7+ (RAG2.γc DKO)-recipient mice were analyzed 9–11 weeks following reconstitution with WT, Puma KO or Bim KO LSK cells. (A) Flow cytometric analysis of bone marrow-derived single-cell suspensions. Dot plots represent three different time points from two independent experiments. (B) Quantification of total cellularity (i), numbers of total B-lineage cells (B220+CD19+) (ii), sIgM− B cell progenitors (B220+CD19+IgM−) (iii) and sIgM+ B cells (B220+CD19+IgM+) (iv) in the bone marrow. Error bars represent the means ± SEMs of two independent experiments (n = 5–7 mice/genotype).
In IL-7+ recipients, and consistent with their dependence on IL-7, the number of total B-lineage cells was markedly increased, with a moderate increase in the numbers of Bim KO B-lineage cells (average fold difference Bim/WT = 2.4) compared with IL-7+ recipients reconstituted with WT or Puma KO stem cells. This augmentation was likely due to the increased numbers of immature/mature-B cells (B220+CD19+sIgM+), as the numbers of sIgM− B cell progenitors (pro-/pre-B cells: B220+CD19+IgM−) were relatively constant among genotypes (Fig. 2B ii–iv). IL-7− mice reconstituted with WT stem cells displayed a 73-fold reduction in the number of total B-lineage cells (average 1.9 × 104 cells) when compared with the number of total B-lineage cells obtained in an IL-7+ recipient transplanted with WT stem cells (average 1.4 × 106 cells). Yet, this marked defect in IL-7− recipients could be substantially ameliorated to a 14-fold reduction when Bim-deficient stem cells were used for transplantation (average 1.3 × 105 cells) (compare Figs 1Bii and 2Bii). This suggests that at least one additional factor, besides Bim and independent from Puma, may be neutralized by IL-7 signaling during B cell maturation. In addition, our data provide the first evidence that lack of Bim in progenitor B cells is sufficient to augment adult B cell lymphopoiesis in the absence of IL-7.
Splenic Bim-deficient B cells accumulate in IL-7 sufficient as well as IL-7-deficient mice
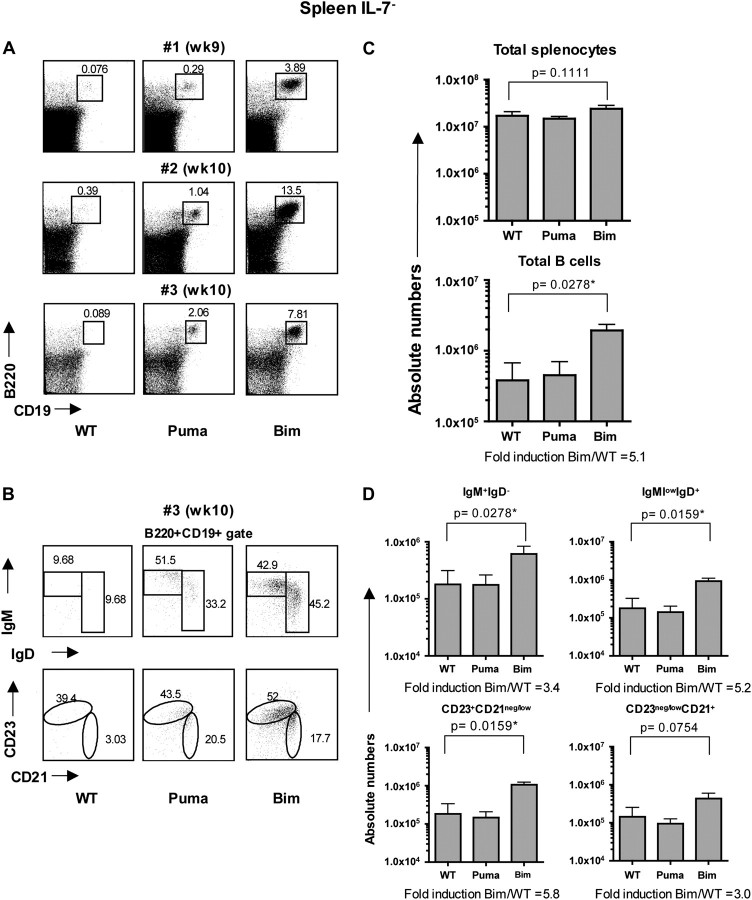
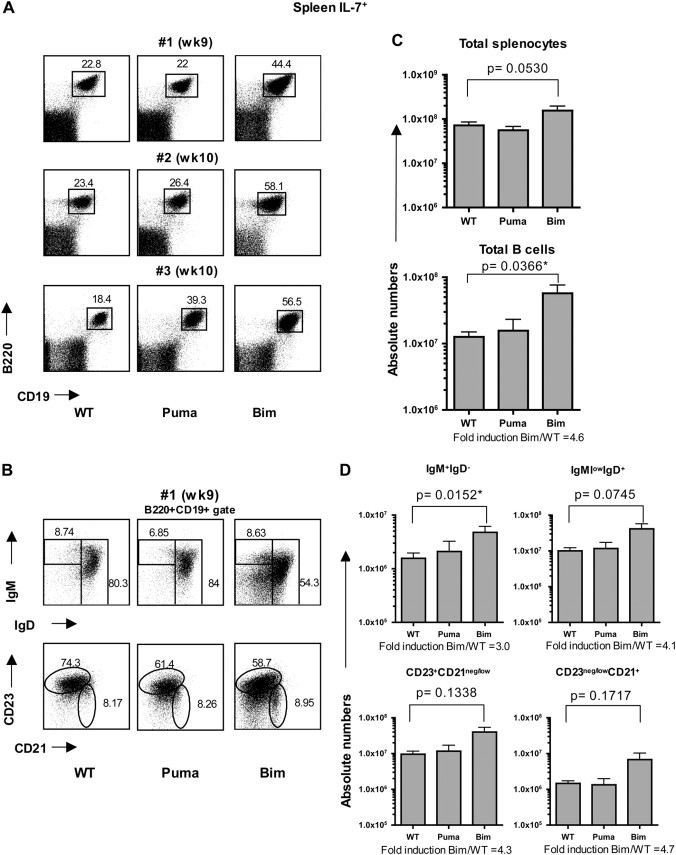
Next, we assessed how the absence of Bim or Puma impacted on the peripheral B cell compartment. As recipient mice were virtually devoid of lymph nodes due to the absence of IL-7 signaling (2, 26, 27), we focused our analysis in the spleen. Consistent with our findings in the bone marrow, WT and Puma KO stem cell-reconstituted IL-7− recipients contained very few splenic B cells. In contrast, a clearly identifiable population of splenic B cells was consistently found in Bim KO stem cell-reconstituted IL-7− recipients (Fig. 3A). Phenotypically, this population comprised all classical B cell subsets, including both immature/transitional (sIgM+sIgD−) and mature (sIgMlowsIgD+) B cells and follicular (CD23+CD21neg/low) and marginal zone (CD23neg/lowCD21+) B cells (Fig. 3B). In IL-7+ recipients, the frequency of B cells was generally similar among WT and Puma KO stem cells-reconstituted mice, yet consistently augmented in Bim KO stem cells-reconstituted counterparts (Fig. 4A). The relative cellular subset distribution in both immature and mature B cells and follicular and marginal zone B cells was similar among the different genotypes (Fig. 4B).
Fig. 3.
Accumulation of Bim-deficient B cells in the spleen of IL-7-deficient mice. Flow cytometric analysis of the spleens from IL-7− (RAG2.IL-7 DKO) recipients reconstituted with WT, Puma KO or Bim KO LSK cells. (A) Dot plots represent three different time points from two independent experiments. (B) B cells (B220+CD19+) were phenotypically characterized with different B cell maturation markers: immature (IgM+IgD−) and mature B cells (IgMlowIgD+) (top row); follicular B cells (CD23+CD21neg/low) and marginal zone B cells (CD23neg/lowCD21+) (bottom row); one representative staining is shown. (C) Quantification of total cellularity and total B cells (B220+CD19+) of the spleen. (D) Quantification of B cell subsets. Error bars represent the means ± SEMs of two independent experiments (n = 5–7 mice/genotype).
Fig. 4.
Accumulation of Bim-deficient B cells in the spleen of IL-7-sufficient mice. Flow cytometric analysis of the spleens from IL-7+ (RAG2.γc DKO) recipients reconstituted with WT, Puma KO, or Bim KO LSK cells. (A) Dot plots represent three different time points from two independent experiments. (B) B cells (B220+CD19+) were phenotypically characterized with different B cell maturation markers: immature (IgM+IgD−) and mature B cells (IgMlowIgD+) (top row); follicular B cells (CD23+CD21neg/low) and marginal zone B cells (CD23neg/lowCD21+) (bottom row); one representative staining is shown. (C) Quantification of total cellularity and total B cells (B220+CD19+) of the spleen. (D) Quantification of B cell subsets. Error bars represent the means ± SEMs of two independent experiments (n = 5–7 mice/genotype).
The total splenic cellularity was augmented in IL-7+ recipients of all genotypes of donors (WT, Puma KO and Bim KO) when compared with IL-7− recipient counterparts and without any statistically significant differences among genotypes (although the cellularity was slightly elevated in Bim KO stem cell-reconstituted IL-7+ animals; Fig. 3C and 4C). As in the bone marrow, the total number of B cells was significantly augmented in Bim KO stem cell-reconstituted IL-7− recipients (average fold difference Bim/WT = 5.1) compared with IL-7− mice reconstituted with WT or Puma KO stem cells, with a marked increased in all B cell subsets analyzed (Fig. 3D). Quantitatively, WT stem cell-reconstituted IL-7− mice displayed a 31-fold reduction in total B cells (0.4 × 106 cells) compared with the number of total B cells found in WT stem cell-reconstituted IL-7+ recipient (average 12 × 106). Similar to the bone marrow, lack of Bim attenuated this defect to a 6-fold reduction (1.9 × 106 cells) (Figs 3C and 4C, total B cells). In IL-7+ recipients, the total number of Bim KO B cells was also markedly increased compared with WT and Puma KO stem cell-reconstituted animals (average 4.6-fold increase). This augmentation was observed in both immature as well as mature B cells (Fig. 4D). Overall, our data suggest that Bim KO B cells mature and persist in both an IL-7-deficient and an IL-7-sufficient environment, indicating that Bim controls peripheral B cell homeostasis in an IL-7-independent fashion.
Augmented serum IgM levels in IL-7-deficient mice reconstituted with hematopoietic stem cells lacking Bim, but not Puma
To assess whether B cells that developed from Bim KO hematopoietic progenitors in the absence of IL-7 were functional, we measured the total IgM and IgG levels in the sera of reconstituted mice and assessed B cell proliferative responses to mitogens. Ig levels in Bim KO or Puma KO stem cell-reconstituted mice were normalized to their WT stem cell-reconstituted counterparts. In accordance with the augmentation in B cell numbers, serum IgM levels were 4- to 5-fold increased in WT stem cell-reconstituted IL-7+ mice compared with the WT stem cell reconstituted with IL-7− recipients (data not shown). Remarkably, in Bim KO stem cell transplanted IL-7− recipients, serum IgM levels were 3- to 4-fold increased compared with WT or Puma KO stem cell-reconstituted IL-7− animals (Fig. 5A). Serum IgG levels were very low or absent in all IL-7− recipients regardless of the genotype of stem cells that had been transferred. This lack of IgG may be explained by the absence of T cells in IL-7− recipients. Deficiency in Bim did not restore the peripheral T cell pool in the absence of IL-7 (T cells were detected in 1/5 Bim KO-reconstituted recipients, data not shown). In contrast, T lymphopoiesis in the thymus and peripheral T cell numbers was normal in IL-7+ recipients reconstituted with stem cells from all genotypes of donors (data not shown). In Bim KO stem cell-reconstituted IL-7+ recipients both IgM and IgG levels were elevated compared with WT or Puma KO counterparts (Fig. 5B). Moreover, Bim KO B cells that developed in IL-7− recipients were able to proliferate in response to LPS and IL-4 in vitro as B cells developed in IL-7+ recipients from all genotypes analyzed (Fig. 5C). Hence, our results indicate that in an IL-7-deficient environment, B cells lacking Bim exhibit intrinsic effector functions.
Fig. 5.
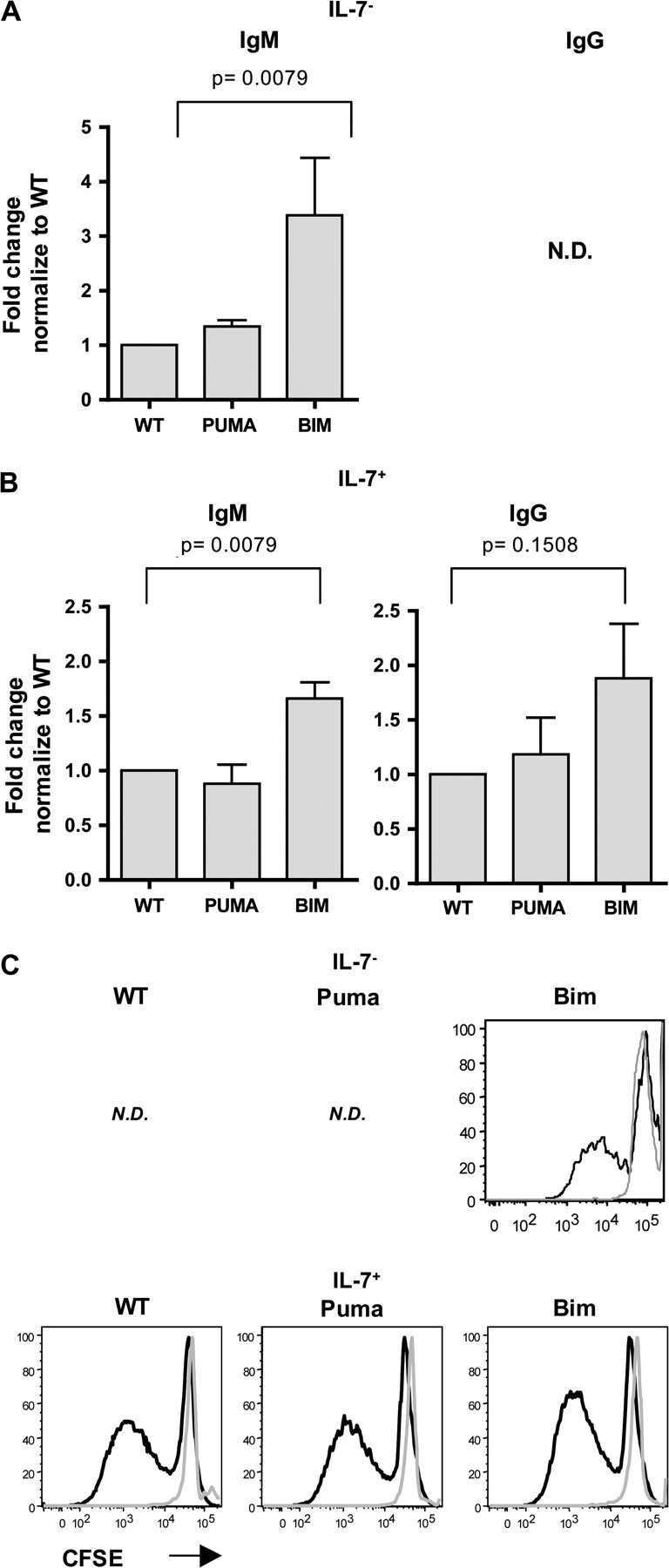
IL-7-deficient mice reconstituted with hematopoietic progenitors lacking Bim have higher serum IgM levels than those reconstituted with WT or Puma counterparts. Ig titers in the sera of (A) IL-7− (RAG2.IL-7 DKO) and (B) IL-7+ (RAG2.γc DKO) recipients were quantified by ELISA 9-11 weeks following reconstitution with WT, Puma KO or Bim KO LSK cells. ND, not detectable. Dilutions were predetermined to produce absorbance readings in the linear range. Values were normalized to the values obtained with IL-7− and IL-7+ recipients reconstituted with WT. Error bars represent the means ± SEMs of two independent experiments (n = 5–7 mice/genotype). (C) In vitro B cell proliferative response. CFSE-labeled splenocytes from IL-7− (upper row) and IL-7+ (lower row) recipients following reconstitution with WT, Puma KO or Bim KO were culture in presence of LPS and IL-4. After 5 days, cultured cells were analyzed by FACS analysis. Black line: gated on B220+CD19+ cells; gray line: gated on B220−CD19− cells. ND, not done. Data are from a representative experiment of two performed.
Discussion
Conceptual models proposed a specific role for BH3-only proteins Puma and Bim in regulating lymphocyte apoptosis (1). While Puma is a prime effector of apoptosis induced by p53-mediated cellular response to DNA damage, Bim is a critical mediator of apoptosis caused by growth factor withdrawal (1). Yet, recent evidence has shown a certain degree of functional overlap between Bim and Puma, as single and combined loss of these BH3-only proteins delayed apoptosis induced by cytokine deprivation (8, 10, 28). Herein, using complementary approaches, we tested a putative functional overlap between these BH3-only proteins in regulating apoptosis during B lymphopoiesis that is caused by the absence of IL-7. Our data provide evidence that loss of Bim, but not loss of Puma, can partially rescue the development and differentiation of B cells in an IL-7-deficient environment.
Three growth factors have been identified that promote B cell development in the mouse: IL-7, Fms-related tyrosine kinase 3 ligand (FLT3L) and thymic stromal lymphopoietin (TSLP) (2, 16, 29, 30). Whereas FLT3L and IL-7 are mandatory in governing B lymphopoiesis in fetal and adult life, TSLP is predominantly important during fetal life (29). Accordingly, we found that adult B lymphopoiesis was significantly compromised in IL-7-deficient recipient mice upon reconstitution with WT hematopoietic progenitors. Strikingly, however, loss of Bim significantly enhanced B cell production under these circumstances (Fig. 1). Augmented B cell numbers resulted from increases in both B cell progenitors (pro-B/pre-B cells: sIgM− B-lineage cells) and immature/mature (CD19+sIgM+) B cells, suggesting the existence of ongoing B lymphopoiesis in the absence of IL-7 (Fig. 1). Cells of non-hematopoietic origin (stromal cells) are the predominant source of IL-7 (31). Although one cannot exclude the possibility that certain cells of hematopoietic origin may produce some minute amounts of IL-7, it is unlikely that those sustain B cell lymphopoiesis in our experimental system. We and others have shown that IL-7-proficient WT stem cell progenitors are not able to produce B cells in irradiated IL-7-deficient adult mice (29, 32). Hence, IL-7 produced by cells of hematopoietic origin, if any, is not sufficient to sustain adult B cell lymphopoiesis. In IL-7-sufficient recipients, lack of Puma or Bim did not significantly affect the number of B cell precursors, suggesting that B lymphopoiesis in the presence of IL-7 is not markedly affected by lack of Bim (Fig. 2). Our data provide evidence for the first time that lack of Bim can partially bypass the requirement for IL-7 during B cell development. Alternatively, the absence of Bim may promote an IL-7-independent pathway for B cell development that is normally concealed by the overt effects of IL-7. In this situation, TSLP, which receptor shares the IL-7Rα chain, emerges as a possible candidate promoting B cell development. Ectopic expression of TSLP appears to sustain adult B lymphopoiesis in the absence of IL-7 (32), although the effects of exogenous TSLP seem to be restricted to the neonatal period in IL-7-sufficient mice (33). Under normal circumstances, TSLP appears to be expressed at low levels in WT- and IL-7-deficient adult mice and these low levels fail to bypass the requirement of IL-7 for adult B lymphopoiesis (29, 32, 33). Yet, the existing levels of TSLP, which may be elevated in lymphopenic conditions (e.g. upon whole-body gamma irradiation) as demonstrated for IL-7 (34), may be sufficient to sustain B cell development in the absence of IL-7, providing that B cell progenitor survival is ensured by lack of Bim. Worth mentioning, the overexpression of Bcl-2 is insufficient to rescue B cell development in IL-7Rα KO mice, which lack both IL-7- and TSLP-mediated signaling (35). These findings suggest that downstream signals of IL-7Rα may inhibit a Bcl-2-independent apoptosis pathway. Alternatively, the co-operative contribution of Bcl-2 with other critical survival Bcl-2-related protein, such as Mcl-1 (binding partner of Bim) (5), may be necessary to inhibit this death cascade. From this viewpoint, it would be interesting to determine whether deficiency of Bim still permits B lymphopoiesis in the combined absence of TSLP and IL-7.
In a recent report, Marrack and colleagues. analyzed B cell development in Bim/IL-7 double-deficient mice. They found that absence of Bim failed to promote the differentiation of B cells (B220+CD19+IgM+) in the absence of IL-7 (14). In contrast, we find that Bim-deficient B cells can partially develop without IL-7. These differences may be explained in part by the distinct experimental models. In the of Oliver et al. (14), absence of Bim may generate defects in non-hematopoietic cells that are important to sustain B cell development (17–19). Bone marrow stromal cells provide additional signals, besides IL-7, that are required for the maturation of B cell progenitors and one of these signals may be compromised in the absence of Bim. Moreover, in Bim.IL-7 DKO mice, the IL-7 deficiency is present throughout fetal and adult B cell development, such that hematopoietic precursors may compensate for the IL-7 deficiency. In contrast, we assessed Bim-deficient precursors that develop in an IL-7-sufficient environment upon transfer to an IL-7-deficient recipient, which might limit compensatory mechanisms. Transfer of IL-7.Bim DKO stem cells into Rag2.IL-7 DKO mice may help to validate this possibility. Finally, the time course was distinct in both studies. While bone marrow of IL-7.Bim DKO mice was analyzed at 6 weeks of age, our analysis was conducted at a later time point (14–17 weeks of age; 9–11 weeks after reconstitution of irradiated recipients). Worth mentioning here, and in line with our data, that Olivier et al. (14) also noted an accumulation of B cells in the spleen of IL-7.Bim DKO mice at 12 weeks of age, which may be due to an increase in B lymphopoiesis and/or peripheral B cell survival (Figs 4, 5 and 6). Bone marrow analysis of older DKO animals might help to address this possibility.
Growth factor withdrawal engages a pro-apoptotic pathway in activated lymphocytes that entails de novo transcription of Bim and Puma (5). In the absence of growth factors (e.g. IL-3), PI3K–AKT/PKB signaling is attenuated leading to activation of forkhead transcription factor FoxO3A, which then translocate to the nucleus to mediate transcription of target genes, including the BH3-only proteins Puma and Bim (8, 10, 36). In this scenario, one may expect that loss of Bim or Puma could provide a survival advantage to B cells that undergo development in the absence of IL-7. Yet, our data pointed to a dominant role of Bim in governing B cell homeostasis in the absence of IL-7, excluding Puma as an essential player in this process. This, however, does not exclude a role for Puma in B cell homeostasis once the B cell developmental program is achieved. Consistently, Bim.Puma DKO mice show a B cell hyperplasia that exceeds the one observed in Bim KO mice. On the other hand, loss of Bim did not restore mature B cells in IL-7− recipients to levels found in IL-7+ mice reconstituted with WT LSK cells (Figs 1 and 2). This clearly indicates that at least one additional factor, presumably another BH3-only protein, may be targeted by IL-7 during B cell maturation. A good candidate for such molecule is the BH3-only protein Bmf. Mice lacking Bmf show B cell-restricted lymphadenopathy, beginning from the pre-B cell stage onwards. Moreover, Bim and Bmf are also found to be co-expressed throughout B cell development and show similar modes of post-translation regulation (37).
The notion of competitive survival has been implicated in the control of peripheral B cell homeostasis, which relies on multiple environmental cues, including membrane-bound self-antigen and the tumor necrosis factor (TNF) cytokine family members, B cell activation factor from the tumor necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) (38, 39). We observed an accumulation of immature as well as mature Bim-deficient B cells in the spleen of IL-7-sufficient and IL-deficient recipients suggesting that an IL-7-independent and Bim-dependent pathway governs peripheral B cell homeostasis. Accordingly, B cells accumulate in the spleen of mice lacking IL-7 (40). The overt accumulation of Bim KO B cell subsets, including follicular and marginal zone B cells (Figs 3 and 4), indicates that Bim is a general regulator of B cell homeostasis in vivo. The effector function of Bim-deficient B cells generated in the absence of IL-7, including the capacity to divide in response to LPS and IL-4 in culture or to produce serum soluble antibodies in vivo, appeared intact (Fig. 5). Bim KO mice display abnormal elevated B cell numbers and serum Ig levels as consequence of disturbed B cell selection and extended B cell survival (11–13). One may envision that mature B cells accumulating in the spleen of both IL-7+ as well as IL-7− recipients may contain some deleterious autoreactive B cells that have escaped negative selection. BAFF is a critical regulator of peripheral B cell homeostasis, providing survival and maturation signals essential to the conversion of peripheral immature into mature recirculating B cells (38, 39). Interestingly, BAFF-mediated signals are able to thwart BCR-mediated apoptosis through suppression of Bim suggesting that concerted signaling through BCR and BAFF receptors modulates mature B cell homeostasis (41). In this context, loss of Bim may also correct the defects in mature B cell homeostasis caused by deficiency in BAFF–BAFF-R signaling.
In conclusion, we provide evidence that adult B cell development could be partially rescued in the absence of IL-7 by the loss of Bim, but not by the loss of Puma, in hematopoietic progenitors. These data suggest that BH3-only proteins in B cell homeostasis operate in a hierarchical order, highlighting the chief role of Bim in governing this process.
Funding
Institute Pasteur and Inserm; European Molecular Biology Organization long-term fellowship (ALTF 630-2006) to N.L.A.
Acknowledgments
We thank Odile Richard-Le Goff for technical support.
Glossary
Abbreviations
- APRIL
a proliferation-inducing ligand
- BAFF
B cell activation factor from the tumor necrosis factor family
- BH
Bcl-2 homology
- DKO
double knockout
- FLT3L
Fms-related tyrosine kinase 3 ligand
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
References
- 1.Strasser A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005;5:189. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 2.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves NL, Arosa FA, van Lier RA. Common gamma chain cytokines: dissidence in the details. Immunol. Lett. 2007;108:113. doi: 10.1016/j.imlet.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Alves NL, van Lier RA, Eldering E. Withdrawal symptoms on display: Bcl-2 members under investigation. Trends Immunol. 2007;28:26. doi: 10.1016/j.it.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 7.Alves NL, Derks IA, Berk E, Spijker R, van Lier RA, Eldering E. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 8.You H, Pellegrini M, Tsuchihara K, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J. Exp. Med. 2006;203:1657. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell. 2006;21:749. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Bauer A, Villunger A, Labi V, et al. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc. Natl Acad. Sci. USA. 2006;103:10979. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 2003;198:1119. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 13.Erlacher M, Labi V, Manzl C, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J. Exp. Med. 2006;203:2939. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliver PM, Wang M, Zhu Y, White J, Kappler J, Marrack P. Loss of Bim allows precursor B cell survival but not precursor B cell differentiation in the absence of interleukin 7. J. Exp. Med. 2004;200:1179. doi: 10.1084/jem.20041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J. Exp. Med. 2004;200:1189. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J. Exp. Med. 2005;201:971. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espina B, Liang M, Russell RG, Hulley PA. Regulation of bim in glucocorticoid-mediated osteoblast apoptosis. J. Cell. Physiol. 2008;215:488. doi: 10.1002/jcp.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang M, Russell G, Hulley PA. Bim, Bak, and Bax regulate osteoblast survival. J. Bone Miner. Res. 2008;23:610. doi: 10.1359/jbmr.080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamura N, Kugimiya F, Oshima Y, et al. Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS ONE. 2007;2:e1058. doi: 10.1371/journal.pone.0001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 21.Colucci F, Soudais C, Rosmaraki E, Vanes L, Tybulewicz VL, Di Santo JP. Dissecting NK cell development using a novel alymphoid mouse model: investigating the role of the c-abl proto-oncogene in murine NK cell differentiation. J. Immunol. 1999;162:2761. [PubMed] [Google Scholar]
- 22.Vosshenrich CA, Ranson T, Samson SI, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 2005;174:1213. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 23.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 24.Huntington ND, Xu Y, Puthalakath H, et al. CD45 links the B cell receptor with cell survival and is required for the persistence of germinal centers. Nat. Immunol. 2006;7:190. doi: 10.1038/ni1292. [DOI] [PubMed] [Google Scholar]
- 25.Liu A, Vosshenrich CA, Lagresle-Peyrou C, et al. Competition within the early B-cell compartment conditions B-cell reconstitution after hematopoietic stem cell transplantation in nonirradiated recipients. Blood. 2006;108:1123. doi: 10.1182/blood-2006-01-0061. [DOI] [PubMed] [Google Scholar]
- 26.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl Acad. Sci. USA. 1995;92:377. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 28.Ekoff M, Kaufmann T, Engstrom M, et al. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood. 2007;110:3209. doi: 10.1182/blood-2007-02-073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat. Immunol. 2003;4:773. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 30.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 31.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 32.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 33.Demehri S, Liu Z, Lee J, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 2001;7:73. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 35.Maraskovsky E, Peschon JJ, McKenna H, Teepe M, Strasser A. Overexpression of Bcl-2 does not rescue impaired B lymphopoiesis in IL-7 receptor-deficient mice but can enhance survival of mature B cells. Int. Immunol. 1998;10:1367. doi: 10.1093/intimm/10.9.1367. [DOI] [PubMed] [Google Scholar]
- 36.Stahl M, Dijkers PF, Kops GJ, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 2002;168:5024. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 37.Labi V, Erlacher M, Kiessling S, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J. Exp. Med. 2008;205:641. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr. Opin. Immunol. 2005;17:282. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Stadanlick JE, Cancro MP. BAFF and the plasticity of peripheral B cell tolerance. Curr. Opin. Immunol. 2008;20:158. doi: 10.1016/j.coi.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J. Exp. Med. 2001;194:1141. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craxton A, Draves KE, Gruppi A, Clark EA. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J. Exp. Med. 2005;202:1363. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]