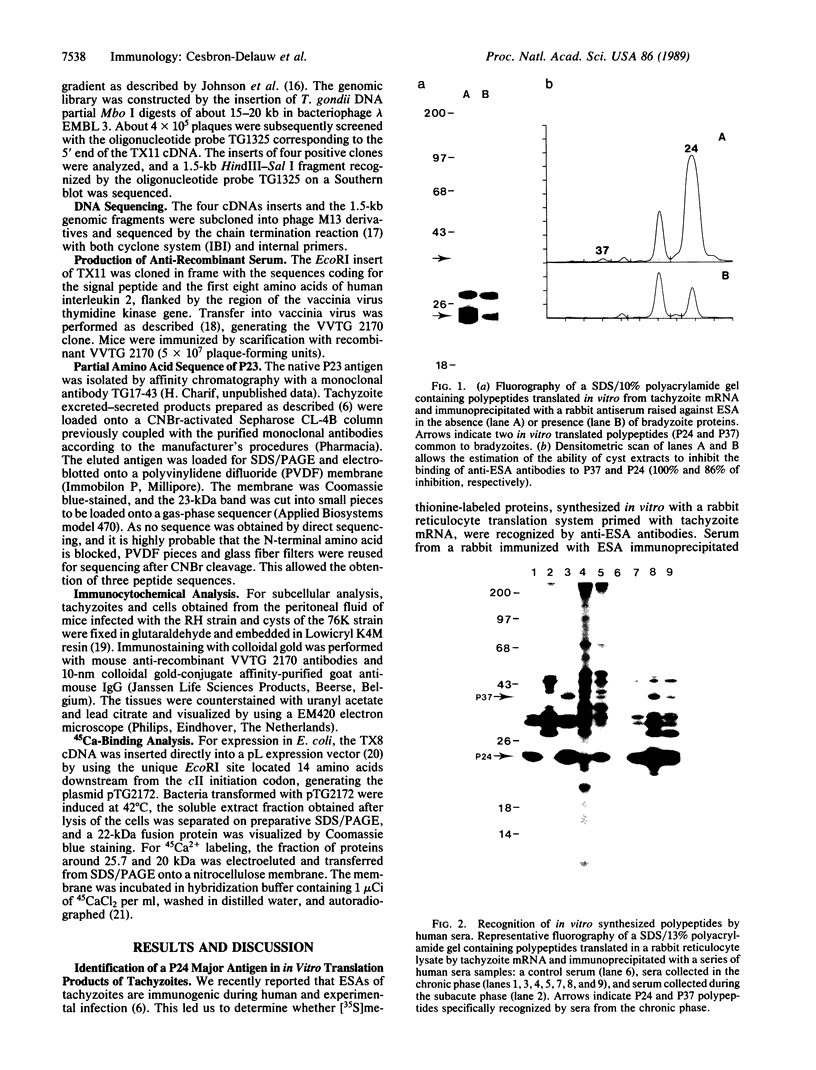
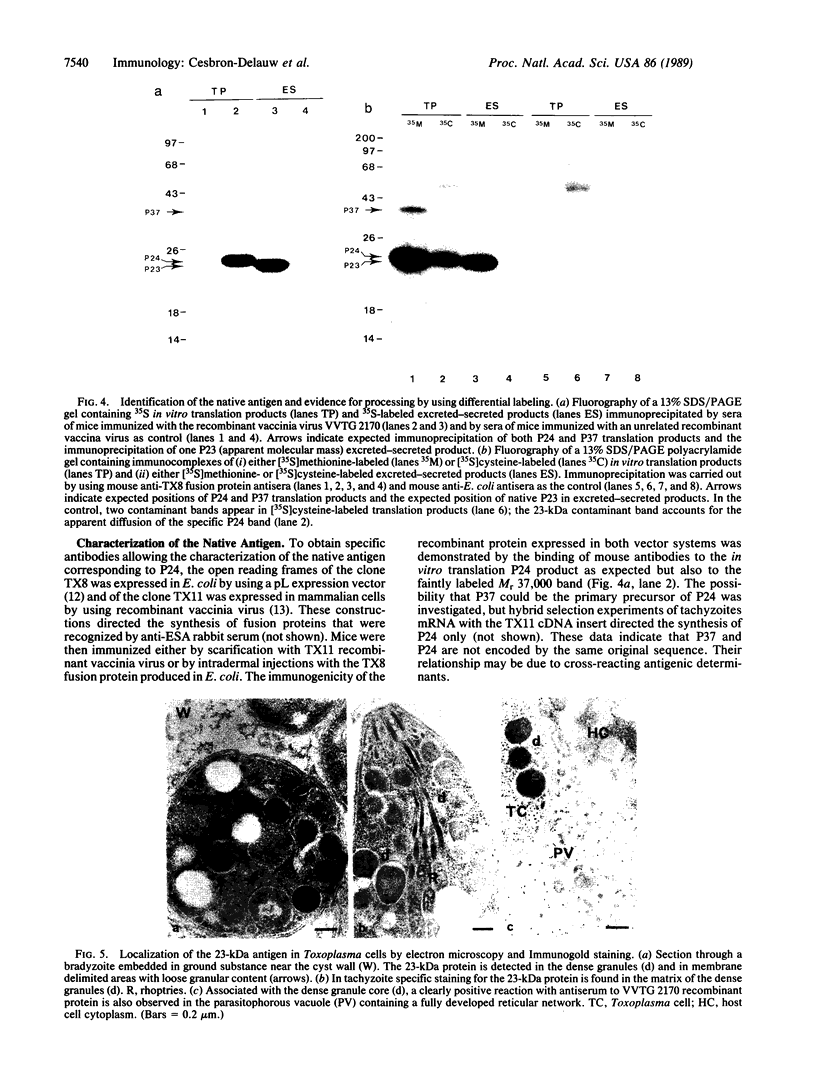
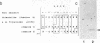Abstract
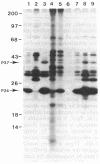
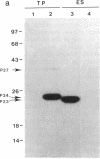
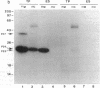
The strategy chosen for cloning potential vaccine antigens of Toxoplasma gondii was based on the hypothesis that the definitive protection observed in natural infection is due to the presence of encysted bradyzoite forms in host tissues throughout life. The antigens released by the bradyzoites would maintain an immune response against the invading tachyzoites. This led us to identify in tachyzoite in vitro translation products a polypeptide of 24 kDa that is an excreted-secreted antigen (ESA) and is cross-reactive with bradyzoites. In addition, the detection of anti-P24 IgG antibodies is correlated with the chronic infection in man. The gene encoding P24 has been isolated, sequenced, and expressed in Escherichia coli and eukaryotic cells. The recombinant proteins were immunogenic in mice, producing anti-native P23 antibodies. Immunocytochemical analysis located the native antigen in the dense granules of both tachyzoite and bradyzoite forms and showed that it is secreted within host-cell-modified phagosome. Moreover 45Ca2+ labeling as well as regional homologies indicate that this protein has Ca2+-binding properties, suggesting its physiological importance in host-cell invasion. P23 is of diagnostic interest as a marker of chronic toxoplasmosis and is proposed as a vaccine component.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley J. K. Toxoplasmosis in animals. Vet Rec. 1976 Aug 14;99(7):123–127. doi: 10.1136/vr.99.7.123. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P. Vaccination against parasitic diseases: some alternative concepts for the definition of protective antigens. Ann Inst Pasteur Immunol. 1988 Jan-Feb;139(1):109–117. doi: 10.1016/0769-2625(88)90135-3. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy F., Deslee D., Santoro F., Charif H., Auriault C., Decoster A., Duquesne V., Capron A. Induction of a protective antibody-dependent response against toxoplasmosis by in vitro excreted/secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol. 1988 Sep;10(5):553–567. doi: 10.1111/j.1365-3024.1988.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Dissous C., Dissous C., Capron A. Isolation and characterization of surface antigens from Schistosoma mansoni schistosomula. Mol Biochem Parasitol. 1981 Aug;3(4):215–225. doi: 10.1016/0166-6851(81)90053-0. [DOI] [PubMed] [Google Scholar]
- Entzeroth R., Dubremetz J. F., Hodick D., Ferreira E. Immunoelectron microscopic demonstration of the exocytosis of dense granule contents into the secondary parasitophorous vacuole of Sarcocystis muris (Protozoa, Apicomplexa). Eur J Cell Biol. 1986 Aug;41(2):182–188. [PubMed] [Google Scholar]
- Frenkel J. K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988 Oct;4(10):273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- Hughes H. P., Connelly C. A., Strangeways J. E., Hudson L. Antigen specific lymphocyte transformation induced by secreted antigens from Toxoplasma gondii. Clin Exp Immunol. 1984 Dec;58(3):539–547. [PMC free article] [PubMed] [Google Scholar]
- Johnson A. M., Dubey J. P., Dame J. B. Purification and characterization of Toxoplasma gondii tachyzoite DNA. Aust J Exp Biol Med Sci. 1986 Aug;64(Pt 4):351–355. doi: 10.1038/icb.1986.37. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med. 1972 Nov 1;136(5):1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Drillien R., Spehner D., Skory S., Schmitt D., Wiktor T., Koprowski H., Lecocq J. P. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984 Nov 8;312(5990):163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laugier M., Quilici M. Intérêt expérimental d'une souche de Toxoplasme peu pathogène pour la souris. Ann Parasitol Hum Comp. 1970 Jul-Aug;45(4):389–403. [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Nagel S. D., Boothroyd J. C. The alpha- and beta-tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol Biochem Parasitol. 1988 Jun;29(2-3):261–273. doi: 10.1016/0166-6851(88)90081-3. [DOI] [PubMed] [Google Scholar]
- Ohno S., Emori Y., Imajoh S., Kawasaki H., Kisaragi M., Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984 Dec 6;312(5994):566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L., Adams G. M., Weidner E. Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. J Cell Biol. 1986 Sep;103(3):867–874. doi: 10.1083/jcb.103.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufty R. M., Kretsinger R. H. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science. 1975 Jan 17;187(4172):167–169. doi: 10.1126/science.1111094. [DOI] [PubMed] [Google Scholar]
- Werk R. How does Toxoplasma gondii enter host cells? Rev Infect Dis. 1985 Jul-Aug;7(4):449–457. doi: 10.1093/clinids/7.4.449. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]