Abstract
Two enzymes have been identified for the oxidative metabolism of carotenoids in mammals. Carotene-15,15’-monooxygenase (CMO-I) primarily centrally cleaves β,β-carotene to form vitamin A. We hypothesize that carotene-9′,10’-monooxygenase (CMO-II) plays a key role in metabolism of acyclic nonprovitamin A carotenoids such as lycopene. We investigated carotenoid bioaccumulation in young adult, male, wild-type (WT) mice or mice lacking CMO-II (CMO-II KO). Mice were fed an AIN-93G diet or identical diets supplemented with 10% tomato powder, 130 mg lycopene/kg diet (10% lycopene beadlets), or placebo beadlets for 4 or 30 d. Lycopene preferentially accumulated in CMO-II KO mouse tissues and serum compared with WT mouse tissues. β-Carotene preferentially accumulated in some CMO-II KO mouse tissues compared with WT mouse tissues. Relative tissue mRNA expression of CMO-I and CMO-II was differentially expressed in mouse tissues, and CMO-II, but not CMO-I, was expressed in mouse prostate. In conclusion, the loss of CMO-II expression leads to increased serum and tissue concentrations of lycopene in tomato-fed mice.
Introduction
Recent landmark discoveries have established that the carotenoid, β-carotene, is metabolized by carotene-15,15’-monooxygenase (CMO-I)8 through central chain cleavage to form vitamin A, with subsequent metabolism resulting in the formation of retinoic acid and other retinoids (1). The metabolism of β-carotene is widely studied, yet very little is known about the metabolism of many other carotenoids and the putative biological significance of resulting carotenoid metabolites (2). Lycopene is the red pigment and predominant carotenoid in tomato products followed by phytoene, phytofluene, and β-carotene. Phytofluene and phytoene are desaturated carotenoid precursors to lycopene and β-carotene in the plant biosynthetic pathway (3).
We and others (4, 5) have identified metabolites of lycopene in rodent tissues and human plasma. Interestingly, in vivo serum and tissue concentrations of these lycopene metabolites are of comparable concentrations to retinoids, suggesting that they may exert biological functions (6, 7). Evidence from an in vitro study suggests that lycopene is metabolized by carotene-9′,10’-monooxygenase (CMO-II), the enzyme catalyzing the eccentric oxidative chain cleavage of carotenoids (5, 8). However, the role of CMO-II for lycopene metabolism lacks functional testing in genetically defined animal models employing KO technology.
We investigated the metabolism of lycopene and other tomato carotenoids in mice with normal genotype [wild-type (WT)] or CMO-II KO mice by quantitatively measuring tissue and serum carotenoid accumulation. In addition, we report the relative tissue expression of genes for the enzymes CMO-I and CMO-II in male mice fed diets containing tomato powder or lycopene beadlets for 4 or 30 d. Our study identifies lycopene as an in vivo substrate for CMO-II metabolism.
Materials and Methods
Mice and diet.
C57BL/6 × 129/SvJ (F1) mice (WT, n = 40) were purchased from Jackson Laboratory. Breeder pairs of B6;129S6-Bcdo2tm1Dnp (CMO-II KO, n = 4) mice were shipped to the University of Illinois courtesy of collaborators Drs. Adrian Wyss at DSM Nutritional Products (Basel, Switzerland) and Johannes von Lintig at Case Western Reserve University (Cleveland, OH). The generation of CMO-II KO mice is described by J. Amengual and J. von Lintig, Case Western University (personal communication). For these studies, CMO-II KO mice were bred at the University of Illinois animal facilities. We confirmed the genotype of this model using genomic DNA from mouse tail biopsies and the Sigma Extract-N-Amp Tissue PCR kit. Mice were housed in shoebox cages with free access to water and were weighed every other day. Male mice were fed an AIN-93G diet for 3 wk prior to randomization to the experimental diets. This study was conducted in 4 cohorts 1 mo apart. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Nine- to 12-wk-old male mice were randomly assigned to 1 of 4 experimental diets: AIN-93G (AIN), 10% tomato powder (TP; Futureceuticals), lycopene (LYC; 10% lycopene beadlet, DSM), or placebo (PB; 10% beadlet, DSM). Mice consumed the experimental diets ad libitum for 4 or 30 d (n = 10 of each genotype in each diet group at both time points). At the conclusion of the study, mice were asphyxiated by CO2 and blood was collected by cardiac puncture. Brain, thymus, lung, liver, spleen, duodenum mucosa, kidney, adrenal gland, seminal vesicle, testis, and prostate were removed and snap-frozen in liquid nitrogen and then stored at −80°C.
All diets were balanced for total fiber, nitrogen, and energy (Table 1) (9). Additionally, vitamin A levels were reduced in all diets to 1.57 μmol retinyl palmitate/kg diet to ensure adequate absorption of carotenoids without resulting in a vitamin A deficiency (7). Fresh diet was provided every 48 h; new diet was made monthly and stored in the dark at 4°C.
TABLE 1.
Composition of diets employed in 4- and 30-d feeding studies of lycopene metabolism in WT and CMO-II KO mice
| Ingredient | AIN | TP1 | PB2 | LYC3 |
| g/kg diet | ||||
| Cornstarch | 398 | 331 | 398 | 398 |
| Casein | 200 | 188 | 200 | 200 |
| Maltodextrin | 102 | 132 | 102 | 102 |
| Sucrose | 100 | 100 | 100 | 100 |
| Nonnutritive cellulose | 50 | 34 | 50 | 50 |
| Mineral mix4 | 35 | 35 | 35 | 35 |
| Vitamin mix5 | 10 | 10 | 10 | 10 |
| l-Cystine | 3 | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean oil | 100 | 65 | 100 | 100 |
| TP | 0 | 100 | 0 | 0 |
| 10% LYC | 0 | 0 | 0 | 0.1 |
| PB | 0 | 0 | 0.1 | 0 |
As measured by HPLC; the TP (FutureCeuticals, Momence, IL) diet contained 5.5 ± 0.5 mg phytoene, 1.4 ± 0.3 mg phytofluene, 0.4 ± 0.0 mg β-carotene, and 110 ± 16 mg lycopene/g diet.
As measured by HPLC; the PB (DSM) diet provided 0 mg carotenoids/g diet.
As measured by HPLC; the LYC (10% DSM) diet provided 130 ± 31 mg lycopene/g diet.
AIN93M-MX formulation (9).
AIN93-VX formulation (9) with reduced vitamin A content (1.57 mol retinyl palmitate/g diet.)
Carotenoid extraction and HPLC analysis.
All chemicals were purchased from Fisher Scientific. Tissue and serum samples were extracted with hexanes as described previously with minor modifications (10). Briefly, 0.08 g tissue was added to 5 mL of ethanol containing 0.01% BHT. Saturated KOH was added and the samples were vortexed and saponified in a water bath at 60°C for 30 min with vortexing every 10 min. Distilled water (2 mL) was added and carotenoids were extracted 3 times with hexanes (6 mL). Serum carotenoids were extracted from 0.15 mL serum in 0.15 mL ethanol/BHT solution with 0.15 mL distilled water and 1.5 mL hexanes.
All extracts were kept on ice under yellow lights and flushed with argon to prevent degradation. For analysis, samples were dissolved in methyl-tert-butyl ether and injected onto a C30 HPLC column (4.6 × 150 mm, 3 μm; YMC) maintained at 18°C. A reverse-phase HPLC-photo-diode array method was utilized and individual carotenoids were identified by comparison of elution times to standards and absorption spectral analysis (4, 11).
Real-time PCR analysis.
Relative tissue expression of CMO-I and CMO-II mRNA was evaluated using a TRIzol (Invitrogen) extraction per the manufacturer’s instructions followed by DNase I treatment (New England Biolab). The concentration and quality of mRNA were determined by spectrophotometry and agarose gel electrophoresis before synthesis of complimentary DNA by Superscript II Reverse Transcriptase (Invitrogen) using random hexamers (Applied Biosystems). Primer pairs were selected to measure CMO-I (NM_021486): forward-5′-ATGGAGATAATATTTGGCCAG and reverse-5′- AACTCAGACACCACGATTC; and CMO-II (NM_1332217): forward-5′- GTTATCTACTTCGAGTTGGACCTGG and reverse-5′-AAGCAACGCCATTCCATCA. Real-time PCR was performed using a 7900HT Fast Real-Time PCR detection system (Applied Biosystems) with the SYBR green fluorescence dye. Threshold cycle (Ct) values for the 18S gene were used as a control and data were expressed as 2−ΔΔCt.
Statistical analysis.
A 2 × 4 factorial design was used with 2 genotypes and 4 diets for carotenoid bioaccumulation and organ and body weights in the 4- and 30-d feeding studies. Interactions between genotype and diet were investigated and all data were analyzed by ANOVA followed by Tukey’s post hoc analysis using SAS 9.1 with α = 0.05. When assumptions were violated, data were transformed by natural log and results were expressed as means ± SEM unless otherwise specified. The 2-way ANOVA for the mRNA expression of CMO-I and CMO-II also compared the effects of the study duration.
Results
Body and organ weights.
The diets did not affect body weight, growth, or organ weight in either the 4- or 30-d feeding study (Supplemental Table 1). Final body weight (P = 0.27) and change in body weight (P = 0.79) did not differ by genotype in the 4-d feeding study, although CMO-II KO mice had enlarged spleens (P < 0.001) and reduced size of testes (P < 0.001) and seminal vesicles (P < 0.001) as a percentage of body weight compared with WT mice (Supplemental Table 1). Body weight (P = 0.44) and change in body weight over the study duration (P = 0.67) were not significantly affected by genotype, although alterations in the weights of several organs were noted (Supplemental Table 1). Again, at the conclusion of the 30-d feeding study, CMO-II KO mice had heavier spleens as a percent of body weight (P < 0.001) but lighter testes (P < 0.001) and seminal vesicles (P < 0.001).
Tissue and serum carotenoids.
Hepatic, testicular, and serum lycopene preferentially accumulated in CMO-II KO mice compared with WT mice (Table 2) after 4 or 30 d of consuming the LYC or TP diets. The percentage of lycopene as cis isomers was also altered in tissues of CMO-II KO mice fed lycopene-containing diets (TP and LYC) for 30 d but was not altered in the 4-d feeding study (Supplemental Table 2).
TABLE 2.
| Genotype | 10% TP diet | Lycopene diet | P-value3 | ||||
| WT | CMO-II KO | WT | CMO-II KO | G | D | G × D | |
| 4 d | nmol/g wet weight | ||||||
| Liver | 17.7 ± 1.02b | 47.1 ± 8.13a | 10.6 ± 1.49b | 63.4 ± 9.23a | <0.001 | 0.483 | 0.036 |
| Testis | 1.82 ± 0.09b | 3.34 ± 0.18a | 1.85 ± 0.08b | 2.62 ± 0.13a | <0.001 | 0.085 | 0.018 |
| Serum | 2.22 ± 0.29b | 4.05 ± 0.66a | 1.54 ± 0.24c | 2.76 ± 0.60b | 0.004 | 0.038 | 0.551 |
| 30 d | |||||||
| Liver | 7.64 ± 2.84b | 38.1 ± 12.50a | 12.2 ± 2.90b | 37.0 ± 6.12a | <0.001 | 0.291 | 0.704 |
| Testis | 0.74 ± 0.16c | 1.76 ± 0.41a | 0.51 ± 0.05d | 1.06 ± 0.13b | <0.001 | 0.137 | 0.156 |
| Serum | 0.63 ± 0.10b | 0.89 ± 0.12a | 0.22 ± 0.04d | 0.46 ± 0.05c | 0.005 | 0.750 | 0.082 |
Values are means ± SEM, = 6. Means within a row without a common letter differ, P < 0.05.
Lycopene was not detected in serum or tissues of mice that were fed the AIN or PB diets.
-values associated with the effects of genotype (G), diet (D), or genotype by diet (G × D) interaction.
Levels of β-carotene in the TP diet were quite low and resulted in marginally detectable levels in WT mouse tissues and serum after 4 or 30 d of feeding (Table 3), although CMO-II KO mice accumulated slightly more serum and tissue β-carotene compared with WT mice.
TABLE 3.
Tissue β-carotene concentrations of CMO-II KO and WT mice that were fed a TP for 4 or 30 d1
| Tissue | Genotype | ||
| WT | CMO-II KO | P-value | |
| 4 d | pmol/g wet weight | ||
| Liver | 3.00 ± 0.00 | 4.00 ± 0.00 | 0.115 |
| Testis | 1.00 ± 0.00 | 2.00 ± 0.00 | <0.001 |
| Serum | ND2 | ND2 | |
| 30 d | |||
| Liver | 2.00 ± 1.00 | 9.00 ± 2.00 | 0.002 |
| Testis | 3.00 ± 2.00 | 1.00 ± 1.00 | 0.003 |
| Serum | ND2 | 1.00 ± 0.00 | 0.235 |
Values are means ± SEM, = 8.
ND, Not determined, limit of detection (1 pmol/g).
After 4 or 30 d of feeding TP, phytoene and phytofluene were not detected in serum or most tissues. On the other hand, the liver accumulated detectable levels of these lycopene precursors, but concentrations did not significantly differ between genotypes (Supplemental Table 3).
We confirmed the enhanced carotenoid bioaccumulation in CMO-II KO mice in a separate, longer term feeding study (60 d). C57BL/6 and CMO-II KO mice were fed a 10% TP diet (0.13 mg lycopene/g diet) and again CMO-II KO mice preferentially accumulated lycopene in most tissues excluding the liver, spleen, and seminal vesicles (Supplemental Table 4). β-Carotene accumulated in slightly higher concentrations in the liver (P = 0.005), adrenals (P = 0.007), kidney (P = 0.009), and prostate (P < 0.001) of CMO-II KO mice compared with WT mice and, notably, accumulation of phytoene and phytofluene did not differ by genotype (data not shown).
mRNA expression of CMO-I and CMO-II.
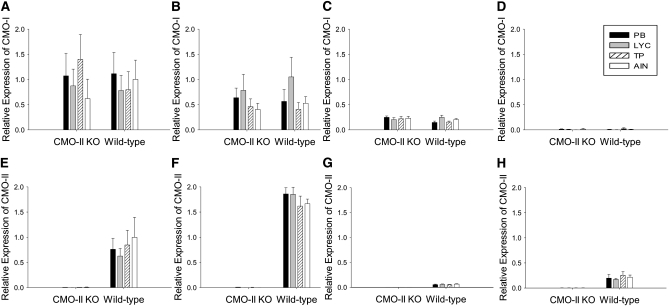
The mRNA expression of CMO-I and CMO-II was measured in tissues of WT and CMO-II KO mice from the 4- and 30-d feeding studies (Fig. 1). Ablation of CMO-II mRNA expression was confirmed in the liver, duodenum, testis, and prostate of CMO-II KO mice. Of the 4 tissues analyzed, the liver had the highest relative mRNA expression of CMO-I whereas the prostate had no detectable expression of CMO-I (Fig. 1). CMO-II was highly expressed in the duodenum followed by the liver, prostate, and testis of WT mice. There was no impact of the diets or feeding duration on the mRNA expression of CMO-I or CMO-II in these tissues (Fig. 1).
FIGURE 1.
Relative mRNA expression of CMO-I is differentially expressed in the liver, (A) duodenum (B), testis (C), and prostate (D) and CMO-II is differentially expressed in the liver (E), duodenum (F), testis (G), and prostate (H) of WT or CMO-II KO mice that were fed the PB, LYC, TP, or AIN diets after 4 or 30 d. Results are expressed as 2−ΔΔCt with 18s as the control gene and all comparisons were made with hepatic expression of CMO-I or CMO-II from mice that were fed the AIN diet. Values are means ± SEM, n = 20. The diets did not affect the mRNA expression of CMO-I or CMO-II in the 4- or 30-d study (P ≥ 0.41). That of CMO-II was lower in CMO-II KO compared with WT mice (P < 0.001). Those of CMO-I and CMO-II did not differ between the 4- and 30-d feeding studies (P ≥ 0.50).
Discussion
Two separate feeding studies were designed to investigate carotenoid tissue bioaccumulation and the expression of carotenoid cleavage enzymes in mice lacking the enzyme, CMO-II. WT and CMO-II KO mice were fed carotenoid-containing diets or their respective control diets for 4 or 30 d. We hypothesized that CMO-II KO mice would accumulate enhanced quantities of tomato carotenoids in their tissues due to reduced metabolic cleavage.
CMO-II KO mice that were fed the LYC or TP diets had significantly higher tissue and serum concentrations of lycopene compared with WT mice after 4 or 30 d (Table 2). The liver, which accumulates substantial quantities of lycopene compared with other tissues (10, 12–15), had 1- to 5-fold more lycopene in CMO-II KO mice than in WT mice. Overall, lycopene preferentially accumulated in CMO-II KO mouse tissues and serum, which supports the hypothesis that lycopene is metabolized by CMO-II in vivo.
The bioaccumulation of the lycopene and β-carotene precursors, phytoene and phytofluene, was quantitatively measured. Although lycopene, phytoene, and phytofluene all have the putative requisite 9′,10’ double bond for the CMO-II enzyme, hepatic phytoene and phytofluene accumulation did not differ between genotypes in either the 4- or 30-d study (Supplementary Table 3) (5). Phytoene and phytofluene did not accumulate in detectable quantities in other tissues or serum. These data suggest that phytoene and phytofluene may not be substrates for the CMO-II enzyme or did not accumulate in high enough concentrations in the liver to induce metabolic cleavage. This finding may also suggest that despite almost identical structures, the reduced number of conjugated double bonds of phytofluene (n = 5) and phytoene (n = 3) compared with lycopene (n = 11) may affect the affinity of the carotenoid for the CMO-II enzyme.
Due to low concentrations of β-carotene in the diet, very little β-carotene could be measured in the liver, testis, or serum of WT or CMO-II KO mice. Nonetheless, some CMO-II KO mouse tissues accumulated significantly more β-carotene compared with WT mouse tissues (Table 3). This suggests that β-carotene is also partly metabolized by CMO-II, although previously published data indicate that CMO-I is the primary cleavage enzyme for β-carotene (1, 7, 8).
The mRNA expression of CMO-I and CMO-II in tissues of WT and CMO-II KO mice that were fed the experimental diets for 4 or 30 d was measured. The carotenoid-containing diets did not alter the expression of CMO-I or CMO-II in tissues of WT or CMO-II KO mice in either study (Fig. 1). The mRNA expression of CMO-I and CMO-II did not differ between the 4- or 30-d feeding studies. This suggests that the carotenoid content of the diet was not high enough to alter mRNA expression, there is not a sensitive biological feedback mechanism for lycopene metabolites, or the activity of CMO-I and CMO-II are regulated at the protein level. Alternatively, we hypothesize that greater concentrations of β-carotene in the diet would have decreased the expression of CMO-I due to the necessary strict regulation of vitamin A synthesis in the body. Of the tissues analyzed for CMO mRNA expression, the liver had the highest relative mRNA expression of CMO-I and CMO-II was most highly expressed in the duodenum. Our current relative mRNA expression data corroborate the data from 2 previous studies in mice, 1 in rats, and 1 in humans (1, 16–19). Together, these data suggest that CMO-I and CMO-II are differentially expressed in tissues, but mRNA expression was not altered by the carotenoid-containing diets.
Notably, CMO-I expression was not detected in the prostate of WT or CMO-II KO mice. These results may imply that retinoids, produced by CMO-I cleavage, may not be locally produced in the prostate of young, adult, male mice. In contrast, because CMO-II was expressed in the prostate, this may suggest that lycopene metabolites could be locally produced in the prostate. Further work regarding relative tissue expression, protein levels, and enzyme activity of carotenoid cleavage enzymes is warranted.
The inverse relationship between prostate cancer incidence and tomato product consumption (20–23) has often been attributed to lycopene. It is unclear whether tomato carotenoids, including lycopene, and/or other tomato components, or their metabolites primarily affect prostate cancer risk. We propose that lycopene metabolites, produced by CMO-II, are partially responsible for this effect (23). Future cancer trials investigating lycopene metabolism may provide insight for this hypothesis.
The bioaccumulation data in these 2 studies provide support for the hypothesis that in vivo, lycopene is metabolized by the CMO-II enzyme. In contrast, phytoene and phytofluene did not preferentially accumulate in CMO-II KO mouse tissues, suggesting that they are either not substrates for the CMO-II enzyme or that these carotenoids did not accumulate in high enough concentrations to induce CMO-II cleavage. Interestingly, we showed that CMO-I and CMO-II are differentially expressed in mouse tissues, the expression of these enzymes is not altered by the carotenoid-containing diets, and the prostate lacks expression of CMO-I. A separate publication will present additional information regarding the generation of CMO-II KO mice and the impact of CMO-II on the metabolism of other carotenoids (J. Amengual and J. von Lintig, Case Western University, personal communication). Further characterization of these mouse models is essential and will provide novel insight into carotenoid metabolism and the effect of carotenoids on health outcomes.
Supplementary Material
Acknowledgments
We thank James Weidner for assisting with hepatic mRNA expression analysis. N.A.F., S.K.C., J.V.L., A.W., and J.W.E Jr designed the research; N.A.F. conducted the research, analyzed data, and performed statistical analysis; J.V.L. and A.W. provided essential materials; N.A.F., S.K.C., J.V.L., and J.W.E. Jr wrote the paper; and N.A.F. and J.W.E. Jr had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grant PHS-1-RO1 CA125384 and National Center for Research Resources National Institutes of Health grant RR020292.
Supplemental Tables 1–4 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AIN, AIN-93G diet; CMO-I, carotene-15,15’-monooxygenase; CMO-II, carotene-9′,10’-monooxygenase; Ct, threshold cycle; KO, knockout; LYC, lycopene diet; PB, placebo beadlet diet; PDA, photo-diode array; TP, tomato powder diet; WT, wild-type.
Literature Cited
- 1.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–61 [DOI] [PubMed] [Google Scholar]
- 2.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr. 2010;30:35–56 [DOI] [PubMed] [Google Scholar]
- 3.Williams RJ, Britton G, Charlton JM, Goodwin TW. The stereospecific biosynthesis of phytoene and polyunsaturated carotenes. Biochem J. 1967;104:767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12'-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136:1552–7 [DOI] [PubMed] [Google Scholar]
- 5.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindshield BL, King JL, Wyss A, Goralczyk R, Lu CH, Ford NA, Erdman JW., Jr Lycopene biodistribution is altered in 15,15'-carotenoid monooxygenase knockout mice. J Nutr. 2008;138:2367–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–6 [DOI] [PubMed] [Google Scholar]
- 9.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 10.Boileau TW, Clinton SK, Zaripheh S, Monaco MH, Donovan SM, Erdman JW., Jr Testosterone and food restriction modulate hepatic lycopene isomer concentrations in male F344 rats. J Nutr. 2001;131:1746–52 [DOI] [PubMed] [Google Scholar]
- 11.Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest Ophthalmol Vis Sci. 1995;36:2756–61 [PubMed] [Google Scholar]
- 12.Boileau TW, Clinton SK, Erdman JW., Jr Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male F344 rats. J Nutr. 2000;130:1613–8 [DOI] [PubMed] [Google Scholar]
- 13.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in Dunning R3327-H prostate adenocarcinomas. Cancer Res. 2007;67:836–43 [DOI] [PubMed] [Google Scholar]
- 14.Zaripheh S, Boileau TW, Lila MA, Erdman JW., Jr 14C-lycopene and 14C-labeled polar products are differentially distributed in tissues of F344 rats prefed lycopene. J Nutr. 2003;133:4189–95 [DOI] [PubMed] [Google Scholar]
- 15.Schmitz HH, Poor CL, Wellman RB, Erdman JW., Jr Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr. 1991;121:1613–21 [DOI] [PubMed] [Google Scholar]
- 16.Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W. Expression pattern and localization of beta,beta-carotene 15,15'-dioxygenase in different tissues. Biochem J. 2001;354:521–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindqvist A, Andersson S. Cell type-specific expression of beta-carotene 15,15'-mono-oxygenase in human tissues. J Histochem Cytochem. 2004;52:491–9 [DOI] [PubMed] [Google Scholar]
- 18.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9′,10'-monooxygenase in human tissues. J Histochem Cytochem. 2005;53:1403–12 [DOI] [PubMed] [Google Scholar]
- 19.Zaripheh S, Nara TY, Nakamura MT, Erdman JW., Jr Dietary lycopene downregulates carotenoid 15,15'-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr. 2006;136:932–8 [DOI] [PubMed] [Google Scholar]
- 20.Etminan M, Takkouche B, Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340–5 [PubMed] [Google Scholar]
- 21.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–76 [DOI] [PubMed] [Google Scholar]
- 22.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev. 2008;66:667–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: are lycopene metabolites bioactive? Arch Biochem Biophys. 2007;458:136–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.