Abstract
Protein synthesis and eukaryotic initiation factor (eIF) activation are increased in skeletal muscle of neonatal pigs parenterally infused with amino acids. Leucine appears to be the most effective single amino acid to trigger these effects. To examine the response to enteral leucine supplementation, overnight food-deprived 5-d-old pigs were gavage fed at 0 and 60 min a: 1) low-protein diet (LP); 2) LP supplemented with leucine (LP+L) to equal leucine in the high-protein diet (HP); or 3) HP diet. Diets were isocaloric and equal in lactose. Fractional protein synthesis rates and translation initiation control mechanisms were examined in skeletal muscles and visceral tissues 90 min after feeding. Protein synthesis rates in longissimus dorsi, gastrocnemius, and masseter muscles, heart, jejunum, kidney, and pancreas, but not liver, were greater in the LP+L group compared with the LP group and did not differ from the HP group. Feeding LP+L and HP diets compared with the LP diet increased phosphorylation of mammalian target of rapamycin (mTOR), 4E-binding protein 1, ribosomal protein S6 kinase-1, and eIF4G and formation of the active eIF4E·eIF4G complex in longissimus dorsi muscle. In all tissues except liver, activation of mTOR effectors increased in pigs fed LP+L and HP vs. LP diets. Our results suggest that leucine supplementation of a low-protein meal stimulates protein synthesis in muscle and most visceral tissues to a rate similar to that achieved by feeding a high-protein meal and this stimulation involves activation of mTOR downstream effectors.
Introduction
The nutritional management of low-birth weight infants (LBW)6 has improved in recent years (1, 2); nonetheless, most are discharged weighing less than the tenth percentile for age (3). Some remain small to adulthood and exhibit adverse long-term developmental outcomes, including learning impairment and reduced work capacity (4, 5). Consumption of a high-protein diet can increase weight and length of LBW infants at discharge (6, 7). However, this is often difficult to achieve due to the potential for adverse events and difficulty with intake volume. Indeed, early studies associated high-protein intakes with azotemia, hyperammonemia, and metabolic acidosis (6). However, provision of moderate compared with low amino acid levels by parenteral infusion has been shown to increase whole body protein synthesis rates in LBW infants (7).
Due to the invasiveness of the techniques required to evaluate the effects of amino acids on protein synthesis, we have used the neonatal pig as a model of the human neonate. Our work suggests that neonatal pigs use dietary amino acids efficiently for growth, because they can increase protein synthesis in response to feeding (8). The protein synthetic response to ingestion of a meal occurs in all tissues, but the greatest effect occurs in skeletal muscle and the response decreases with development (8). This response to feeding is independently mediated by the postprandial rise in amino acids and insulin (9, 10). Moreover, parenteral infusion of the branched-chain amino acid (BCAA), leucine, to achieve levels similar to those seen during the postprandial state acutely increases protein synthesis in skeletal muscle of the neonatal pig (11). This effect appears to be unique to leucine and not to the other BCAA (12, 13), although the response to leucine is not sustained, likely due to the leucine-induced reduction in circulating amino acids as they are utilized for protein synthesis. Recently, we demonstrated that when the decrease in circulating amino acid levels was prevented by parenteral infusion of a balanced amino acid mixture devoid of leucine to maintain baseline levels, the stimulation of muscle protein synthesis was maintained for a more prolonged period (14, 15).
Protein synthesis requires both amino acids as precursors and a substantial amount of metabolic energy (9, 12, 16). Amino acids, especially leucine, serve not only as substrates for protein synthesis but also as nutrient signals to stimulate protein synthesis through activation of the intracellular signal transduction pathway that regulates mRNA translation (11, 14, 17). Leucine positively regulates the mammalian target of rapamycin (mTOR) signaling, promoting eukaryotic initiation factor (eIF) 4E-binding protein 1 (4E-BP1) phosphorylation, inhibiting 4E-BP1·eIF4E complex formation, and increasing the formation of the active eIF4E·eIF4G complex, which, in association with eIF4A, mediates the binding of the mRNA to the 43S ribosomal complex. mTOR also phosphorylates ribosomal protein S6 kinase-1 (S6K1), an activator of S6, and this protein has been reported to promote mRNA translation of proteins involved in the regulation of translation (18).
Previously, we demonstrated that parenteral infusion of a balanced amino acid mixture or leucine alone stimulates the intracellular signaling proteins that regulate protein synthesis in neonates (19, 20, 11). Recently, we showed that the reduced muscle growth and protein synthesis that occur with feeding a low-protein diet result from a decrease in translation initiation factor activation (16). However, the effects of enteral delivery of leucine on the activation of these pathways in the neonate has not previously been determined, even though enteral feeding presents fewer risks than parenteral feeding (21). Therefore, the aim of this study was to determine whether leucine supplementation of a low-protein meal can enhance rates of skeletal muscle and visceral tissue protein synthesis in neonatal pigs to levels similar to those achieved with a high-protein meal. We further examined the mechanism involved by measuring the acute effect of enteral leucine on the activation of amino acid signaling proteins and translation initiation factors.
Methods
Pigs and housing.
Two multiparous crossbred (Yorkshire × Landrace × Hampshire × Duroc) pregnant sows obtained from the Agricultural Headquarters of the Texas Department of Criminal Justice (Huntsville, TX) were brought to the animal facility of the Children’s Nutrition Research Center prior to their due date. After birth, the piglets (n = 21) were allowed to suckle ad libitum to 4 d of age and were not given supplemental creep feed. The sow and her piglets were housed in a free-standing lactation crate in environmentally controlled rooms and were managed as previously described (11, 13). At 2 d of age, piglets were anesthetized and indwelling catheters were surgically inserted into the jugular vein and carotid artery by use of sterile techniques (22). The Animal Care and Use Committee of Baylor College of Medicine approved all experimental procedures. This study was conducted in accordance with the NRC’s Guide for the Care and Use of Laboratory Animals.
Treatments and infusion.
Overnight food-deprived 5-d-old piglets weighing 2.0 ± 0.3 kg body weight were randomly assigned to 1 of the 3 treatment groups: low-protein (LP), low-protein supplemented with leucine (LP+L), or high-protein (HP) diets (n = 7 pigs/3 diets; total = 21) (Table 1). The LP+L diet provided an equivalent amount of leucine to the HP diet. All piglets were gavage fed their respective diet at a rate of 40 mL· kg−1 at time 0 min and at 60 min. Protein provided in the meal was 1.3 and 6.4 g · kg body weight−1 for the low and high-protein diet groups, respectively. Diets were isocaloric and contained the same level of lactose. Blood samples were collected every 30 min from 0 to 90 min for measurement of individual plasma amino acids, insulin, and glucose concentrations. At 60 min, piglets were injected with l-[4-3H] phenylalanine and killed 30 min later. Samples of longissimus dorsi, gastrocnemius, and masseter muscles, heart, liver, pancreas, kidney, and jejunum were removed after killing for measurements of protein synthesis rate and nutrient signaling activation.
TABLE 1.
Composition of LP, LP+L, and HP diets
| LP | LP+L | HP | |
| % of premix | |||
| Ingredients | |||
| Whey protein1 | 1.85 | 1.85 | 45.4 |
| Dried skim milk2 | 16 | 16 | 16 |
| Fat Pak 803 | 35.5 | 33.8 | 12.4 |
| Water | 42 | 40.3 | 22.3 |
| Vitamin premix4 | 0.35 | 0.35 | 0.35 |
| Trace mineral premix4 | 0.25 | 0.25 | 0.25 |
| Xanthan gum4 | 1 | 1 | 1 |
| Dicalcium | 3 | 3 | 3 |
| MgSO4 | 0.01 | 0.01 | 0.01 |
| KCl | 0.01 | 0.01 | 0.01 |
| Nutrition composition5 | |||
| Energy, MJ/kg | 13.7 | 13.7 | 13.7 |
| Protein, % | 8.3 | 8.3 | 41.6 |
| Fat, % | 28.6 | 27.3 | 12.0 |
| Lactose, % | 10.5 | 10.5 | 10.5 |
| Leucine, % | 0.7 | 4.0 | 4.0 |
Hilmartm 8000 (Hilmar Ingredients).
International Ingredient Corporation.
MSC Company.
DyetsR Inc. Vitamin premix provided (g/kg): thiamine HCl, 0.1; riboflavin, 0.375; pyridoxine HCl, 0.1; niacin, 1; calcium pantothenate, 1.2; folic acid, 0.13; biotin, 0.02; cobalamin, 1.5; retinyl palmitate, 0.8; cholecalciferol, 0.05; tocopheryl acetate, 8.8; menadione sodium bisulfate, 0.08. Trace mineral premix provided (g/kg): calcium phosphate, dibasic, 187; calcium carbonate, 279; sodium chloride, 85; potassium phosphate monobasic, 155; magnesium sulfate, anhydrous, 44; manganous carbonate, 0.93; ferric citrate, 10; zinc carbonate, 1.84; cupric carbonate, 0.193; potassium iodate, 0.005; sodium selenite, 0.007.
Calculated nutrient composition.
Plasma hormones and substrate assays.
The concentration of whole blood glucose was analyzed using a YSI 2300 STAT Plus (Yellow Springs Instruments). Plasma total BCAA were analyzed by rapid enzymatic kinetic assay (23). Individual amino acids from frozen plasma samples were measured with a HPLC method (PICO-TAG reverse-phase column; Waters) as previously described (24). Plasma radioimmunoreactive insulin concentrations were measured using a porcine insulin RIA kit (Linco).
Tissue protein synthesis in vivo.
The fractional rate of protein synthesis was measured with a flooding dose of 1.5 mmol·kg body weight−1 of l[4-3H]phenylalanine (18.5 MBq·kg body weight−1; Amersham Biosciences) injected 30 min prior to the end of the infusion (25). Piglets were killed at 90 min. Muscle and visceral tissue samples were obtained, immediately frozen in liquid nitrogen, and stored at −70°C until analyzed as previously described (26). Previous studies have demonstrated that, after a flooding dose of 3H-phenylalanine is administered, the specific radioactivity of tissue free phenylalanine is in equilibrium with the aminoacyl-tRNA specific radioactivity, and therefore the tissue free phenylalanine is a valid measure of the tissue precursor pool specific radioactivity (27). The majority of RNA in tissues is ribosomal; hence, the RNA:protein ratio (mg RNA·g protein−1) was used as an estimate of protein synthetic capacity. RNA content was determined as previously described (26). Protein synthetic efficiency was estimated as the total protein synthesized in 1 d per total RNA (g protein·d−1·g RNA−1). Total protein was measured using the bicinchoninic acid protein assay kit (Pierce Biotechnology).
Protein immunoblot analysis.
Proteins from tissue homogenates were separated on PAGE. For each assay, all samples were analyzed at the same time on triple-wide gels to eliminate inter-assay variation. Proteins were electrophoretically transferred to polyvinylidene difluoride transfer membranes (Pall Corporation), which were incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody as previously described (28).
For normalization, immunoblotting preformed with antiphospho-specific antibodies were stripped in stripping buffer (Pierce Biotechnology) and reprobed with corresponding nonphospho-specific antibodies. Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences), visualized, and analyzed using a ChemiDoc-It Imaging System (UVP). Primary antibodies that were used in the immunoblotting were protein kinase B (PKB) (total and Ser473, Cell Signaling), mTOR (total and Ser2448, Cell Signaling), 4E-BP1 (total, Bethyl Laboratories and Thr70, Cell Signaling), eIF4G (total and Ser1180, Cell Signaling), and S6K1 (total and Thr398, Cell Signaling).
Quantification of eIF4E#x22C54E-BP1 and eIF4E#x22C5eIF4G complexes.
These complexes were immunoprecipitated using an anti-eIF4E monoclonal antibody (courtesy of Dr. Leonard Jefferson, Penn State University College of Medicine, Hershey, PA) from aliquots of fresh tissue homogenates followed by immunoblotting with either 4E-BP1 antibody (Bethyl Laboratories) or eIF4G antibody (Cell Signaling) as previously described (13).
Calculations and statistics.
The fractional rate of protein synthesis (percentage of protein mass synthesized in 1 d) was calculated as Ks (%/d) = [(Eb/Ea) × (1440/t)] × 100, where Eb (in dpm·nmol−1) is the specific radioactivity of the protein-bound phenylalanine, Ea (in ·nmol−1) is the specific radioactivity of the tissue free phenylalanine at the time of tissue collection, t is the time of labeling in min, and 1440 is the minute to day conversion.
Statistical analysis was carried out in SPSS (version 17.0). A protected post hoc least significant differences t test was used to determine differences between groups if, after performing a 1-way ANOVA, we found a significant difference (P < 0.05). Analysis of glucose and insulin across time was carried out with SPSS General Linear Model using the repeated measures test for within-subject effects. Differences of P < 0.05 were considered significant for all comparisons and data are presented as means ± SEM.
Results
Plasma insulin, glucose, and amino acids.
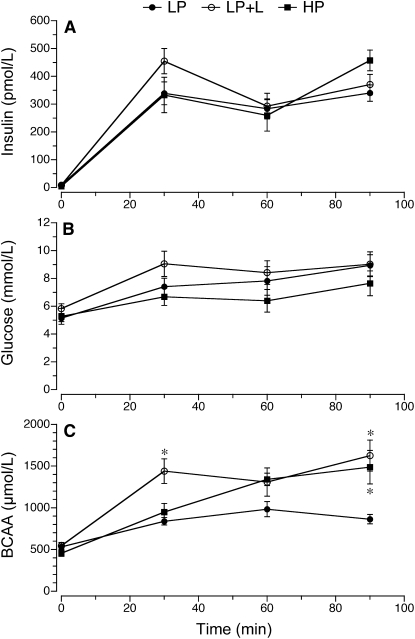
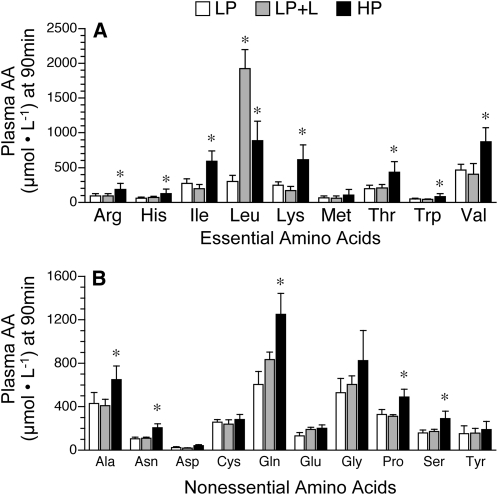
Plasma insulin concentrations did not differ between groups (Fig. 1A). Glucose concentrations were in the 6–8 mmol/L range expected for fed pigs and did not differ between groups (Fig. 1B). BCAA concentrations increased over time in the 3 treatment groups (P < 0.05) (Fig. 1C). The concentration of BCAA at 30 min in the LP+L group was greater than in the other 2 treatment groups (P < 0.05). However, at 90 min, both LP+L and HP groups had greater circulating BCAA concentrations than the LP group (P < 0.05). Plasma levels of most of the essential and nonessential amino acids increased over time in the 3 treatment groups (data not shown), but by 90 min, they were similar in both LP and LP+L groups and greater in the HP group (P < 0.05; Fig. 2).
FIGURE 1.
Circulating plasma insulin (A), whole blood glucose (B), and plasma BCAA (C) concentrations in piglets fed a LP, LP+L, or HP meal. Piglets were gavage fed at time 0 and 60 min. Values are means ± SEM, n = 7. *Different from LP, P < 0.05.
FIGURE 2.
Plasma concentrations of essential amino acids (A) and nonessential amino acids (B) in piglets fed a LP, LP+L, or HP meal. Values are means ± SEM, n = 7. *Different from LP, P < 0.05.
Protein synthesis in fast-twitch, glycolytic muscle.
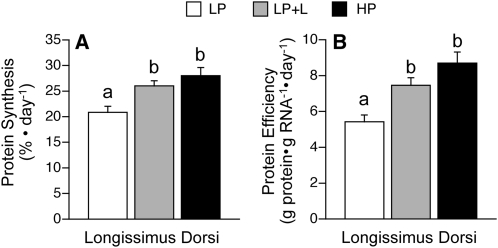
In the longissimus dorsi, a muscle containing primarily fast-twitch, glycolytic fibers, fractional rates of protein synthesis were greater in the LP+L compared with the LP group (P < 0.05; Fig. 3A). Fractional protein synthesis rates in the longissimus dorsi muscle did not differ in the LP+L pigs from the HP pigs. The efficiency with which the ribosomes translated mRNA into protein, i.e. the total protein synthesized per total RNA, did not differ between the LP+L and HP groups but was greater than in the LP group (P < 0.05; Fig. 3B).
FIGURE 3.
Fractional protein synthesis rates and protein synthetic efficiency in the longissimus dorsi muscle of piglets fed a LP, LP+L, or HP meal. Values are means ± SEM, n = 7. Means without a common letter differ, P < 0.05.
Translation initiation factors in fast-twitch, glycolytic muscle.
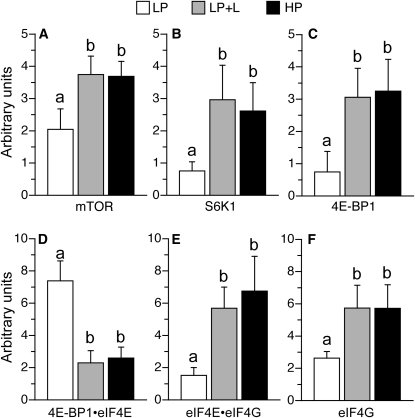
To examine the mechanism by which leucine supplementation increased protein synthesis in the longissimus dorsi muscle, markers of translation initiation signaling were measured. The phosphorylation of mTOR, S6K1, and 4E-BP1 in the longissimus dorsi muscle was enhanced in the LP+L and HP groups compared with the LP group (P < 0.05; Fig. 4A–C). The abundance of the translational repressor 4E-BP1·eIF4E complex was lower in the LP+L and HP groups than in the LP group (P < 0.05; Fig. 4D). The abundance of the active eIF4E·eIF4G complex and the phosphorylation of eIF4G in the longissimus dorsi muscle were greater (P < 0.05) in the LP+L and HP groups compared with the LP group (P < 0.05; Fig. 4E,F). The phosphorylation of PKB, a signaling component that resides upstream of mTOR, did not differ between groups (data not shown).
FIGURE 4.
mTOR phosphorylation (A), S6K1 phosphorylation (B), 4E-BP1 phosphorylation (C), inactive 4E-BP1·eIF4E complex abundance (D), active eIF4Ehe abundance of the translational repressoreIF4G complex abundance (E), and eIF4G phosphorylation (F) in the longissimus dorsi muscle of piglets fed a LP, LP+L, or HP meal. Values are means ± SEM, n = 7. Means without a common letter differ, P < 0.05.
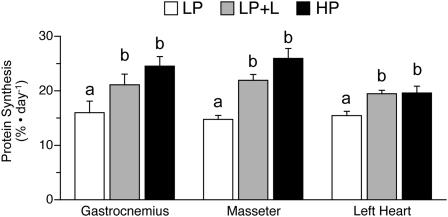
Protein synthesis and protein synthetic efficiency in muscles of oxidative fiber types.
Fractional rates of protein synthesis and translational efficiency in gastrocnemius (mixed glycolytic and oxidative fibers), masseter, and heart (oxidative fibers) muscles of neonatal pigs that received the LP+L diet were greater than in those fed the LP diet (P < 0.05; Fig. 5). However, these muscles in the HP group achieved similar protein synthesis rates and translational efficiency compared with those in the LP+L group.
FIGURE 5.
Fractional protein synthesis rates in the gastrocnemius, masseter, and heart muscles of piglets fed a LP, LP+L, or HP meal. Values are means ± SEM, n = 7. Means without a common letter differ, P < 0.05.
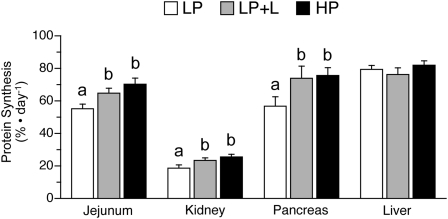
Protein synthesis and protein synthetic efficiency in visceral tissues.
To determine whether the effects of leucine supplementation are specific to muscle, fractional rates of protein synthesis and translational efficiency were determined in the jejunum, kidney, pancreas, and liver. In response to the meal, protein synthesis rates in the jejunum, kidney, and pancreas, but not the liver, were greater in the neonatal pigs that received the LP+L and HP diets compared with those that received the LP diet (Fig. 6). In the jejunum, kidney, and pancreas, translational efficiency was higher in the LP+L and HP groups compared with the LP group (P < 0.05; Table 2).
FIGURE 6.
Fractional protein synthesis rates in the jejunum, kidney, pancreas, and liver of piglets fed a LP, LP+L, or HP meal. Values are means ± SEM, n = 7. Means without a common letter differ, P < 0.05.
TABLE 2.
Translational RNA efficiency in piglets fed a LP, LP+L, or HP meal1
| Tissue | LP | LP+L | HP |
| g protein · g RNA−1 · d−1 | |||
| Gastrocnemius | 3.99 ± 0.53a | 6.25 ± 0.57b | 6.44 ± 0.55b |
| Masseter | 4.90 ± 0.64a | 7.02 ± 0.79b | 7.65 ± 1.13b |
| Heart | 4.04 ± 0.26a | 4.68 ± 0.12b | 4.62 ± 0.29b |
| Liver | 7.22 ± 0.37 | 7.22 ± 0.60 | 7.71 ± 0.32 |
| Jejunum | 6.73 ± 0.54a | 8.24 ± 0.53b | 8.85 ± 0.49b |
| Pancreas | 4.66 ± 0.57a | 6.75 ± 0.74b | 6.85 ± 0.65b |
| Kidney | 2.83 ± 0.31a | 3.47 ± 0.22b | 3.73 ± 0.34b |
Values are means ± SEM, = 7. Means in a row with superscripts without a common letter differ, P < 0.05.
Activation of translation in oxidative muscles and visceral tissues in response to leucine supplementation.
The phosphorylations of S6K1 and 4E-BP1 were used as indices of mTOR activation in oxidative muscles and visceral tissues. S6K1 and 4E-BP1 phosphorylation in the gastrocnemius, masseter, heart, jejunum, kidney, and pancreas was greater in the LP+L and HP groups compared with the LP group (P < 0.05; Table 3). However, S6K1 and 4E-BP1 phosphorylation in liver did not differ in the LP, LP+L, and HP groups (Table 3).
TABLE 3.
mTOR effectors, S6K1 and 4E-BP1, in muscles and visceral tissues of piglets fed a LP, LP+L, or HP meal1
| S6K1 |
4E-BP1 |
|||||
| Tissue | LP | LP+L | HP | LP | LP+L | HP |
| arbitrary units | ||||||
| Gastrocnemius | 1.62 ± 0.23a | 3.39 ± 0.65b | 3.13 ± 0.53b | 0.73 ± 0.12a | 1.30 ± 0.20b | 1.42 ± 0.26b |
| Masseter | 0.23 ± 0.04a | 0.46 ± 0.10b | 0.50 ± 0.05b | 0.13 ± 0.03a | 0.23 ± 0.03b | 0.26 ± 0.04b |
| Heart | 0.07 ± 0.02a | 0.15 ± 0.03b | 0.14 ± 0.02b | 1.74 ± 0.27a | 3.30 ± 0.67b | 4.20 ± 0.89b |
| Liver | 13.46 ± 1.63 | 13.94 ± 2.24 | 12.93 ± 2.49 | 0.66 ± 0.08 | 1.08 ± 0.21 | 1.07 ± 0.28 |
| Jejunum | 0.38 ± 0.07a | 1.36 ± 0.32b | 1.36 ± 0.21b | 0.49 ± 0.08a | 1.33 ± 0.17b | 1.42 ± 0.24b |
| Pancreas | 0.61 ± 0.16a | 1.23 ± 0.16b | 1.70 ± 0.32b | 0.35 ± 0.06a | 0.78 ± 0.10b | 0.93 ± 0.19b |
| Kidney | 0.56 ± 0.09a | 0.93 ± 0.14b | 0.95 ± 0.08b | 0.53 ± 0.10a | 1.04 ± 0.20b | 1.22 ± 0.14b |
Values are means ± SEM, = 7. Means in a row for either S6K1 or 4E-BP1 with superscripts without a common letter differ, P < 0.05.
Discussion
By using neonatal pigs as a model of human neonates, we have shown that feeding stimulates protein synthesis in skeletal muscle of neonates (8) and that this response is independently mediated by the rise in insulin and amino acids after a meal (9, 20). Leucine appears to be the most effective single amino acid to activate protein synthesis in muscle (29–31), because parenteral infusion of leucine acutely increases protein synthesis in skeletal muscle of neonatal pigs (11, 13, 32). Because parenteral leucine infusions bypass portal amino acid extraction, we examined in the current study the stimulatory effects of leucine supplemented enterally on protein synthesis in different muscles and visceral tissues. Optimization of milk formulas with leucine are relevant, because the advancement of enteral feedings in neonates with growth failure is often limited by the infant’s ability to tolerate complete enteral feeds that fulfill protein requirements necessary for growth (21, 33). The results of the current study show that leucine supplementation of a milk formula with low-protein content acutely increases protein synthesis in skeletal muscle as well as visceral tissues of the neonatal pig to a similar rate as that achieved by a high-protein meal.
Effects of enterally administered leucine on circulating insulin and amino acids.
Previously, we showed that when leucine is given parenterally as a continuous infusion, it induces a reduction in the circulating concentrations of other amino acids and this effect can limit the increase in protein synthesis (11, 15). However, when the decrease in other amino acids is prevented, the stimulation of protein synthesis by leucine is maintained (15). These findings imply that other amino acids are needed as a substrate to maintain the stimulation of protein synthesis by leucine and other anabolic agents (11, 15, 34). In the current study, feeding high- and low-protein meals increased circulating amino acids, but the greatest increase occurred in the HP group. Even though we provided a low-protein meal in the LP+L group, circulating amino acid levels did not fall in the presence of leucine supplementation and thus likely did not limit the leucine-induced stimulation of protein synthesis, but high-protein delivery was not required to achieve similar effects.
Dietary protein, specifically leucine, can induce an increase in plasma insulin levels in humans and rats (35). Some studies have suggested that the increase in plasma insulin is necessary for the leucine-induced enhancement of protein synthesis (20, 35, 36). In our study, feeding increased circulating insulin levels in all groups, but enteral leucine supplementation of a low-protein milk formula did not produce a greater increase in insulin than in the other 2 treatment groups. The lack of difference in insulin levels among groups suggests that the stimulation of protein synthesis by enteral leucine supplementation is independent of insulin, in agreement with previous studies (15).
Enteral leucine supplementation stimulates protein synthesis in fast-twitch, glycolytic muscles.
Initially, we examined the effects of enteral leucine supplementation on the longissimus dorsi muscle, which contains primarily fast-twitch, glycolytic fibers. We (11, 15) and others (37) have found that the response of muscle protein synthesis to anabolic agents is greater in muscles of predominantly fast fiber than in those of slow fiber type. In the current study, we found that supplementation of a low-protein meal with leucine increased protein synthesis in the longissimus dorsi muscle compared with a low-protein meal alone and this increase was similar to that with a high-protein diet meal. The increase in protein synthesis was due to an increase in translational efficiency, i.e. the rate of translation of mRNA via alterations in peptide change initiation, similar to our previous reports during parenteral leucine infusion (11, 15). Oral leucine administration in food-deprived adult rats has also been shown to stimulate protein synthesis in the skeletal muscle through activation of mTOR effectors (30).
In our study, PKB activation did not differ among groups in agreement with the lack of difference among groups in circulating insulin concentrations, indicating that the increased mTOR signaling activation by leucine supplementation is not due to alterations in the activation of the insulin signaling pathway. Moreover, feeding a leucine-supplemented, low-protein milk formula increased the activation of mTOR and its downstream effectors, S6K1 and 4E-BP1. This resulted in a decreased abundance of the inactive 4E-BP1·eIF4E complex and an increased abundance of the active eIF4E·eIF4G complex, whose formation may be enhanced by the increase in eIF4G phosphorylation. Previously, we demonstrated that 1 h after the parenteral infusion of leucine, activation of 4E-BP1 and S6K1 and protein synthesis are increased, but the stimulation in protein synthesis is not sustained after 2 h of leucine infusion despite 4E-BP1 and S6K1 remaining activated (11). This effect was thought to be due to the decrease in the circulating concentrations of other amino acids that are needed as substrates for protein synthesis. When hypoaminoacidemia was prevented, the prolonged infusion of leucine for 24 h was able to maintain the leucine-induced stimulation of protein synthesis in fast-twitch, glycolytic muscles in the presence of increased 4E-BP1 and S6K1 phosphorylation and eIF4E·eIF4G formation (15). Thus, although leucine is a positive regulator of mTOR signaling, chronic stimulation of protein synthesis in fast-twitch, glycolytic muscles requires the provision of all amino acids as a substrate.
Enterally supplemented leucine in a milk formula stimulates protein synthesis in oxidative muscles.
In the current study, a gastric meal of a low-protein formula supplemented with leucine increased protein synthesis in the gastrocnemius and masseter muscles that are more of the oxidative fiber type compared with the LD muscle. As in the LD muscle, this increase in protein synthesis was also mediated by an increase in the activation of the mTOR signaling pathway, as indicated by enhanced phosphorylation of 4E-BP1 and S6K1, resulting in increased translational efficiency. Previous studies have indicated that growth and protein synthesis rates in neonatal pigs are influenced by dietary protein intake (16). Indeed, when piglets were fed diets containing different protein levels, protein synthesis in the gastrocnemius and masseter muscles was increased with feeding a medium- to high-protein diet compared with a low-protein diet (16). In the current study, the fractional protein synthesis rates, translational efficiency, and activation of mTOR signaling proteins in the group that received the LP+L meal were comparable to that achieved in those fed the HP meal.
Leucine supplementation of a low-protein meal increased protein synthesis rates in the left ventricular wall to rates similar to those achieved with feeding a high-protein meal. The increase in protein synthesis was due to an increase in translation efficiency and mediated by an increase in mTOR activation. Previously, we reported that acute parenteral infusion of leucine increased protein synthesis in the left ventricular wall (13). The left ventricle is subject to an increase in hemodynamic workload right after birth and an increase in mass due to hypertrophy and hyperplasia during the first 10 d of postnatal life (38).
Enteral leucine supplementation of a milk formula increases protein synthesis and enhances mTOR signaling in visceral tissues.
The effects of leucine on visceral tissues are not well understood, especially in the jejunum. Hence, determining the effects of leucine on the intestine is important, because one of the problems that neonates confront is the intolerance to feeding and the predisposition to intestinal problems like necrotizing enterocolitis (39–41). In the current study, leucine supplementation of a low-protein meal increased protein synthesis and the activation of the mTOR effectors, S6K1 and 4E-BP1, in the pancreas, kidney, and jejunum. Translational efficiency in the jejunum, pancreas, and kidney increased in response to leucine supplementation. It has been reported that the requirements for BCAA are 56% higher in neonatal piglets fed enterally than in neonates that received BCAA through the parenteral infusion route (42, 43). This is in part because BCAA are extracted by first-pass splanchnic metabolism (42–44). This physiological metabolism at the intestinal level emphasizes the importance of optimizing the amount and quality of ingredients in milk formula to ensure adequate nutrition.
When we examined the effects of leucine supplementation in the liver, we did not see an increase in protein synthesis or the activation of S6K1 and 4E-BP1. Acute infusion of leucine in neonatal pigs and oral administration in mature rats in the food-deprived state did not find a stimulatory effect of leucine in the liver, although 4E-BP1 and S6K1 were activated in some studies (11, 45, 46). Because amino acids are extracted by first-pass splanchnic metabolism, we postulate that the failure of leucine supplementation of a low-protein meal as well as feeding a high-protein meal to increase protein synthesis beyond that achieved with feeding a low-protein meal may be due to a high sensitivity of neonatal liver to the level of amino acids provided in the low-protein meal. Thus, the amino acid levels provided by the low-protein meal were sufficient to maximally activate protein synthesis.
Summary.
In conclusion, a gastric meal of a low-protein formula supplemented with leucine stimulates protein synthesis similar to that achieved by feeding a meal of a high-protein milk formula. The stimulation of protein synthesis achieved by leucine supplementation occurs by the activation of translation initiation factors through the mTOR signaling pathway that leads to protein synthesis and this effect is independent of the activation of the insulin signaling pathway. Importantly, leucine supplementation of a low-protein meal acutely stimulated protein synthesis in all muscular and visceral tissues described in this study with the exception of the liver, which may be maximally stimulated by the low-protein meal. These near-global effects of leucine supplementation of a low-protein meal on protein synthesis are supportive of the potential for use of leucine supplementation as an adjunct in the nutritional management of infants unable to tolerate full protein delivery in their enteral feeds. However, further studies are needed to evaluate the effects of long-term supplementation of leucine on protein synthesis and growth in muscle and visceral tissues as well as to identify possible adverse events of long-term leucine supplementation.
Acknowledgments
We thank William Heird and Douglas Burrin for helpful discussions, Rosemarie Almonaci for technical assistance, Jerome Stubblefield and Rickey Bryant for care of animals, E. O’Brian Smith for statistical assistance, Adam Gillum for graphics, and Linda Weiser for secretarial assistance. R.M.T., A.S., M.L.F, and T.A.D. designed the research; R.M.T., A.S., M.C.G., R.A.O., S.E., J.W.F., and H.V.N. conducted the research; R.M.T., A.S., H.V.N., and T.A.D. analyzed the data; R.M.T. and T.A.D wrote the paper; and T.A.D. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by Ajinomoto Amino Acid Research Program, NIH R01 AR44474, NIH KO8 AR051563, and USDA 58-6250-6-001. This work is a publication of the USDA, Agricultural Research Service Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or politics of the USDA, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Abbreviations used: BCAA, branched-chain amino acid; 4E-BP1, 4E binding protein-1; eIF, eukaryotic initiation factor; HP, high-protein diet; LBW, low birth weight; LP, low-protein diet; LP+L, low-protein diet supplemented with leucine; mTOR, mammalian target of rapamycin; PKB, protein kinase B; S6K1, ribosomal protein S6 kinase-1.
Literature Cited
- 1.Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics. 1991;87:587–97 [PubMed] [Google Scholar]
- 2.Hack M, Fanaroff AA. Outcomes of extremely-low-birth-weight infants between 1982 and 1988. N Engl J Med. 1989;321:1642–7 [DOI] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, Katsikiotis V, Tyson JE, Oh W, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–9 [DOI] [PubMed] [Google Scholar]
- 4.Ford GW, Doyle LW, Davis NM, Callanan C. Very low birth weight and growth into adolescence. Arch Pediatr Adolesc Med. 2000;154:778–84 [DOI] [PubMed] [Google Scholar]
- 5.Peralta-Carcelen M, Jackson DS, Goran MI, Royal SA, Mayo MS, Nelson KG. Growth of adolescents who were born at extremely low birth weight without major disability. J Pediatr. 2000;136:633–40 [DOI] [PubMed] [Google Scholar]
- 6.Johnson JD, Albritton WL, Sunshine P. Hyperammonemia accompanying parenteral nutrition in newborn infants. J Pediatr. 1972;81:154–61 [DOI] [PubMed] [Google Scholar]
- 7.Thureen PJ, Melara D, Fennessey PV, Hay WW., Jr Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003;53:24–32 [DOI] [PubMed] [Google Scholar]
- 8.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7 than in 26-day-old pigs. Am J Physiol. 1996;270:E802–9 [DOI] [PubMed] [Google Scholar]
- 9.O'Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–9 [DOI] [PubMed] [Google Scholar]
- 10.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab. 2002;282:E880–90 [DOI] [PubMed] [Google Scholar]
- 11.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–21 [DOI] [PubMed] [Google Scholar]
- 12.Kimball SR, Jefferson LS. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care. 2001;4:39–43 [DOI] [PubMed] [Google Scholar]
- 13.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–21 [DOI] [PubMed] [Google Scholar]
- 14.Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab. 2007;293:E1615–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr. 2010;140:264–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E225–33 [DOI] [PubMed] [Google Scholar]
- 17.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131:S856–60 [DOI] [PubMed] [Google Scholar]
- 18.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–8 [DOI] [PubMed] [Google Scholar]
- 19.Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab. 2007;293:E1597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–53 [DOI] [PubMed] [Google Scholar]
- 21.Thureen PJ. Early aggressive nutrition in very preterm infants. Nestle Nutr Workshop Ser Pediatr Program. 2007;59:193–204 [DOI] [PubMed] [Google Scholar]
- 22.Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol. 1997;273:E305–14 [DOI] [PubMed] [Google Scholar]
- 23.Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC. Spectrophometric assay for measuring branched-chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem. 1996;240:48–53 [DOI] [PubMed] [Google Scholar]
- 24.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol. 1993;265:R334–40 [DOI] [PubMed] [Google Scholar]
- 25.Garlick PJ, Mcnurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980;192:719–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis TA, Fiorotto MF, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of sucking rats. Am J Physiol. 1989;257:R1141–6 [DOI] [PubMed] [Google Scholar]
- 27.Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol. 1999;277:E103–9 [DOI] [PubMed] [Google Scholar]
- 28.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2000;279:E1226–34 [DOI] [PubMed] [Google Scholar]
- 29.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–45 [DOI] [PubMed] [Google Scholar]
- 30.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9 [DOI] [PubMed] [Google Scholar]
- 31.Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr. 2004;134:1704–10 [DOI] [PubMed] [Google Scholar]
- 32.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neu J, Huang Y. Nutrition of premature and critically ill neonates. Nestle Nutr Workshop Ser Clin Perform Programme. 2003;8:171–81 [DOI] [PubMed] [Google Scholar]
- 34.Wilson FA, Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Gazzaneo MC, Davis TA. Fed levels of amino acids are required for the somatotropin-induced increase in muscle protein synthesis. Am J Physiol Endocrinol Metab. 2008;295:E876–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–101 [DOI] [PubMed] [Google Scholar]
- 36.Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E–BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–36 [DOI] [PubMed] [Google Scholar]
- 37.Vary TC, Lynch CJ. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007;137:1835–43 [DOI] [PubMed] [Google Scholar]
- 38.Anversa P, Olivetti G, Loud AV. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. I. Hypertrophy, hyperplasia, and binucleation of myocytes. Circ Res. 1980;46:495–502 [DOI] [PubMed] [Google Scholar]
- 39.Neu J. Neonatal necrotizing enterocolitis: an update. Acta Paediatr Suppl. 2005;94:100–5 [DOI] [PubMed] [Google Scholar]
- 40.Neu J, Chen M, Beierle E. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:137–44 [DOI] [PubMed] [Google Scholar]
- 41.Bjornvad CR, Thymann T, Deutz NE, Burrin DG, Jensen SK, Jensen BB, Molbak L, Boye M, Larsson LI, et al. Enteral feeding induces diet-dependent mucosal dysfunction, bacterial proliferation, and necrotizing enterocolitis in preterm pigs on parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1092–103 [DOI] [PubMed] [Google Scholar]
- 42.Elango R, Goonewardene LA, Pencharz PB, Ball RO. Parenteral and enteral routes of feeding in neonatal piglets require different ratios of branched-chain amino acids. J Nutr. 2004;134:72–8 [DOI] [PubMed] [Google Scholar]
- 43.Elango R, Pencharz PB, Ball RO. The branched-chain amino acid requirement of parenterally fed neonatal piglets is less than the enteral requirement. J Nutr. 2002;132:3123–9 [DOI] [PubMed] [Google Scholar]
- 44.Saenz de PM, VanBeek RH, Quero J, Perez J, Wattimena DJ, Sauer PJ. Effect of minimal enteral feeding on splanchnic uptake of leucine in the postabsorptive state in preterm infants. Pediatr Res. 2003;53:281–7 [DOI] [PubMed] [Google Scholar]
- 45.Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr. 2001;131:1171–6 [DOI] [PubMed] [Google Scholar]
- 46.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:S227–31 [DOI] [PubMed] [Google Scholar]