Abstract
Previously, we showed that supplementation of diets with short-chain inulin (P95), long-chain inulin (HP), and a 50:50 mixture of both (Synergy 1) improved body iron status and altered expression of the genes involved in iron homeostasis and inflammation in young pigs. However, the effects of these 3 types of inulin on intestinal bacteria remain unknown. Applying terminal restriction fragment length polymorphism analysis, we determined the abundances of luminal and adherent bacterial populations from 6 segments of the small and large intestines of pigs (n = 4 for each group) fed an iron-deficient basal diet (BD) or the BD supplemented with 4% of P95, Synergy 1, or HP for 5 wk. Compared with BD, all 3 types of inulin enhanced (P < 0.05) the abundance of beneficial bifidobacteria and lactobacilli in the microbiota adherent to intestinal mucus of various gut segments of pigs. These changes were seen as proximal as in the jejunum with P95 but did not appear until the distal ileum or cecum with HP. Similar effects of inulin on bacterial populations in the lumen contents were found. Meanwhile, all 3 types of inulin suppressed the less desirable bacteria Clostridium spp. and members of the Enterobacteriaceae in the lumen and mucosa of various gut segments. Our findings suggest that the ability of dietary inulin to alter intestinal bacterial populations may partially account for its iron bioavailability-promoting effect and possibly other health benefits.
Introduction
Prebiotics are carbohydrates that resist digestion in the small intestine. After escaping to the large intestine, prebiotics undergo fermentation by the resident microflora and promote a favorable enteric microbial balance. This is mediated through selective enhancements of beneficial Bifidobacterium spp. and Lactobacillus spp., with concomitant reductions in less desirable populations such as Clostridium spp. (1, 2). Recently, prebiotics have also been shown to enhance mineral bioavailability (3–7).
Inulin, a prebiotic commonly added to foods, encompasses all β (2→1) linear fructans of varying chain lengths (8, 9). Earlier, our group observed an improved iron status in young pigs following supplementation with a 50:50 mixture of short- and long-chain inulin (Synergy 1, Orafti) into a corn-soybean meal basal diet (BD)7 (3). Analysis of digesta from various segments of the small and large intestines revealed measureable inulin in the jejunum and ileum, but not in the cecum and colon of pigs (10). More recently, we have shown that supplementation with short-chain or long-chain inulin produced effects on dietary iron utilization similar to those of the 50:50 mixture of these 2 types of inulin (11). Furthermore, all 3 types of inulin exerted similar effects on expression of the genes involved in iron storage and proinflammatory cytokines in the intestinal mucosa and/or liver. These effects were more pronounced in the lower than the upper gut. Inulin is not hydrolyzed by mammalian digestive enzymes, but significant degradation does occur in the ileum, presumably by the microflora resident in this part of the intestine. In fact, no inulin was recovered in segments distal to the ileum in pigs fed diets containing supplemental inulin (10, 11).
Although the prebiotic effect of inulin-type fructans in rats and humans is well documented (12–14), past studies on the roles of inulin-type fructans in microbial populations have been limited to collections of feces, colonic luminal contents through colonoscopy and effluents of ileostomy patients, or samples taken from cannulated animals (15–17). Only a few studies have documented positive effects of inulin-type fructans on the bacterial populations of the lumen and the mucosa (18–20). Consequently, it remained largely unclear as to the specific effect of different types of inulin (short chain, long chain, or a mixture of the 2) on populations of enteric microbiota in the lumen or associated with the mucosa in various segments of the intestine. Therefore, we used terminal restriction fragment (TRF) length polymorphism (TRFLP) analysis to genotype the mucosal and luminal enteric bacteria from various segments of the intestines in pigs fed 3 types of inulin.
Materials and Methods
BD, inulin, and pigs.
Details of diets, pigs, and experimental protocols are found elsewhere (11). Briefly, the BD consisted of corn and soybean meal and contained adequate concentrations of all nutrients (21) except for iron (no supplemental inorganic iron). Inulin was supplemented into the designated diet (40 mg/kg) at the expense of corn starch, which was present at this level in the BD. The 3 inulin sources were as follows: Raftiline HP (long chain; degree of polymerization = 10–60, average of 25), Raftilose P95 (short chain; degree of polymerization = 2–7, average of 4), and Synergy 1 (50:50 mixture of HP and P95). Experiments were conducted at the Cornell University Swine Farm and the protocol was approved by the Cornell Institutional Animal Care and Use Committee. Experimental pigs (n = 32, 17 males and 15 females, 6 wk old, body weight = 9.2 ± 0.4 kg) were individually penned and fed the BD or the BD plus 4% of Synergy 1, HP, or P95 for 5 wk (11).
Sample collection.
At the end of the trial, pigs were killed by electrical stunning followed by exsanguination. The entire gastrointestinal tract was quickly removed from the body. Segments (5 cm each) of the jejunum, ileum, cecum, proximal colon, mid colon, and distal colon were harvested from 16 pigs [n = 4/treatment group based on body weight and blood hemoglobin concentration (11)] and were subjected to 2 washing procedures as previously described (22, 23). The first wash with saline was to recover the microbial communities inhabiting the lumen and the second wash with Triton-X 100 was to isolate those populations adherent to the intestinal wall (either associated with the mucus lining or adherent to the epithelial cells).
Enteric microbial community analysis.
The TRFLP analysis of luminal and adherent microbial populations from the jejunum, ileum, cecum, proximal colon, mid colon, and distal colon was carried out as previously described (22). Putative identifications of TRF were obtained by ribosomal database mining (http://mica.ibest.uidaho.edu/) and by comparisons with pure bacterial controls. To determine the abundance of individual TRF within a microbial community profile from each intestinal segment, we first totaled the fluorescence emitted by the entire community and then calculated the relative percent contribution of individual peaks (or TRF) to the total. The abundance of peaks corresponding to Bifidobacterium spp. and Lactobacillus spp. was added to determine the overall abundance change of these 2 favorable intestinal populations.
Statistics.
All data were analyzed by 1-way ANOVA and post hoc Bonferroni t tests (MINITAB release 14.20), with significance defined as P ≤ 0.05. Values are expressed as means ± SE.
Results
Growth, iron status, and inulin fate.
Details of growth performance, body iron status, and site of disappearance of the 3 types of inulin in pigs were described in our previous publication (11). Although the initial blood hemoglobin concentrations (at wk 0) were similar among the 4 groups of pigs, the final blood hemoglobin concentrations in pigs fed inulin, irrespective of the type, were 6–14% higher (P < 0.05) than those fed BD. Growth performance of the pigs did not differ among treatment groups. Segments distal to the ileum in pigs fed any type of inulin had no detectable inulin, but the small intestine showed variable degradations of the 3 types of inulin.
Changes in abundance of Bifidobacterium spp. and Lactobacillus spp.
The total number of TRF detected in the intestinal segments of pigs fed the BD did not differ from those of pigs fed the different types of inulin (data not shown). Compared with those fed BD, pigs fed the 3 types of inulin tended to have a greater relative abundance of Bifidobacterium spp. and Lactobacillus spp. in the lumen digesta contents of all intestinal segments (P = 0.05–0.07) (Fig. 1).
FIGURE 1.
Effects of different types of supplemental inulin on relative abundances of phylotypes corresponding to the sum of Bifidobacterium spp. and Lactobacillus spp. in the lumen contents of selected intestinal segments in pigs. Values are means ± SE, n = 4. Within a segment, means without a common letter tended to differ, P = 0.05–0.07.
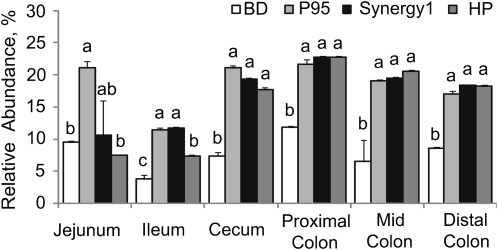
There was a strong effect (P < 0.05) of dietary inulin treatment on the relative abundance of Bifidobacterium spp. and Lactobacillus spp. in the microbial communities associated with intestinal mucus and adherent to the epithelia (Fig. 2). Compared with that in pigs fed BD, the abundance of these bacteria was greater (P < 0.05) in all intestinal segments of pigs fed P95 and in all segments except the jejunum of pigs fed Synergy 1 or HP, respectively. The relative abundance of these 2 bacteria was also higher (P < 0.05) in the jejunum and ileum of pigs fed P95 and the ileum of pigs fed Synergy 1 than that of pigs fed HP, respectively. Detailed representative profiles of luminal and adherent microbial communities from all intestinal segments from each treatment group are presented in Supplemental.
FIGURE 2.
Effects of different types of supplemental inulin on relative abundances of phylotypes corresponding to the sum of Bifidobacterium and Lactobacillus spp. in the mucosa of selected intestinal segments in pigs. Values are means ± SE, n = 4. Within a segment, means without a common superscript letter differ, P < 0.05.
Changes in the abundance of other bacterial populations.
Putative identification of other TRF from the microbial community profiles showed marked decreases in less favorable populations in pigs fed the 3 types of inulin compared with those fed BD (Supplemental ). These changes, seen in all intestinal segments, included reductions in Clostridium spp., Streptococcus spp./Prevotella spp. and Enterobacteriaceae/Pseudomonas spp./Bacillus spp. In all these cases, the observed changes were not only specific to the communities inhabiting the intestinal mucus or adhering to the epithelia but also within luminal communities. Notably, several TRF detected from each segment could not be assigned an identification and might represent novel species that were modulated by inulin supplementation.
Discussion
Using TRFLP, we demonstrated that supplementation with 3 types of inulin promoted populations of beneficial Bifidobacterium spp. and Lactobacillus spp. and suppressed the less desirable bacteria, including Clostridium spp. and members of the Enterobacteriaceae, in various intestinal segments of pigs. Our finding is very relevant to human nutrition and health, because these bacteria are similarly found in the human gut (24, 25). Overall, 3 important clues come from the present study in elucidating the physiological effects of inulin-type fructans. First, enhancements of the abundance of beneficial Bifidobacterium spp. and Lactobacillus spp. by dietary inulin seemed to be stronger and more consistent in the adherent bacteria than in the luminal populations. Such changes of the mucosa-associated bacterial populations and their intimate interactions with the intestinal lining may be a critical factor for the underlying mode of action of the inulin-type fructans. Presumably, adherent bacteria might influence functions of the epithelium and possibly other organs of the body. Second, significant increases in these bacterial populations appeared in segments as proximal as the small intestine (jejunum) in pigs fed the short-chain inulin but not until the cecum in pigs fed the long-chain inulin. It is likely that the short-chain inulin was degraded to a greater extent than the long-chain inulin in the small intestine. Third, unlike in the small intestine, the abundance of these bacterial populations throughout the mucosa of the large intestine were similarly enhanced by all 3 types of inulin. Thus, the impacts of inulin on the colonic bacterial populations, like those on body hemoglobin concentration and tissue gene expression (11), were little affected by their chain length.
The inulin-resultant specific promotion of the mucosa-associated Bifidobacterium spp. and Lactobacillus spp. is in agreement with several other reports (18–20). Kleessen et al. (19) demonstrated an increase in mucosal Bifidobacterium spp. in rats harboring a human fecal flora, even though the stimulation was not significant in the intestinal lumen. Humans given 15 g/d Synergy 1 for 2 wk had increased Bifidobacterium spp. and Lactobacillus spp. in their mucosa (20). In the present study, supplemental inulin modulated several populations in the porcine gastrointestinal tract that could not be identified using the available ribosomal databases. A similar scenario has also been observed from culture studies of human gastrointestinal tract samples (24). Future identification and characterization of these potential novel populations will be needed to determine whether they play important roles in maintaining intestinal health and a beneficial microbial balance.
Our previous study showed a downregulation of inflammation-related genes, including tumor necrosis factor, transferrin receptor, and NRAMP1 (SLC11A1), in the colonic mucosa of pigs fed the 3 types of inulin (11). Tumor necrosis factor is a cytokine involved in systemic inflammation and is mainly produced by resident macrophages in response to lipopolysaccharides present in the outer membrane of Gram-negative bacteria (26, 27). In the present study, feeding the 3 types of inulin enhanced the abundance of the Gram-positive Bifidobacterium spp. and Lactobacillus spp. Because cell walls of Gram-positive bacteria do not contain lipopolysaccharides, pigs fed inulin may have less exposure to these stimuli, resulting in the observed downregulation of inflammation-related genes.
The short-chain P95 seemed to be fermented to some extent in the jejunum and ileum by the resident microbiota that were composed of more aerotolerant species such as lactobacilli (11). In contrast, HP did not appear to be degraded until reaching the distal ileum or the cecum where the bacterial population shifts to more anaerobic species such as bifidobacteria, Bacteroides, and clostridia that can contribute to the complete fermentation of the prebiotic in this region. Presumably, profiles or changes in the colonic microbiota reflect cross-feeding processes and transit of fermentation metabolites through the intestine. However, an examination of the inulin content in the digesta of these same pigs using HPLC revealed no inulin in the cecum or colon of any of the dietary groups despite varying degrees of degradation and changes in the concentrations of inulin byproducts and other sugars in the small intestine (11). Although this result alone suggests that all 3 types of inulin were fermented to virtual completion during the transit from ileum to cecum, caution should still be taken in associating inulin fermentation with microbiota in different intestinal segments. This is because the HPLC measurement of digesta inulin contents may lack the sensitivity or specificity required to detect small concentrations of inulin. Likely, certain undetectable quantities of inulin might still be present in the cecum and perhaps the proximal colon of these animals. Resolving this uncertainty will require a more sensitive inulin assay than is currently available.
Pigs used in the present study were iron deficient at the beginning of the experiment and were fed diets that were relatively low in iron (no supplemental inorganic iron). Thus, the initial microflora of our iron-deficient pigs might be somewhat different from that found in iron-adequate pigs of the same age. Tompkins et al. (28) demonstrated an increase in anaerobes, microaerophiles, lactobacilli, and enterococci in the large intestines of iron-deprived mice compared with iron-replete controls. Moreover, the efficacy of prebiotics such as inulin may be affected by the initially existing microflora (29, 30), i.e. prebiotics tend to be more efficacious when the initial counts of bifidobacteria and lactobacilli are quite low. If our iron-deficient pigs had increased counts of these bacteria compared with their iron-replete counterparts, as in the reported mouse case (28), the efficacy of inulin might have been somewhat constrained in this study. If so, supplementation of iron-adequate pigs with inulin might produce greater enhancement of bifidobacteria and lactobacilli in the lumen and adherent microbiota, respectively.
In conclusion, all 3 types of inulin promoted a favorable intestinal microbial balance with increases in beneficial Bifidobacterium spp. and Lactobacillus spp. and concomitant decreases in less desirable populations such as clostridia and members of the Enterobacteriaceae. This effect was stronger in the microbiota adhering to the mucosa, with modest changes observed in the lumen. Modulation of the adherent microbial populations of the small intestine of pigs fed P95 suggests that short-chain inulin was fermented to a meaningful extent in these regions. Because there is tremendous heterogeneity in the environmental conditions among various segments of the gastrointestinal tract, further studies will be needed to characterize bacterial populations at the species level and their impact on intestinal epithelial cell physiology. Results from those studies will help us in understanding the mode of microbiota action of inulin for its positive effect on body iron status and gut health.
Supplementary Material
Acknowledgments
X.G.L., D.D.M., and R.M.W. designed the research; J.K.P. and K.Y. conducted the experiments and analyzed data; J.K.P., K.Y., D.D.M., and X.G.L. wrote the paper; and J.K.P., D.D.M., and X.G.L. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by a grant from Harvest-Plus, International Food Policy Research Institute, Centro International Agriculture Tropical, and the USDA (USDA/ National Research Initiative grant 2006-35200-16583).
Supplemental are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BD, basal diet; HP, long-chain inulin; P95, short-chain inulin; Synergy1, 50:50 mixture of HP & P95; TRF, terminal restriction fragment; TRFLP, terminal restriction fragment length polymorphism.
Literature Cited
- 1.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75 [DOI] [PubMed] [Google Scholar]
- 2.Rastall RA, Gibson GR, Gill HS, Guarner F, Klaenhammer TR, Pot B, Reid G, Rowland IR, Sanders ME. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: an overview of enabling science and potential applications. FEMS Microbiol Ecol. 2005;52:145–52 [DOI] [PubMed] [Google Scholar]
- 3.Yasuda K, Roneker KR, Miller DD, Welch RM, Lei XG. Supplemental dietary inulin affects the bioavailability of iron in corn and soybean meal to young pigs. J Nutr. 2006;136:3033–8 [DOI] [PubMed] [Google Scholar]
- 4.Ohta A, Ohtsuki M, Baba S, Takizawa T, Adachi T, Kimura S. Effects of fructooligosaccharides on the absorption of iron, calcium and magnesium in iron-deficient anemic rats. J Nutr Sci Vitaminol (Tokyo). 1995;41:281–91 [DOI] [PubMed] [Google Scholar]
- 5.Coudray C, Bellanger J, Castiglia-Delavaud C, Remesy C, Vermorel M, Rayssignuier Y. Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men. Eur J Clin Nutr. 1997;51:375–80 [DOI] [PubMed] [Google Scholar]
- 6.Delzenne N, Aertssens J, Verplaetse H, Roccaro M, Roberfroid M. Effect of fermentable fructo-oligosaccharides on mineral, nitrogen and energy digestive balance in the rat. Life Sci. 1995;57:1579–87 [DOI] [PubMed] [Google Scholar]
- 7.Griffin IJ, Hicks PMD, Heaney RP, Abrams SA. Enriched chicory inulin increases calcium absorption mainly in girls with lower calcium absorption. Nutr Res. 2003;23:901–9 [Google Scholar]
- 8.Roberfroid MB. Introducing inulin-type fructans. Br J Nutr. 2005;93Suppl 1:S13–25 [DOI] [PubMed] [Google Scholar]
- 9.Niness KR. Inulin and oligofructose: what are they? J Nutr. 1999;129:S1402–6 [DOI] [PubMed] [Google Scholar]
- 10.Yasuda K, Maiorano R, Welch RM, Miller DD, Lei XG. Cecum is the major degradation site of ingested inulin in young pigs. J Nutr. 2007;137:2399–404 [DOI] [PubMed] [Google Scholar]
- 11.Yasuda K, Dawson HD, Wasmuth EV, Roneker CA, Chen C, Urban JF, Welch RM, Miller DD, Lei XG. Supplemental dietary inulin influences expression of iron and inflammation related genes in young pigs. J Nutr. 2009;139:2018–23 [DOI] [PubMed] [Google Scholar]
- 12.Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–82 [DOI] [PubMed] [Google Scholar]
- 13.Campbell JM, Fahey GC, Jr, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127:130–6 [DOI] [PubMed] [Google Scholar]
- 14.Kruse HP, Kleessen B, Blaut M. Effects of inulin on faecal bifidobacteria in human subjects. Br J Nutr. 1999;82:375–82 [DOI] [PubMed] [Google Scholar]
- 15.Branner GR, Bohmer BM, Erhardt W, Henke J, Roth-Maier DA. Investigation on the precaecal and faecal digestibility of lactulose and inulin and their influence on nutrient digestibility and microbial characteristics. Arch Anim Nutr. 2004;58:353–66 [DOI] [PubMed] [Google Scholar]
- 16.Houdijk JG, Bosch MW, Tamminga S, Verstegen MW, Berenpas EB, Knoop H. Apparent ileal and total-tract nutrient digestion by pigs as affected by dietary nondigestible oligosaccharides. J Anim Sci. 1999;77:148–58 [DOI] [PubMed] [Google Scholar]
- 17.Bohmer BM, Branner GR, Roth-Maier DA. Precaecal and faecal digestibility of inulin (DP 10–12) or an inulin/Enterococcus faecium mix and effects on nutrient digestibility and microbial gut flora. J Anim Physiol Anim Nutr (Berl). 2005;89:388–96 [DOI] [PubMed] [Google Scholar]
- 18.Poxton IR, Brown R, Sawyerr A, Ferguson A. Mucosa-associated bacterial flora of the human colon. J Med Microbiol. 1997;46:85–91 [DOI] [PubMed] [Google Scholar]
- 19.Kleessen B, Hartmann L, Blaut M. Fructans in the diet cause alterations of intestinal mucosal architecture, released mucins and mucosa-associated bifidobacteria in gnotobiotic rats. Br J Nutr. 2003;89:597–606 [DOI] [PubMed] [Google Scholar]
- 20.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NRC Nutrition requirements of swine. 10th ed. Washington, DC: National Academies Press; 1998 [Google Scholar]
- 22.Patterson JK, Rutzke MA, Fubini SL, Glahn RP, Welch RM, Lei X, Miller DD. Dietary inulin supplementation does not promote colonic iron absorption in a porcine model. J Agric Food Chem. 2009;57:5250–6 [DOI] [PubMed] [Google Scholar]
- 23.Patterson J, Chapman T, Hegedus E, Barchia I, Chin J. Selected culturable enteric bacterial populations are modified by diet acidification and the growth promotant Tylosin. Lett Appl Microbiol. 2005;41:119–24 [DOI] [PubMed] [Google Scholar]
- 24.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol. 2004;70:6113–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beutler B, Cerami A. The biology of cachectin/TNF: a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–55 [DOI] [PubMed] [Google Scholar]
- 27.Ziegler-Heitbrock HW, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–5 [DOI] [PubMed] [Google Scholar]
- 28.Tompkins GR, O'Dell NL, Bryson IT, Pennington CB. The effects of dietary ferric iron and iron deprivation on the bacterial composition of the mouse intestine. Curr Microbiol. 2001;43:38–42 [DOI] [PubMed] [Google Scholar]
- 29.Tuohy KM, Kolida S, Lustenberger AM, Gibson GR. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides: a human volunteer study. Br J Nutr. 2001;86:341–8 [DOI] [PubMed] [Google Scholar]
- 30.De Preter V, Vanhoutte T, Huys G, Swings J, Rutgeerts P, Verbeke K. Baseline microbiota activity and influence responses to prebiotic initial bifidobacteria counts dosing in healthy subjects. Aliment Pharmacol Ther. 2008;27:504–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.