Abstract
Prematurity and overfeeding in infants are associated with insulin resistance in childhood and may increase the risk of adult disease. Total parenteral nutrition (TPN) is a major source of infant nutritional support and may influence neonatal metabolic function. Our aim was to test the hypothesis that TPN induces increased adiposity and insulin resistance compared with enteral nutrition (EN) in neonatal pigs. Neonatal pigs were either fed enteral formula orally or i.v. administered a TPN mixture for 17 d; macronutrient intake was similar in both groups. During the 17-d period, we measured body composition by dual-energy X-ray absorptiometry scanning; fasting i.v. glucose tolerance tests (IVGTT) and hyperinsulinemic-euglycemic clamps (CLAMP) were performed to quantify insulin resistance. On d 17, tissue was collected after 1-h, low-dose CLAMP for tissue insulin signaling assays. TPN pigs gained less lean and more body fat and developed hepatic steatosis compared with EN pigs. After 7 and 13 d, IVGTT showed evidence of insulin resistance in the TPN compared with the EN group. Fasting plasma glucose and insulin also were higher in TPN pigs. CLAMP showed that insulin sensitivity was markedly lower in TPN pigs than in EN pigs. TPN also reduced the abundance of the insulin receptor, insulin receptor substrate 1, and phosphatidylinositol 3 kinase in skeletal muscle and liver and the proliferation of total pancreatic cells and β-cells. Hepatic proinflammatory genes as well as c-Jun-N-terminal kinase 1 phosphorylation, plasma interleukin 6, and tumor necrosis factor-α were all higher in TPN pigs than in EN pigs. The results demonstrate that chronic TPN induces a hepatic inflammatory response that is associated with significant insulin resistance, hepatic steatosis, and fat deposition compared with EN in neonatal pigs. Further studies are warranted to establish the mechanism of TPN-induced insulin resistance and hepatic metabolic dysfunction and whether there are persistent metabolic consequences of this lifesaving form of infant nutritional support.
Introduction
Many infants are not fed enterally because of feeding intolerance, risk of necrotizing enterocolitis, or other medical conditions. In these circumstances, infants receive parenteral nutrition (PN)8 for several days or sometimes months, which thus provides critical nutritional support for survival (1). However, evidence from clinical studies suggests that very low birth weight (VLBW) infants frequently experience growth failure and this has been linked to poor neurodevelopmental outcomes (2). These outcomes have prompted clinicians to adopt more aggressive nutrition regimens to optimize growth, which translates to increased rates of PN administration (3). Aggressive nutritional support of premature infants, especially PN, may increase the risk of hyperglycemia, because these infants have poor control of glycemia (4–7). Hyperglycemia is a particular concern for VLBW infants, because this has been associated with increased morbidity and mortality (8, 9). As a result, the clinical practice of insulin therapy has been implemented to normalize glycemia while enabling aggressive glucose administration in premature infants (10). Hyperglycemia in VLBW infants has been linked to the inability to inhibit endogenous hepatic glucose production and reduced peripheral glucose utilization during parenteral administration of glucose and insulin (11, 12). The etiology of persistent hepatic glucose production and possible insulin resistance in premature neonates is unknown.
A critical goal of nutritional support of parenterally fed premature and VLBW infants is early introduction of partial enteral nutrition [i.e. minimal enteral nutrition (EN)] and rapid advancement to full enteral feeding (13). This situation is also true for term infants that receive PN due to intestinal failure secondary to congenital abnormalities or extensive surgical resection (14, 15). Minimal enteral feeding accelerates the maturation of gastrointestinal function and the tolerance to full enteral feeding of premature and VLBW infants and thereby reduces the duration of hospitalization (16). Few studies have examined the effect of enteral compared with PN on glucose tolerance and utilization in neonates, but 1 report suggests that chronic PN leads to impaired insulin secretory response (17). Under normal enteral feeding conditions, the gut and liver remove a significant amount (30–40%) of the dietary glucose load by first-pass metabolism (18–20). Studies in mature animals and adult humans indicate that delivery of glucose via the enteral compared with parenteral route preferentially increases hepatic and peripheral glucose utilization (18, 21, 22). Most of the evidence demonstrating the stimulatory effect of enteral glucose infusion on glucose disposal is based on acute studies (<6 h), although chronic (14 d) studies in rats showed that those fed a complete elemental diet parenterally experienced insulin resistance and markedly higher insulin concentrations than those fed enterally (23). Studies in dogs indicated that chronic (5 d) total PN (TPN) increased peripheral glucose disposal at a lower circulating insulin concentration, suggesting increased insulin sensitivity (24, 25). Thus, there is conflicting evidence of the role of PN support in glucose utilization and insulin sensitivity; however, to our knowledge, no studies have been done in neonatal animals or infants.
The current study tested the hypothesis that TPN compared with intermittent, enteral formula feeding (EN) results in insulin resistance and glucose intolerance in neonatal pigs. We characterized endpoints of whole-body insulin sensitivity, liver and muscle insulin signaling, pancreatic β-cell function, and body composition. We used the neonatal pig given its relevance as an animal model of the human neonate based on comparative aspects of metabolism, body composition, organ function, and stage of development (26–29). With respect to glucose metabolism, the fasted newborn pig (26) and VLBW infant (7, 30) are both susceptible to glycemic extremes and the relationship between body weight and glucose turnover in the 2 species is strikingly similar. Importantly, the larger body size of the neonatal pig compared with the neonatal rodent allows investigation of the effect of PN.
Research Design and Methods
Animals and study design.
The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals [DHHS publication no. (NIH) 85–23, revised 1985, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205]. Newborn (2 d old), crossbred (female) pigs (n = 30) obtained from the Texas Department of Criminal Justice were transported to the animal facility at the Children’s Nutrition Research Center (Houston, TX). Upon arrival (d 0), piglets were weighed and placed in cages in a heated room (~30°C). The study design is shown in Supplemental Figure 1. We studied a total of 30 pigs that were randomly assigned to 1 of 2 treatment groups, either EN or TPN. The study design was replicated in 2 groups of pigs (17 pigs in group 1 and 13 pigs in group 2) at different times such that there were 15 pigs per treatment group (n = 15). On d 0 and 16, body composition was determined by dual-energy X-ray absorptiometry (DXA) as described below. Subgroups of piglets also were subjected to i.v. glucose tolerance tests (IVGTT) on d 7 (n = 6) and 13 (n = 8) and hyperinsulinemic-euglycemic clamps (CLAMP) on d 15 (n = 6–7) and before euthanasia on d 17 (n = 6–7) as described below.
Following DXA analysis on d 0, piglets were fed a cow milk-based formula until surgery.
The following day, piglets were implanted with silastic catheters into the jugular vein and carotid artery as previously described (31, 32). After surgery, all piglets received TPN at 50% of their requirement for 24 h and then were assigned to 1 of 2 treatments to receive either TPN or intermittent enteral (oral) feedings of formula (EN) until the end of the study period. Thereafter, intakes of TPN and EN piglets were increased to 100% within the next 48 h. TPN piglets were administered 240 mL·kg−1·d−1 providing 25 g·kg−1·d−1 glucose, 13 g·kg−1·d−1 l-amino acids, 5 g·kg−1·d−1 lipid (Intralipid 20%; Fresenius Kabi), and electrolytes, (trace) minerals, and vitamins according to their requirement as described previously (33). EN piglets were fed a cow milk-based formula for baby pigs (Litter Life, Merrick’s) at 50 g·kg−1·d−1, suspended in 240 mL water, providing 25 g·kg−1·d−1 lactose, 12.5 g·kg−1·d−1 protein, 5 g·kg−1·d−1 fat, and electrolytes, (trace) minerals, and vitamins. TPN piglets received their nutrition continuously, whereas EN piglets were orally fed individual meals 3–4 times daily. A detailed list of the diet compositions is provided in Supplemental Table 1. Piglets were weighed daily and their intakes adjusted accordingly.
Body composition analysis.
On d 0 and 16, pigs were briefly anesthetized with isoflurane and their body composition was determined by DXA using a fan-beam densitometer (Hologic QDR4500A, Hologic) in the infant whole body scan mode as described previously (34). Scan results provided values for total body bone mineral content (BMC), bone mineral density, nonbone lean tissue, and total body fat mass. Summing BMC and lean tissue values provided a measure of fat-free mass.
IVGTT and plasma analysis.
On d 7 and d 13, subgroups of pigs were feed deprived for 8 h and then submitted to an IVGTT. Glucose (40% solution, 0.5 g·kg−1) was injected via the jugular vein catheter within 60 s and arterial blood samples were obtained before (0 min) and after the injection at 10, 20, 30, and 60 min. Plasma was frozen in liquid nitrogen, then stored at −80°C until analysis for glucose (glucose oxidase; Sigma) and insulin (Millipore). Plasma samples were also collected after 14 d for assay of tumor necrosis factor-α (TNFα; Invitrogen) and interleukin-6 (IL-6; R&D Systems). Prior to CLAMP on d 15, pigs were feed deprived for 12 h and plasma samples were collected for assay of glucose and insulin.
CLAMP.
On d 15, after being feed deprived for 12 h, 4-h CLAMP were performed, targeting circulating insulin concentrations in the physiological fed state (69 pmol·L−1) and hyperinsulinemia (1042 pmol·L−1). On d 17, after being feed deprived for 12 h, before killing, liver and skeletal muscle samples were collected after a 1-h CLAMP, targeting circulating insulin concentrations of 69 pmol·L−1. CLAMP were performed in subgroups (n = 6–7) of TPN and EN pigs as previously described (35). Briefly, 30 min before the clamp procedure was initiated, 5 blood samples were obtained to establish the average basal (fasting) glucose concentration. Blood glucose during the clamp protocol was rapidly determined using a glucose analyzer (YSI 2300 STAT Plus Glucose Analyzer; YSI Incorporated). After a 10-min priming infusion, porcine insulin (Sigma-Aldrich) was infused for 2 h at 5.2 pmol·kg−0.66·min−1 (low dose; 86.1 nmol·L−1, 0.9% saline, 0.1% human serum albumin) and then for 2 h at 52 pmol·min−1·kg−0.66 (high dose; 861 nmol·L−1, 0.9% saline, 0.1% human serum albumin) to achieve plasma insulin levels of 69 and 1042 pmol·L−1, respectively. During the infusion, arterial blood samples were obtained in 5-min intervals and immediately analyzed for glucose. Subsequently, glucose infusion rates (20% solution for low-dose insulin, 50% solution for high-dose insulin) were adjusted to maintain blood glucose concentrations within ±10% of the average basal glucose concentration. Additional blood samples were acquired for later analysis of insulin. After completing the CLAMP, pigs were returned to their respective treatment until d 17, when we repeated the low-dose CLAMP protocol to examine the ability of insulin to acutely activate tissue-specific insulin signaling. This CLAMP protocol was similar to that described above, but insulin and glucose were infused for only 1 h. Immediately at the end of the 1-h infusion period, animals were killed and liver, skeletal muscle (Longissimus dorsi), and pancreatic tissue samples were quickly excised and frozen in liquid nitrogen.
Liver and pancreas tissue analyses and histology.
Fresh liver was fixed in O.C.T. Compound (Sakura Finetek), frozen in liquid nitrogen, and then frozen sections were stained with Oil Red O. Liver triglyceride concentrations were also determined by extraction of frozen tissue with ethanolic KOH (2 parts ethanol:1 part 30% KOH) for 24 h at 55°C and measuring glycerol content using an enzymatic assay involving sequential reactions with glycerol kinase and glycerol phosphate oxidase to produce hydrogen peroxide (Infinity Triglycerides Reagent, Thermo Fisher Scientific). Liver myeloperoxidase activity was assayed using frozen tissue as previously described (36). Tissue protein and DNA concentration were determined as previously described (32). Organ weight and composition were expressed relative to body weight in kilograms.
Fresh pancreas tissue was fixed in 4% buffered formalin for 24 h and then stored in 70% ethanol at room temperature. Paraffin sections (5 μm) were unmasked by microwave heating in 10 mmol/L citrate buffer (pH = 6.0) for 20 min, washed with PBS, and then permeabilized in PBS-T (0.3% Triton-100 in PBS) for 45 min at room temperature. Sections were blocked for 1 h with 10% normal goat serum in 0.1% PBS-T at room temperature and then incubated for 18 h at 4°C with primary antibody at 1:200 dilution in 10% normal goat serum with 0.1% PBS-T. Primary antibodies were mouse, anti-human Ki-67 (BD Biosciences), and guinea pig, anti-human insulin (Millipore). Secondary antibodies (Alex Fluor 546 goat anti-mouse and Alex Fluor 488 goat anti-guinea pig, Invitrogen Molecular Probes) at 1:1000 dilution were incubated for 2 h at room temperature and then nuclei were counterstained with Topro-3 (1:1000 in PBS) for 10 min (Invitrogen). Sections were visualized using a laser scanning microscope (Olympus Fluoview FV 300). Multiple images (15–20) from each sample were captured at 100×, 200×, and 400× magnification for quantitative analysis of insulin-positive area to represent β-cells and Ki-67-positive cells to represent proliferating cells. Insulin and Ki-67 double-positive cells were also quantified to represent proliferating β-cells. Total cell densities were quantified based on topro-3 and used to adjust Ki-67 and insulin-positive cell numbers. Captured digital Tiff images were subjected to computer-assisted analysis after thresholding for intensity using Image Pro Plus Version 5.1 software (Media Cybernetics).
Hepatic inflammatory gene expression.
Quantitative real-time PCR was performed as described (37). Briefly, total RNA was extracted from liver tissue using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The design of porcine-specific primers, real-time PCR protocol, and primer sequences are described in the online appendix. Data were normalized to glyceraldehyde-3-phosphate dehydrogenase expression and the 2−ΔΔCT-method was used to compare gene expression levels between samples.
Tissue insulin signaling.
Immunoblotting was performed on tissue extracts of frozen liver and muscle tissue to detect phosphatidylinositol 3 kinase (PI3-K), insulin receptor substrates 1 and 2 (IRS1, 2), insulin receptor (IR), and c-Jun-N-terminal kinase 1 (JNK 1) as well as their phosphorylated forms as described in the online appendix. All Western blots were run with 6 pigs from each treatment group and used for statistical analysis. Treatment means and SE are shown as bar graphs. The abundances of PI3-K are expressed relative to that of tubulin measured after stripping and reprobing membranes. To quantify the phosphorylated forms of specific proteins, we expressed the calculated densitometric band intensities of the phosphorylated form relative to that of the total protein on the same immunoblot; the latter was determined after stripping and reprobing the membranes. These values are expressed as arbitrary units for each treatment group in the figures.
Statistical analysis.
Data for the 2 treatment groups were analyzed using Minitab statistical software. Data were first analyzed by 1-way ANOVA with nutritional treatment as a main effect. Measurements of plasma substrates and insulin during the IVGTT and at intervals during the 17-d period were analyzed by 2-way ANOVA with main effects of treatment and time. Differences compared with the initial time point were determined by Tukey’s means comparison. The area under the curve (AUC) for glucose and insulin data derived from the IVGTT tests were calculated using GraphPad statistical software. Results are expressed as mean ± SEM and P < 0.05 was considered significant.
Results
Body and organ composition.
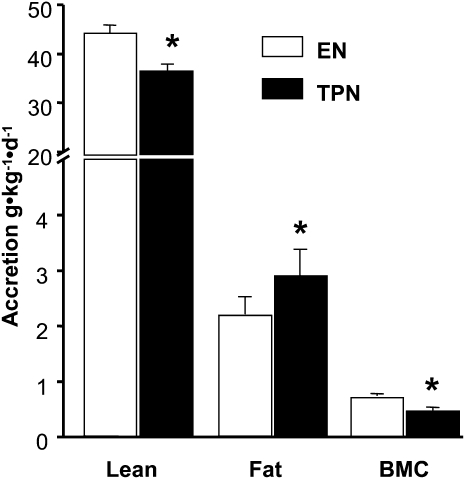
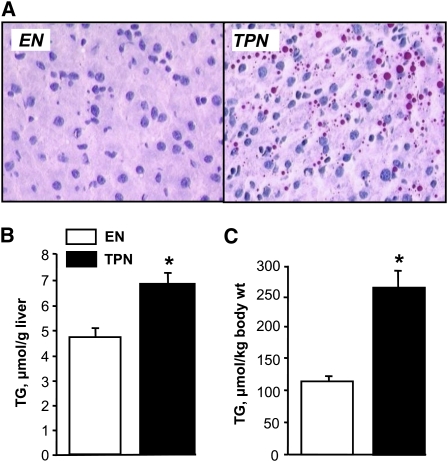
Initial body weight and body composition did not differ between the 2 treatment groups; the composition of the piglets was dominated by lean tissue (~90%) with relatively low body fat (4–6%) and BMC (1.5%) (Table 1). However, daily weight gain was lower and final body weight tended to be lower (P = 0.08) in TPN compared with EN pigs (Table 1). Body composition analysis based on DXA scanning indicated that TPN piglets gained significantly less lean (37.9 ± 1.8 vs. 43.1 ± 1.3 g·kg−1·d−1) and bone mass (0.473 ± 0.026 vs. 0.741 ± 0.029 g·kg−1·d−1) and more fat mass (2.86 ± 0.23 vs. 2.13 ± 0.18 g·kg−1·d−1) compared with EN piglets (Fig. 1). TPN induced hepatomegaly based on increased relative liver weight and protein and DNA mass relative to EN (Table 1). TPN also induced steatosis compared with EN pigs as evidenced by a greater lipid concentration (6.84 ± 0.43 vs. 4.71 ± 0.34 μmol/g liver) and microscopic lipid droplets (Fig. 2). There was no difference in pancreas relative weight or cellular composition between groups; however, the spleen was significantly enlarged in TPN compared with EN pigs (Table 1).
TABLE 1.
Body and organ weights and tissue composition in neonatal pigs chronically fed EN or TPN1
| EN | TPN | |
| Initial body weight, g | 1894 ± 77 | 1950 ± 82 |
| Initial body lean, g | 1760 ± 70 | 1812 ± 78 |
| Initial body fat, g | 102 ± 7 | 104 ± 4 |
| Initial body BMC, g | 32.4 ± 2.1 | 33.6 ± 1.8 |
| Final body weight, g | 4451 ± 263 | 4039 ± 152 |
| Daily weight gain,2g·kg BW−1 | 46.0 ± 1.2 | 41.2 ± 1.7* |
| Liver weight, g·kg BW−1 | 25.3 ± 0.7 | 37.9 ± 1.9* |
| Liver protein content, mg·kg BW−1 | 4399 ± 281 | 6102 ± 267* |
| Liver DNA content, mg·kg BW−1 | 161 ± 16 | 191 ± 12* |
| Pancreas weight, g·kg BW−1 | 1.42 ± 0.04 | 1.34 ± 0.08 |
| Pancreas protein content, mg·kg BW−1 | 236 ± 12 | 210 ± 12 |
| Pancreas DNA content, mg·kg BW−1 | 8.54 ± 0.53 | 8.01 ± 0.45 |
| Spleen weight, g·kg BW−1 | 3.33 ± 0.7 | 5.40 ± 1.9* |
Values are means ± SEM, = 15. *Different from EN, P < 0.05.
BW, body weight.
FIGURE 1.
Accretion of lean mass, fat mass, and BMC in neonatal pigs fed EN or TPN over a 16-d period. Results are expressed as mean ± SEM, n = 15. *P < 0.05.
FIGURE 2.
Hepatic lipid content in neonatal pigs fed EN or TPN for 17 d. (A) Oil Red O-stained liver sections showing lipid droplets (red) in livers from EN or TPN pigs after 17 d. (B) Hepatic triglyceride (TG) content per g tissue and (C) per kg body weight (BW) in neonatal pigs fed EN or TPN for 17 d. Results are expressed as mean ± SEM, n = 15. *P < 0.05.
Hepatic and systemic inflammation.
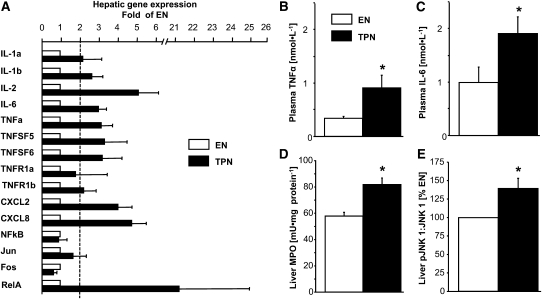
Given the evidence of hepatic steatosis, we quantified various measures of hepatic inflammation and found that myeloperoxidase activity and the expression of several proinflammatory cytokines and chemokines were significantly upregulated in TPN compared with EN pigs, especially IL-2, CXCL2, CXCL8, TNFα, IL-6, and Rel A (Fig. 3). The phosphorylation of JNK 1, also known as mitogen-activated protein kinase 8, also was higher in livers from TPN than those in EN pigs. Circulating plasma concentrations of both TNFα and IL-6 were significantly increased in TPN compared with EN pigs, indicating systemic inflammation.
FIGURE 3.
Hepatic and plasma measurements of inflammation in neonatal pigs fed EN or TPN for 17 d. (A) Hepatic gene expression of proinflammatory cytokines and chemokines in TPN compared with EN pigs (n = 6). (B) Plasma TNFα. (C) Plasma IL-6 (n = 8). Hepatic myeloperoxidase (MPO) (n = 8) (D) and pJNK 1 (B) in TPN and EN pigs (n = 7). pJNK 1 is presented as a ratio and TPN is expressed as percentage of EN (control). Results are expressed as mean ± SEM. *P < 0.05.
Pancreatic growth and β-cell responses.
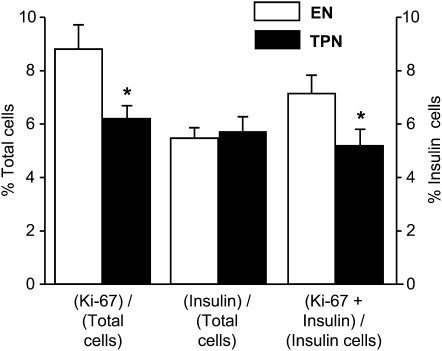
We determined the number of total cells and β-cells within the pancreas in the 2 groups. As indicated, the total weight and DNA content of the pancreas was not different (Table 1) and this was confirmed histologically (Fig. 4); representative staining is shown in Supplemental Figure 2. The total number of insulin-positive, β-cells also did not differ between the 2 groups; however, the number of Ki67 positive, proliferating cells, and especially β-cells was lower in TPN compared with EN pigs.
FIGURE 4.
Quantitative analysis of pancreatic tissue from TPN and EN pigs determined total proliferating (Ki-67 positive) and β-cells (insulin-positive) as a percentage of total cells (left axis). Also shown are proliferating β-cells (Ki-67 + insulin) as a percentage of total β-cells (right axis). Results are expressed as mean ± SEM, n = 11. *P < 0.05.
Whole body insulin sensitivity and glucose tolerance.
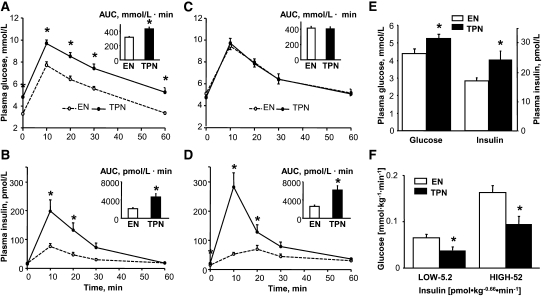
We examined the impact of mode of nutrition on whole body glucose tolerance based on an IVGTT after 7 and 13 d of treatment. Plasma glucose (7 d) and insulin responses were significantly higher in TPN piglets than in EN piglets (Fig. 5A–D); the AUC estimates were also higher in TPN piglets. Also after 15 d of treatment, we assessed insulin sensitivity using the CLAMP technique based on infusion of low and high doses of insulin to achieve plasma insulin levels of 69 and 1042 pmol/L, respectively. The steady-state plasma insulin concentrations were not different between EN (n = 5) and TPN (n = 7) pigs during the low (64 ± 9 and 65 ± 5 pmol/L) and high (986 ± 76 and 1076 ± 76 pmol/L) insulin infusion clamp periods, respectively. Similarly, the steady-state plasma glucose concentrations did not differ between EN and TPN pigs during the low (4.23 ± 0.32 and 3.74 ± 0.25 mmol·L−1) and high (4.69 ± 0.29 and 5.17 ± 0.95 mmol·L−1) insulin infusion clamp periods, respectively. At both the low (physiological) and high (hyperinsulinemic) insulin concentrations, the glucose infusion rates necessary to maintain euglycemia at the fasting glucose concentrations were significantly lower in TPN piglets compared with EN piglets (Fig. 5F). After 17 d of treatment during the basal period before the CLAMP was initiated, the overnight fasting glucose and insulin concentrations were significantly higher in TPN piglets than in EN piglets as measured (Fig. 5E).
FIGURE 5.
Plasma glucose and insulin concentrations and respective AUC values during IVGTT on d 7 (n = 6) (A,B) and on d 13 (n = 8) (C,D) in TPN and EN pigs. (E) Fasting (12-h) plasma glucose and insulin concentrations in TPN and EN pigs on d 15 (n = 6). (F) Glucose and insulin infusion rates during CLAMP on d 15 in TPN (n = 5) and EN (n = 7) pigs. Results are expressed as mean ± SEM. *P < 0.05.
Tissue insulin signaling.
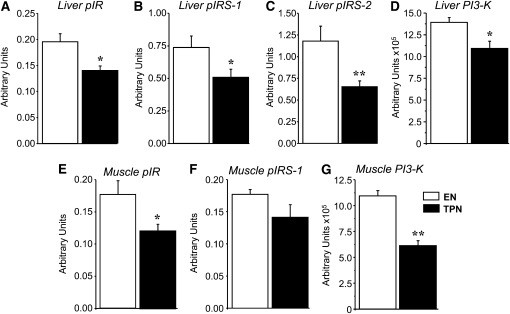
To further assess the effects of TPN on tissue-specific insulin resistance, we measured the liver and skeletal muscle tissue abundance of the IR, IRS-1/2, and PI3K. The hepatic abundance of PI3-K protein and phosphorylation of IR, IRS-1, and IRS-2 was significantly reduced in TPN compared with EN piglets (Fig. 6A–D). Similarly, the skeletal muscle abundance of PI3-K protein and phosphorylation of IR was depressed in TPN piglets compared with EN piglets (Fig. 6 E,F).
FIGURE 6.
Relative abundance and phosphorylation of proteins in liver and skeletal muscle tissue samples obtained from EN (n = 6) and TPN (n = 6) pigs after a 1-h CLAMP on d 17. Liver tissue relative abundance of phospho-IR (pIR) (A), phospho-IRS-1 (pIRS-1) (B), and phospho-IRS-2 (pIRS-2) (C) and PI3-K (D). Muscle tissue relative abundance of p-IR (E), IRS-1 (F), and PI3-K (G). Phosphorylated proteins expressed relative to total protein and PI3-K protein expressed relative to tubulin. Results are expressed in arbitrary units as mean ± SEM for EN and TPN. *P < 0.05 and **P < 0.01.
Discussion
The aim of the study was to characterize the metabolic response to chronic PN in neonatal piglets. We were especially interested in the impact on glucose metabolism given the evidence of derangements in glycemia in parenterally fed VLBW infants (4–7). We examined the effect on TPN on the liver, because it plays an integral role in glucose metabolism, and previous reports, including our own in piglets, indicate that TPN induces hepatic steatosis and cholestasis (38–40). We used the neonatal pig because of its relevance as a model of the human neonate with respect to glucose metabolism, and its larger body size compared with neonatal rodents allows investigation of the effect of PN (26, 7, 30). Our results show that chronic TPN induces a dysfunctional metabolic phenotype in neonatal pigs marked by hepatic inflammation and steatosis and is associated with significant insulin resistance and increased body fat deposition. Tissue analysis also revealed defects in liver and skeletal muscle insulin signaling and suppressed pancreatic islet cell proliferation consistent with deterioration of glucose regulatory capacity.
An important finding in the study was the alteration in body composition associated with TPN. Based on our previous studies in neonatal pigs, we anticipated that TPN would result in increased liver mass and steatosis, but the finding of increased fat and reduced lean mass accretion was unexpected (38). The changes in liver mass were not due to increased lipid content but rather substantial increases in protein and DNA content, suggestive of increased hepatic protein synthesis. We are not aware of any studies that have examined the influence of PN compared with EN on either growth rate or body composition in human premature infants despite the fact that PN can represent a significant proportion of the nutrition intake in this population. This issue may be significant given the current recommendations to pursue aggressive early nutrition in premature and VLBW infants to maintain normal neurodevelopment (2, 3), because an emerging body of evidence suggests that rapid catch-up growth in these infants may predispose to increased adiposity and later obesity (41).
The changes in liver growth resulting from TPN compared with EN prompted us to examine in more detail evidence for altered inflammatory response, because our previous report in TPN-fed neonatal pigs showed increased proinflammatory markers along with hepatic steatosis. Our results showed a marked increase in the expression of several proinflammatory genes, including both proinflammatory cytokines and chemokines. This finding was corroborated by increased hepatic tissue myeloperoxidase activity, which is a peroxidase enzyme marker found in macrophages, such as infiltrating neutrophils and resident Kupffer cells during liver inflammatory injury. We further explored a key signaling pathway activated by cellular inflammation, namely JNK 1 (42). We found that phosphorylation of JNK 1 was modestly upregulated in TPN compared with EN, consistent with its role in nutrient overload and metabolic stress associated with inflammation. We finally quantified the circulating plasma concentrations of TNFα and IL-6, key proinflammatory cytokines, and found both were increased 2- to 3-fold in TPN-fed piglets. The proinflammatory response combined with hepato- and splenomegaly may be indicative of an acute-phase response that occurs during infection or endotoxemia.
A dominant metabolic response observed in TPN-fed piglets was insulin resistance that was evident at the whole body and cellular level. Insulin resistance was observed early, after only 7 d, when we found increased glucose excursion and insulinemia during the IVGTT. Insulin resistance was confirmed in TPN piglets compared with EN piglets based on CLAMP at both physiological and supra-physiological insulin concentrations and increased fasting plasma glucose and insulin at 14 d. The combined results suggest that the TPN induced insulin resistance and was mainly due to peripheral tissue resistance, likely skeletal muscle. Our current results contrast with previous studies in dogs showing that TPN increased hepatic but reduced peripheral muscle glucose uptake compared with EN (24, 25). The latter is consistent with the portal glucose signal phenomenon revealed in dog studies showing that intraportal glucose infusion stimulates preferential hepatic glucose uptake but suppresses peripheral skeletal muscle glucose uptake (43, 44). However, recent dog studies show that portal coinfusion of GLP-1 and glucose increases peripheral glucose utilization (45). Based on our previous studies, we predict that circulating (and portal venous) levels of both GIP and GLP-1 would be higher in EN piglets than in TPN piglets and may explain the increased insulin sensitivity (32). Our results showed that phosphorylation of the IR and IRS-1 were significantly depressed in the liver and skeletal muscle from TPN-fed piglets. The dampening of insulin signaling we observed in TPN-fed piglets maybe linked to increased circulating proinflammatory cytokines, namely TNFα and IL-6, secreted by the liver Kupffer cells or adipose macrophages (46, 47).
We also characterized the cellularity of the pancreas, especially the β-cell volume, given its key role in the regulation of glucose metabolism. The suppressed β-cell proliferation is unexpected, because we found hyperinsulinemia in the TPN group secondary to insulin resistance. This finding raises questions about the long-term consequence of TPN on metabolic function, because proliferation of β-cells occurs during infancy and thus the neonatal period is a critical stage of β-cell development (48).
The picture that emerges from our study in neonatal pigs is that TPN leads to a dysfunctional metabolic phenotype that includes hepatic inflammation and steatosis coupled with marked insulin resistance and glucose intolerance that is evident at the liver and peripheral muscle tissue sites. The phenotype we observed in the TPN-fed neonatal pig includes some of the same metabolic markers found in metabolic syndrome, type 2 diabetes, and obesity. The underlying mechanism(s) that explains this dysfunctional metabolic phenotype is not clear. One logical explanation is the mode of nutrition support per se, EN versus PN. The provision of nutrition during TPN is in an elemental molecular form, i.e. glucose and amino acids, via the parenteral route. Thus, although this is not physiological from the perspective of enteral formula feeding, it is not profoundly different from nutritional support in utero via the umbilical circulation. However, the absence of EN and secretion of key incretin gut hormones, such as gastric inhibitory peptide and glucagon-like peptide 1, may play a role, as these are both trophic for insulin secretion and β-cell proliferation (49). Another plausible explanation is that impaired intestinal barrier function secondary to TPN (36) results in gut-derived endotoxemia and hepatic inflammation and this triggers a metabolic dysfunction leading to deranged hepatic lipid metabolism, steatosis, and insulin resistance, similar to that observed in chronic diseases such as obesity and type 2 diabetes (42). A final explanation could be that a component of TPN is toxic and this induces metabolic dysfunction. A TPN component that has been specifically linked to liver disease is the lipid emulsion (i.e. Intralipid). Intralipid is a soybean oil-based emulsion that is enriched with (n-6) PUFA and phytosterols that have been linked to proinflammatory signaling and cholestatic liver disease (39, 50).
This work also may have clinical relevance to long-term metabolic disease. Recent reports showed that both prepubertal children and young adults born appropriate-for-gestational age, but premature, showed signs of insulin resistance and glucose intolerance (51, 52). It is unknown why prematurity is associated with adolescent and young adult-onset insulin resistance. However, a major determinant of clinical outcomes for premature infants is nutritional support and most premature infants receive PN after birth. There is limited information describing the impact of chronic PN on insulin sensitivity and glucose metabolism in human neonates. One recent report in young children showed that chronic PN resulted in diminished glucose-induced insulin secretory capacity (17), contrary to our finding of hyperinsulinemia with PN. Growing evidence supports the idea that nutritional imbalance or overload during the perinatal period can have long-term metabolic effects (53, 54). This series of studies reported by Patel and others has demonstrated that feeding neonatal rodents a high-carbohydrate diet and the resultant hyperinsulinemia that occurs can translate into long-term pancreatic islet dysfunction and obesity (53, 54). Whether the metabolic effects of TPN in our model persist beyond the neonatal period warrants further study.
Supplementary Material
Acknowledgments
We thank Mareah Sedenquist, Hanh Nguyen, Roman Shypailo, and Nancy Benight for technical assistance with this study. D.G.B., D.A.H., and B.S. designed the experiments; D.G.B., D.A.H., B.S., L.C., X.C., A.K., A.M. collected data, D.G.B., D.A.H., B.S., L.C., X.C., A.K., and A.M. analyzed data; D.G.B. and B.S. wrote the manuscript; and K.J.E., D.L.H., A.S., and T.A.D. provided important advice and technical assistance. All authors read and approved the final manuscript.
Footnotes
Supported by the USDA, Agricultural Research Service under Cooperative Agreement no. 58-6250-6-001 (to D.B.), Texas Medical Center Digestive Diseases Center (NIH grant no. P30 DK-56338 to D.B.), and NIH grants K12HD043397 and RHD059142 (to A.M.).
Detailed description of Methods where indicated, as well as Supplemental Table 1 and Supplemental Figures 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AUC, area under the curve; BMC, bone mineral content; CLAMP, hyperinsulinemic-euglycemic clamp; CXCL-2, chemokine (C-X-C motif) ligand 2; CXCL8, interleukin-8; DXA, dual-energy X-ray absorptiometry; EN, enteral nutrition; IL-2, interleukin-2; IR, insulin receptor; IRS, insulin receptor substrate; IL-6, interleukin 6; IVGTT, intravenous glucose tolerance test; JNK 1, c-Jun-N-terminal kinase 1; PI3-K, phosphatidylinositol 3 kinase; RelA, v-rel reticuloendotheliosis viral oncogene homolog A; (T)PN, (total) parenteral nutrition; TNFα, tumor necrosis factor-α; VLBW, very low birth weight;
Literature Cited
- 1.Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol. 2007;27:284–90 [DOI] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61 [DOI] [PubMed] [Google Scholar]
- 3.Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birthweight infant. Clin Perinatol. 2002;29:225–44 [DOI] [PubMed] [Google Scholar]
- 4.Miranda LE, Dweck HS. Perinatal glucose homeostasis: the unique character of hyperglycemia and hypoglycemia in infants of very low birth weight. Clin Perinatol. 1977;4:351–65 [PubMed] [Google Scholar]
- 5.Lilien LD, Rosenfield RL, Baccaro MM, Pildes RS. Hyperglycemia in stressed small premature neonates. J Pediatr. 1979;94:454–9 [DOI] [PubMed] [Google Scholar]
- 6.Sinclair JC, Bottino M, Cowett RM. Interventions for prevention of neonatal hyperglycemia in very low birth weight infants. Cochrane Database Syst Rev. 2009;CD007615. [DOI] [PubMed] [Google Scholar]
- 7.Sunehag AL, Haymond MW. Glucose extremes in newborn infants. Clin Perinatol. 2002;29:245–60 [DOI] [PubMed] [Google Scholar]
- 8.Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol. 2006;26:730–6 [DOI] [PubMed] [Google Scholar]
- 9.Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118:1811–8 [DOI] [PubMed] [Google Scholar]
- 10.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, Midgley P, Thompson M, Thio M, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359:1873–84 [DOI] [PubMed] [Google Scholar]
- 11.Cowett RM, Oh W, Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest. 1983;71:467–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunehag A, Gustafsson J, Ewald U. Very immature infants (< or = 30 Wk) respond to glucose infusion with incomplete suppression of glucose production. Pediatr Res. 1994;36:550–5 [DOI] [PubMed] [Google Scholar]
- 13.Berseth CL. Feeding methods for the preterm infant. Semin Neonatol. 2001;6:417–24 [DOI] [PubMed] [Google Scholar]
- 14.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47Suppl 1:S33–6 [DOI] [PubMed] [Google Scholar]
- 15.Wessel JJ, Kocoshis SA. Nutritional management of infants with short bowel syndrome. Semin Perinatol. 2007;31:104–11 [DOI] [PubMed] [Google Scholar]
- 16.Tyson JE, Kennedy KA. Trophic feedings for parenterally fed infants. Cochrane Database Syst Rev. 2005;CD000504. [DOI] [PubMed] [Google Scholar]
- 17.Beltrand J, Colomb V, Marinier E, Daubrosse C, Alison M, Burcelin R, Cani PD, Chevenne D, Marchal CL. Lower insulin secretory response to glucose induced by artificial nutrition in children: prolonged and total parenteral nutrition. Pediatr Res. 2007;62:624–9 [DOI] [PubMed] [Google Scholar]
- 18.Moore MC, Cherrington AD, Wasserman DH. Regulation of hepatic and peripheral glucose disposal. Best Pract Res Clin Endocrinol Metab. 2003;17:343–64 [DOI] [PubMed] [Google Scholar]
- 19.Pagliassotti MJ, Cherrington AD. Regulation of net hepatic glucose uptake in vivo. Annu Rev Physiol. 1992;54:847–60 [DOI] [PubMed] [Google Scholar]
- 20.Stoll B, Burrin DG, Henry J, Yu H, Jahoor F, Reeds PJ. Substrate oxidation by the portal drained viscera of fed piglets. Am J Physiol. 1999;277:E168–75 [DOI] [PubMed] [Google Scholar]
- 21.Vella A, Camilleri M, Rizza RA. The gastrointestinal tract and glucose tolerance. Curr Opin Clin Nutr Metab Care. 2004;7:479–84 [DOI] [PubMed] [Google Scholar]
- 22.Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr Opin Clin Nutr Metab Care. 2004;7:471–8 [DOI] [PubMed] [Google Scholar]
- 23.Colomb V, Leturque A, Guihot G, Loizeau M, Lavie S, Colomer S, Ricour C, Girard J. Route of nutrient delivery affects insulin sensitivity and liver glucose transporter expression in rat. Am J Physiol. 1995;269:E827–33 [DOI] [PubMed] [Google Scholar]
- 24.Chen SS, Donmoyer C, Zhang Y, Hande SA, Lacy DB, McGuinness OP. Impact of enteral and parenteral nutrition on hepatic and muscle glucose metabolism. JPEN J Parenter Enteral Nutr. 2000;24:255–60 [DOI] [PubMed] [Google Scholar]
- 25.Chen SS, Torres-Sanchez CJ, Hosein N, Zhang Y, Lacy DB, Chang C, McGuinness OP. Route-dependent effect of nutritional support on liver glucose uptake. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1319–27 [DOI] [PubMed] [Google Scholar]
- 26.Flecknell PA, Wootton R, John M. Total body glucose metabolism in the conscious, unrestrained piglet and its relation to body- and organ weight. Br J Nutr. 1980;44:193–203 [DOI] [PubMed] [Google Scholar]
- 27.Moughan PJ, Birtles MJ, Cranwell PD, Smith WC, Pedraza M. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev Nutr Diet. 1992;67:40–113 [DOI] [PubMed] [Google Scholar]
- 28.Book SA, Bustad LK. The fetal and neonatal pig in biomedical research. J Anim Sci. 1974;38:997–1002 [DOI] [PubMed] [Google Scholar]
- 29.Schook L, Beattie C, Beever J, Donovan S, Jamison R, Zuckermann F, Niemi S, Rothschild M, Rutherford M, et al. Swine in biomedical research: creating the building blocks of animal models. Anim Biotechnol. 2005;16:183–90 [DOI] [PubMed] [Google Scholar]
- 30.Cornblath M, Hawdon JM, Williams AF, Aynsley-Green A, Ward-Platt MP, Schwartz R, Kalhan SC. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141–5 [DOI] [PubMed] [Google Scholar]
- 31.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology. 2005;146:22–32 [DOI] [PubMed] [Google Scholar]
- 32.Burrin DG, Stoll B, Jiang RH, Chang XY, Hartmann B, Holst JJ, Greeley GH, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–10 [DOI] [PubMed] [Google Scholar]
- 33.Stoll B, Chang X, Fan MZ, Reeds PJ, Burrin DG. Enteral nutrient intake level determines intestinal protein synthesis and accretion rates in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2000;279:G288–94 [DOI] [PubMed] [Google Scholar]
- 34.Koo WW, Hammami M, Shypailo RJ, Ellis KJ. Bone and body composition measurements of small subjects: discrepancies from software for fan-beam dual energy X-ray absorptiometry. J Am Coll Nutr. 2004;23:647–50 [DOI] [PubMed] [Google Scholar]
- 35.Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol. 1997;273:E305–14 [DOI] [PubMed] [Google Scholar]
- 36.Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou CN, Harvey R, Burrin D. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1162–70 [DOI] [PubMed] [Google Scholar]
- 37.Shaik SS, Soltau TD, Chaturvedi G, Totapally B, Hagood JS, Andrews WW, Athar M, Voitenok NN, Killingsworth CR, et al. Low intensity shear stress increases endothelial ELR+ CXC chemokine production via a focal adhesion kinase-p38{beta} MAPK-NF-{kappa}B pathway. J Biol Chem. 2009;284:5945–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Khaoustov VI, Krishnan B, Cai W, Stoll B, Burrin DG, Yoffe B. Total parenteral nutrition induces liver steatosis and apoptosis in neonatal piglets. J Nutr. 2006;136:2547–52 [DOI] [PubMed] [Google Scholar]
- 39.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:277–87 [DOI] [PubMed] [Google Scholar]
- 40.Lloyd DA, Gabe SM. Managing liver dysfunction in parenteral nutrition. Proc Nutr Soc. 2007;66:530–8 [DOI] [PubMed] [Google Scholar]
- 41.Dunger DB, Salgin B, Ong KK. Session 7: early nutrition and later health early developmental pathways of obesity and diabetes risk. Proc Nutr Soc. 2007;66:451–7 [DOI] [PubMed] [Google Scholar]
- 42.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore MC, Hsieh PS, Neal DW, Cherrington AD. Nonhepatic response to portal glucose delivery in conscious dogs. Am J Physiol Endocrinol Metab. 2000;279:E1271–7 [DOI] [PubMed] [Google Scholar]
- 44.Galassetti P, Shiota M, Zinker BA, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient decreases skeletal muscle glucose uptake. Am J Physiol. 1998;275:E101–11 [DOI] [PubMed] [Google Scholar]
- 45.Johnson KM, Edgerton DS, Rodewald T, Scott M, Farmer B, Neal D, Cherrington AD. Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. Am J Physiol Endocrinol Metab. 2007;293:E1085–91 [DOI] [PubMed] [Google Scholar]
- 46.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6 [DOI] [PubMed] [Google Scholar]
- 48.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85:1171–84 [DOI] [PubMed] [Google Scholar]
- 51.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, Cutfield WS. Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–86 [DOI] [PubMed] [Google Scholar]
- 52.Hovi P, Andersson S, Eriksson JG, Jarvenpaa AL, Strang-Karlsson S, Makitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:2053–63 [DOI] [PubMed] [Google Scholar]
- 53.Vadlamudi S, Hiremagalur BK, Tao L, Kalhan SC, Kalaria RN, Kaung HL, Patel MS. Long-term effects on pancreatic function of feeding a HC formula to rats during the preweaning period. Am J Physiol. 1993;265:E565–71 [DOI] [PubMed] [Google Scholar]
- 54.Patel MS, Srinivasan M. Metabolic programming due to alterations in nutrition in the immediate postnatal period. J Nutr. 2010;140:658–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.