Abstract
Standard therapy for severe acute malnutrition (SAM) is home-based therapy with ready-to-use therapeutic food (RUTF) containing 25% milk. In an effort to lower the cost of RUTF and increase availability, some have suggested that a portion of milk be replaced with soy. This trial was designed to determine whether treating children with SAM with 10% milk RUTF containing soy would result in a similar recovery rate compared with the 25% milk RUTF. This was a randomized, double-blind, controlled, clinical, quasi-effectiveness trial of isoenergetic amounts of 2 locally produced RUTF to treat SAM in Malawi among children aged 6–59 mo. A total of 1874 children were enrolled. Children were assessed every fortnight and participated in the study until they clinically recovered or received 8 wk of treatment. The primary outcome was recovery (weight-for-height Z score > −2 and no edema). Secondary outcomes were rates of weight and height gain. Survival analysis was used to compare the recovery rates. Recovery among children receiving 25% milk RUTF was greater than children receiving 10% milk RUTF, 64% compared with 57% after 4 wk, and 84% compared with 81% after 8 wk (P < 0.001). Children receiving 25% milk RUTF also had higher rates of weight and height gain compared with children receiving 10% milk RUTF. Treating children with SAM with 10% milk RUTF is less effective compared with treatment with the standard 25% milk RUTF. These findings also emphasize that clinical evidence should be examined before recommending any changes to the formulation of RUTF.
Introduction
Ready-to-use therapeutic food (RUTF)8 has greatly improved the recovery rate of children with severe acute malnutrition (SAM) in sub-Saharan Africa (1). RUTF is a lipid peanut paste that resists bacterial contamination, contains very little water, does not require cooking, is energy dense (23 kJ/g), and meets the compositional requirement specified by the WHO for therapeutic food (2). Use of RUTF in home-based therapy has increased recovery rates from 25–50% to 80–90% (3, 4) and has been adopted as the standard of the joint United Nation’s agencies for treating uncomplicated SAM (5).
RUTF contains 25% milk powder, an expensive ingredient that is not readily available worldwide. Soy is known to have a favorable amino acid profile and has been successfully substituted for animal products in a variety of other foods, including infant formula (6). Substituting soy for much of the milk in RUTF might reduce its cost and/or increase its availability. This notion has led some food producers and nutritionists to advocate inclusion of soy in RUTF and offer alternative formulations of RUTF with less milk (7–9). However, children with SAM suffer from profound metabolic derangements, rendering their clinical condition unstable (10, 11), and there is no substantial clinical evidence to support the use of soy-containing foods for the treatment of SAM. Children with SAM have not been successfully treated with plant-based diets, and large amounts of milk have always been a key component in the recommended treatment regimens (10). Given the precarious clinical status of children with SAM, changes in the formulation of RUTF should be evaluated in a clinical trial before they are used on a widespread basis.
This trial was conducted to test the hypothesis that RUTF made with 10% milk and 15% soy would result in a similar recovery rate for children with SAM as RUTF made with 25% milk.
Methods
Study area and participants.
Fifteen rural study sites were identified in the southern region of Malawi based on census reports of severely malnourished children provided by the World Food Program. Children aged 6–59 mo presenting at these sites from July 2008 to April 2009 were screened for eligibility. Eligible children were those with SAM and a good appetite. SAM was defined as having a weight-for-height Z-score (WHZ) < −3 and/or having bipedal pitting edema (10). A good appetite was defined as being able to consume 30 g of RUTF upon presentation. Children known to have chronic illness, including HIV, cardiac disease, congenital abnormalities, cerebral palsy, or cancer, or those who had participated in a treatment program for SAM within the past 12 wk were not eligible for the study. Oral and written informed consent was obtained from all caretakers. The study was approved by the College of Medicine Research and Ethics Committee, University of Malawi, and the Human Studies Committee of Washington University School of Medicine.
Study design.
This study was a randomized, double-blind, clinical, quasi-effectiveness trial of 2 locally produced foods for the treatment of SAM. Children were randomly assigned with equal probability to either 25% milk RUTF or 10% milk RUTF as home-based therapy for up to 8 wk. Randomization was blocked for the entire study rather than at each study site. To allocate children to a food group, caretakers chose a sealed envelope that contained 1 of 6 letters: 3 of these letters corresponded to the 25% milk formulation and 3 to the 10% milk formulation. The letter was recorded separately from the child’s clinical measurements and field workers involved in the randomization process did not know which letter corresponded to which food. The field assistants who distributed the food did not assess the participants. Caretakers, field workers, and investigators assessing the children remained unaware of what type of food each child received for the duration of the study.
If the child was a twin, an additional supply of food was given to the caretaker to ensure that the child received a full ration and to limit sharing between the children. If there were 2 study participants in the same household, both children were given the same type of food, thereby eliminating the possibility of confounding study foods. If there was a moderately malnourished child and a child with SAM in the same household, the caretaker was instructed to treat both children as if they had SAM and the children were given the same type of food.
Children participated in the study until they recovered or received 8 wk of therapeutic feeding. Recovery was defined as having a WHZ > −2 without edema. Children were assessed once per fortnight. The primary outcome was recovery. Secondary outcomes included the rates of weight and height gain. The outcomes were compared using survival analysis. The sample size was estimated to be 1800 children divided equally between the 2 foods. This was chosen to detect a 5% difference in recovery rates between the 2 foods with 95% sensitivity and 90% power, assuming that the standard recovery rate is 85% and using a 1-sided difference of equivalence (Fisher’s exact test).
Participation.
Upon initial presentation and at every subsequent visit, each child was assessed for edema and had his/her weight, length or standing height, and mid-upper arm circumference (MUAC) measured by trained study staff. Length was measured for children < 2 y of age and height measured for children > 2 y of age. Weight was measured using an electronic scale (SECA model 334; precision, 5 g, calibrated weekly). Length was measured in triplicate using a canvas measure mat and the mean value was used as the length (SECA model 210; precision, 0.25 cm). Height was measured in triplicate and the mean value was used (SECA model 412; precision, 1 mm). MUAC was measured with a standard insertion tape (UNICEF, precision, 2 mm). All anthropometric measurements were made by 1 of the 2 senior field workers who had completed 3 y of medical school and were trained for the study in a standard manner by the senior investigator. At enrollment, the caretaker of the participating child was interviewed to obtain basic demographic information, including the child’s date of birth, sex, history of physical illness, and family background. On enrollment and at each follow-up visit, mothers were asked how many days in the last 2 wk the child had had fever, cough, and diarrhea. Children returned every fortnight for reassessment and were given additional RUTF until they either clinically recovered or had received 8 wk of therapeutic feeding without recovery. Children were not followed after recovery. If the child remained wasted after 4 return visits or clinically worsened during treatment, the child was referred for medical evaluation and inpatient treatment. Worsening condition was defined as weight loss > 400 g, increasing severity of edema, anorexia, or caretaker’s report that the child was having vomiting, diarrhea, or oral thrush such that they consumed <75% of the RUTF.
Food products.
The macro- and micronutrient content of the 2 foods was similar. The only difference was replacement of some of the milk with extrusion-cooked, unprocessed soy flour in the 10% milk RUTF (Table 1). Concentrated vitamins and minerals from Nutriset were added to both types of RUTF so that the micronutrient content of each RUTF was similar. The protein content of the soy flour was 39% and the milk powder was 37%. All children received rations of 733 kJ/(kg⋅d). Both foods were predominantly sweet, with the color and texture of the peanut paste. The quantity of food provided, determined by the child’s body weight, was based on meeting the estimated energy requirement for catch-up growth in SAM (11).
TABLE 1.
Ingredient and nutrient content of the study foods
| Nutrient | 25% milk RUTF | 10% milk RUTF |
| Dried skimmed milk, g/100 g | 25 | 10 |
| Peanut paste, g/100 g | 26 | 26 |
| Whole soy flour, g/100 g | 0 | 15 |
| Energy, kJ/100 g | 2000 | 2000 |
| Protein, g/100 g | 15 | 15 |
| Fat, g/100 g | 40 | 40 |
| Retinyl acetate, μg/100 g | 910 | 913 |
| Cholecalciferol, μg/100 g | 16 | 16.3 |
| α-Tocopherol, mg/100 g | 20 | 20 |
| Phylloquinone, μg/100 g | 21 | 21 |
| Thiamin, mg/100 g | 0.6 | 0.6 |
| Riboflavin, mg/100 g | 1.8 | 1.8 |
| Vitamin B-6, mg/100 g | 0.6 | 0.58 |
| Vitamin B-12, μg/100 g | 1.8 | 1.85 |
| Vitamin C, mg/100 g | 53 | 53.3 |
| Biotin, μg/100 g | 65 | 65 |
| Folate, μg/100 g | 210 | 210 |
| Niacin, mg/100 g | 5.3 | 5.3 |
| Pantothenate, mg/100 g | 3.1 | 3.1 |
| Potassium, mg/100 g | 1111 | 1110 |
| Magnesium, mg/100 g | 92 | 92 |
| Iron, mg/100 g | 11.53 | 11.5 |
| Zinc, mg/100 g | 14 | 14 |
| Copper, mg/100 g | 1.78 | 1.74 |
| Iodine, μg/100 g | 110 | 100 |
| Selenium, μg/100 g | 30 | 30 |
| Calcium, mg/100 g | 320 | 300 |
| Phosphorus, mg/100 g | 394 | 300 |
Senior research nurses instructed the caretakers on how much of the food to feed the child each day, emphasizing that the food should not be shared with other members of the household, that it should be treated as medical therapy for the affected child, that no other foods were required for recovery, and that it should not be mixed or diluted in porridge.
The foods were packaged in 245-g plastic jars and procured from Project Peanut Butter). The food production facility produced these foods in accordance with the WHO Codex Alimentarius and is certified by UNICEF and the Malawi Bureau of Standards. The cost of the food in Malawi at the time of the study for locally manufactured 25% milk RUTF was US$4.92/kg and US$3.80/kg for 10% milk RUTF.
Statistical analyses.
Summary enrollment characteristics were calculated as means ± SD for continuous measures and as n (%) for categorical measures. Anthropometric indices were calculated using WHO 2006 standards (12). The anthropometric index weight-for-height/-length was determined using length measurements for children < 2 y and standing height for children > 2 y and is referred to as WHZ in Results. The rate of weight gain was expressed as g/(kg⋅d), calculated over the duration of therapy. This denominator normalizes the rate of weight gain to the initial body weight. Height and MUAC gain were calculated over the entire duration of therapy. Intention-to-treat analysis was used.
To assess the effect of the therapeutic food on recovery, survival analysis was used. Cox regression models that estimated the proportional hazard associated with receiving 10% RUTF were created (SPSS 17.0 for Windows) (13). Covariates in all models included age, sex, twin status, whether the child’s mother and father were alive, edema, enrollment WHZ, enrollment height-for age Z-score (HAZ), the study site, the presence of fever, cough, or diarrhea on enrollment, and whether the child had previously been treated for malnutrition. Coefficients in the regression models with 95% CI that did not include one were considered to be significant.
The outcomes upon completion of therapy and the rates of recovery between the 2 study groups were compared using Student’s t test for continuous measures and Fisher’s exact test categorical measures.
To identify risk factors among children with poor outcomes, including death, backward binary logistic regression was used. P-values < 0.01 were considered significant. This more stringent P-value was chosen to identify the most important risk factors for poor outcome, assuming that risk factors with P-values > 0.01 and < 0.05 would have significance but less clinical importance.
To compare the case fatality rate in this study to international standards, the predicted case fatality rate, determined using the method of Prudhon (14), was compared with the actual case fatality rate.
Results
A total of 1961 children were identified as having SAM and 1874 children were enrolled in the study; 929 received 10% milk RUTF and 945 received 25% milk RUTF (Table 2; Supplemental Fig. 1). Among those enrolled, 78% had edema (Table 2). Among the 1458 children with edema, 194 (13%) had WHZ ≤ −3, 431 (30%) had WHZ > −3 and ≤ −2, 594 (41%) had WHZ > −2 and ≤ −1, and 239 (16%) had WHZ > −1. All children with edema had weight-for-age Z-scores (WAZ) ≤ −2.
TABLE 2.
Enrollment characteristics of children treated for severe malnutrition1
| 10% milk RUTF | 25% milk RUTF | |
| n | 929 | 945 |
| Male, n (%) | 388 (42) | 432 (46) |
| Age, mo | 19.5 ± 9.7 | 19.2 ± 9.9 |
| Weight, kg | 7.50 ± 1.67 | 7.49 ± 1.75 |
| Height, cm | 72.7 ± 7.1 | 72.7 ± 7.2 |
| MUAC, cm | 12.2 ± 1.3 | 12.1 ± 1.3 |
| WHZ | −2.0 ± 1.2 | −2.1 ± 1.2 |
| WAZ | −3.1 ± 1.2 | −3.1 ± 1.2 |
| HAZ | −3.0 ± 1.5 | −3.0 ± 1.5 |
| Mother alive,2n/N (%) | 894/929 (96) | 907/944 (96) |
| Father alive, n/N (%) | 880/922 (95) | 908/938 (97) |
| Breast-feeding, n/N (%) | 539/925 (58) | 555/938 (59) |
| Edema, n (%) | 721 (78) | 737 (78) |
| Mother with HIV, n (%) | 34 (7) | 44 (9) |
| Prior treatment for malnutrition, n/N (%) | 145/922 (16) | 156/937 (17) |
| Twins, n/N (%) | 69/923 (8) | 65/943 (7) |
| Fever on admission, n (%) | 549 (59) | 524 (55) |
| Cough on admission, n (%) | 462 (50) | 441 (47) |
| Diarrhea on admission, n (%) | 387 (42) | 419 (44) |
| Vomiting on admission, n (%) | 209 (23) | 221 (23) |
Values are mean ± SD or (%).
N in the total number of observations available for these variables.
Survival analysis indicated that receiving 25% milk RUTF was associated with a higher rate of recovery compared with receiving 10% milk RUTF (Fig. 1; Table 3). The rates of weight and height gain were greater among children receiving 25% milk RUTF than those among children receiving 10% milk RUTF (Table 4). Children without edema were less likely to recover (Table 3).
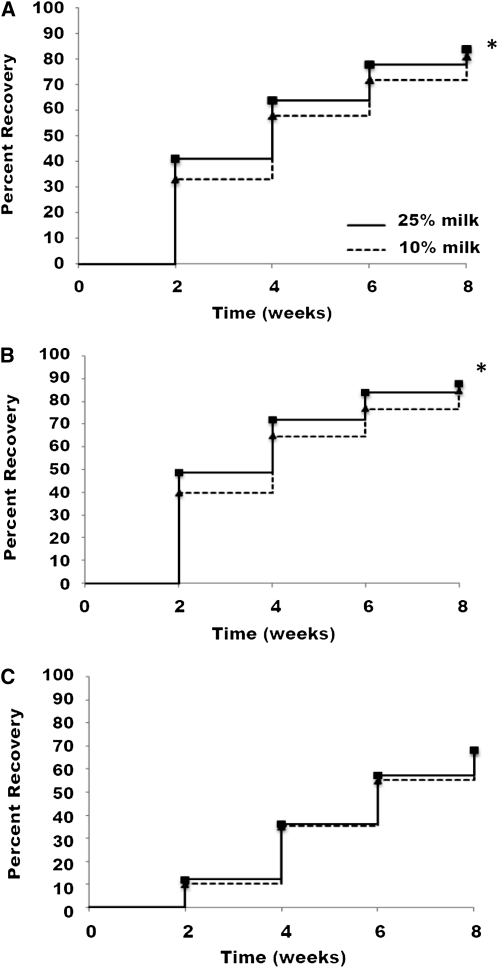
FIGURE 1.
Recovery rates of the Malawian children who received 25% milk RUTF or 10% milk RUTF. All severely malnourished children (n = 1874) (A), children with kwashiorkor (n = 1458) (B), and children with marasmus (n = 416) (C). *Greater than 10% RUTF, P < 0.05.
TABLE 3.
Hazard ratios for recovery for severely malnourished Malawian children receiving either 25% milk RUTF or 10% milk RUTF
| Cox regression model terms | Hazard ratio (95% CI) |
| Categorical | |
| Receiving 10% milk RUTF | 0.873 (0.789, 0.966) |
| Having no edema | 0.802 (0.685,0.939) |
| Female sex | 0.852 (0.764, 0.951) |
| Being a twin | 0.993 (0.815, 1.210) |
| Having mother alive | 0.807 (0.597, 1.090) |
| Having father alive | 0.851 (0.637, 1.138) |
| Having malnutrition previously | 1.060 (0.919, 1.224) |
| Having fever on enrollment | 0.986 (0.882, 1.101) |
| Having cough on enrollment | 1.009 (0.905, 1.124) |
| Having diarrhea on enrollment | 0.969 (0.871, 1.078) |
| Continuous | |
| Age, mo | 0.999 (0.993, 1.006) |
| MUAC, cm | 1.026 (0.961, 1.096) |
| WHZ | 1.291 (1.203, 1.386) |
| HAZ | 1.007 (0.969, 1.046) |
TABLE 4.
Outcomes of severely malnourished Malawian children who received 25% milk RUTF or 10% milk RUTF for up to 8 wk1
| Outcome | 25% milk RUTF | 10% milk RUTF | P-value2 |
| n | 945 | 929 | |
| Recovered | 790 (84) | 754 (81) | 0.18 |
| Remained wasted | 73 (8) | 83 (9) | 0.36 |
| Referred for inpatient treatment | 20 (2) | 39 (4) | 0.01 |
| Died | 34 (4) | 30 (3) | 0.07 |
| Lost to follow up | 28 (3) | 23 (3) | 0.57 |
| Good appetite after 2 wk | 906 (96) | 901 (97) | 0.36 |
| WHZ upon completion | −1.5 ± 1.1 | −1.5 ± 1.1 | — |
| HAZ upon completion | −3.1 ± 1.5 | −3.2 ± 1.5 | 0.15 |
| WAZ upon completion | −2.7 ± 1.2 | −2.8 ± 1.2 | 0.07 |
| Rate weight gain, g/(kg⋅d) | 2.44 ± 2.77 | 1.94 ± 2.70 | <0.0001 |
| Rate height gain, mm/d | 0.23 ± 0.29 | 0.19 ± 0.25 | 0.001 |
| Rate MUAC gain, mm/d | 0.17 ± 0.26 | 0.13 ± 0.25 | <0.0001 |
Values are means ± SD, or (%).
Comparisons made using Fisher's exact test for categorical measures and Student's test for continuous measures.
In children that failed treatment compared with those that recovered, significant risk factors associated with treatment failure were absence of edema at admission; lower enrollment values for weight, MUAC, and WHZ; and the absence of a mother at home (r = 0.31 for the model; P < 0.01 for each covariate listed) .
A total of 64 children (3%) died while enrolled in the study, 34 (3.6%) in the 25% milk RUTF group and 30 (3.2%) in the 10% milk RUTF group. The expected number of fatalities based on the method of Prudhon et al. (14) was 58. The study was not powered to detect differences in the mortality rate between the 2 groups of <2%.
Discussion
Treating children with SAM with 10% milk RUTF resulted in a lower rate of recovery compared with the standard 25% milk RUTF.
This trial was conducted in Malawi, which has a rural, primarily agrarian population whose staple food is corn. Kwashiorkor is the predominant form of SAM. Results may not be generalized to populations where severe wasting is the predominate form of malnutrition, to populations in which many of the children have HIV infection, or to children living in an urban setting. Additionally, because this is a clinical quasi-effectiveness trial and not a strict efficacy trial, it cannot be assumed that children were given only the study food or that sharing within the household did not occur. It is very likely some sharing did occur. Another limitation of the study is that no physiological or functional outcomes that mark the recovery from malnutrition were assessed, such as lean body mass or measures of renal or hepatic function. The large numbers of children managed outside of the hospital setting precluded the inclusion of functional outcomes. The 2 forms of RUTF had a very similar nutrient composition and thus differences in functional outcomes seem to be less likely than when comparing different types of foods.
The number of children lost to follow-up was low, just 3% of the total children treated; those lost were more likely to be younger and marasmic. Default rates < 10% are considered acceptable by international standards (15) and the numbers of children lost to follow-up were similar in each treatment group. Thus, children lost to follow-up are very unlikely to bias the findings in any way.
The results of this study are consistent with other reports concerning the use of home-based therapy with RUTF to treat SAM (1, 3, 4). The recovery rate was 83% in the current study and other studies in Malawi have documented recovery rates between 79% and 90%. In a large program in Niger, Medecins Sans Frontieres reported 91% recovery, although the prevalence of kwashiorkor in their population was only 3% (16).
Those who benefited from more milk in the RUTF were children with kwashiorkor, exhibiting an 88% recovery rate when receiving 25% milk RUTF compared with 85% when receiving 10% milk RUTF. The differences in recovery rates among children with kwashiorkor between the 2 RUTFs were 9% after 2 wk and 7% after 4 and 6 wk (Fig. 1). The recovery rate in marasmic children did not differ. The 2 diets were isonitrogenous and greatly exceeded the estimated protein requirement for a well-nourished child aged 1–3 y. The RUTF provided 5.0 g/(kg⋅d), whereas the protein requirement for a well nourished child is 1.3 g/(kg⋅d). Therefore, assuming the children were consuming about the same amount of RUTF, these findings suggest that it might have been the milk protein that enhanced the resolution of edema in kwashiorkor.
The soy used in this study was not dehulled. Unprocessed soy contains antinutrients that may have contributed to the lower recovery and growth rates for the 10% milk RUTF. Trypsin inhibitors have been shown to affect protein degradation and absorption (17), and phytic acid inhibits zinc and iron absorption (18). Soybeans also contain certain classes of polyphenols, such as the isoflavone genistein, which can affect DNA methylation (19). These observations were largely made in food science laboratories rather than in in vivo human experiments, and thus it is speculative that the antinutrients in soy contributed to the lower recovery rate. Furthermore, it has been suggested that adding milk to fortified foods could improve weight gain, linear growth, and recovery from malnutrition, but direct evidence for this is lacking (20).
It is possible that over the course of treatment the children consumed less 10% RUTF than 25% milk RUTF, perhaps due to subtle differences in taste or color. However, similar numbers of children receiving 25% milk RUTF were referred for inpatient care because they refused to eat the food as those receiving 10% milk RUTF (10/945 vs. 7/929). Additionally, acceptability tests completed prior to the study did not reveal any differences and when questioned during follow-up visits, mothers did not indicate that children consumed less 10% milk RUTF, although this method of assessing intake has not been validated.
Approximately 15% of the children initially treated with home-based therapy either did not recover and were referred for inpatient therapy or died. Failure was seen most frequently in the first 2 wk of therapy, marked by weight loss or worsening of edema. The most significant risk factor for failure to recover was the presence of marasmus, characterized by lower MUAC and WHZ, as well as the absence of the child’s mother from the home. In a country such as Malawi where kwashiorkor predominates, children with marasmus or without a mother in the home might benefit from more frequent or careful follow-up. Alternatively, a home visit by the village health aid to children with marasmus within the first week of therapy might identify those not responding well to treatment. Identification alone, however, is unlikely to improve the outcome of these children unless additional interventions are readily available.
Because milk is more costly than soy, by 11-fold at the time this study was conducted, and milk is the most expensive ingredient in RUTF, the cost of RUTF would be reduced if soy could be substituted for some of the milk (an absolute reduction of $0.55/kg RUTF and a relative reduction of 15% in RUTF cost). If we assume that therapeutic food represents 70% of the cost of treatment of SAM, this would reduce the cost for treatment of a child with SAM by 10%. Although this current study does not support the substitution of 15% soy for 15% milk in RUTF because of an inferior recovery rate, future research investigating formulation changes in RUTF that reduces the cost of the product may improve access to RUTF among children with SAM.
Although nearly identical in macro- and micronutrient composition, 10% milk RUTF and 25% milk RUTF differ in clinical effectiveness. Caution is necessary in the treatment of SAM when altering the composition of therapeutic foods without clinical evidence of equivalency, no matter how slight the alteration may be. Further studies are needed to investigate the role of animal protein compared with plant protein in recovery from malnutrition, specifically in the context of kwashiorkor. This will help to determine under what circumstances, if any, substitution for the milk in the RUTF recipe can be justified in the treatment of SAM.
Acknowledgments
K.M. and M.M. designed research; E.O., J.R., H.S., and M.M. conducted research; E.O., J.R., H.S., G.K., and M.M. analyzed data; I.T. provided the statistical analyses; E.O., H.S., G.K., I.T., and M.M. wrote the paper; and M.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the Hickey Family Foundation. This study was also supported by the Office of Health, Infectious Disease, and Nutrition, Bureau for Global Health, United States Agency for International Development (USAID), under terms of Cooperative Agreement No. GHN-A-00-08-00001-00, through the FANTA-2 Project, operated by the Academy for Educational Development (AED). The contents are the responsibility of AED and do not necessarily reflect the views of USAID or the United States Government. Dr. Trehan is supported by NIH T32 HD049338.
This trial was registered at Current Controlled Trials (UK) as ISRCTN54186063.
Supplemental Results are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: HAZ, height-for age Z-score; MUAC, mid-upper arm circumference; RUTF, ready-to-use therapeutic food; SAM, severe acute malnutrition; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score.
Literature Cited
- 1.Manary MJ, Ndekha MJ, Ashorn P, Maleta K, Briend A. Home based therapy for severe malnutrition with ready-to-use food. Arch Dis Child. 2004;89:557–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manary MJ. Local production and provision of ready-to-use therapeutic food (RUTF) spread for the treatment of severe malnutrition. Food Nutr Bull. 2006;27:S83–8 [DOI] [PubMed] [Google Scholar]
- 3.Ciliberto MA, Sandige H, Ndekha MJ, Ashorn P, Briend A, Ciliberto HM, Manary MJ. A comparison of home-based therapy with ready-to-use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. Am J Clin Nutr. 2005;81:864–70 [DOI] [PubMed] [Google Scholar]
- 4.Linneman Z, Matilsky D, Ndekha M, Manary MJ, Maleta K, Manary MJ. A large-scale operational study of home-based therapy with ready to use therapeutic food in childhood malnutrition in Malawi. Matern Child Nutr. 2007;3:206–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO World Food Programme, United Nations System Standing Committee on Nutrition, United Nations Children#x2019s Fund: Community Based Management of Severe Acute Malnutrition, a Joint Statement, May 2007. Geneva: WHO [Google Scholar]
- 6.Badger TM, Gilchrist JM, Pivik RT, Andres A, Shanker K, Chen JR, Ronis MJ. The health implications of soy infant formula. Am J Clin Nutr. 2009;89:S1668–72 [DOI] [PubMed] [Google Scholar]
- 7.Collins S, Henry J. Alternative RUTF formulations. : Khara T, Collins S, Community-based therapeutic care (CTC). Emergency Nutrition Network supplement 2. Oxford: Emergency Nutrition Network; 2004. p. 35–7 [Google Scholar]
- 8.Bachmann MO. Cost effectiveness of community-based therapeutic care for children with severe acute malnutrition in Zambia: decision tree model. Cost Eff Resour Alloc. 2009;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FANTA Project Training guide for community-based management of acute malnutrition (CMAM) Fanta Project 2008 [cited 2009 Nov 17]. Available from: http://www.fantaproject.org
- 10.WHO Management of severe malnutrition: a manual for physicians and other senior health workers. England: WHO library cataloguing in publication data [cited 2009 Nov 17]. Available from: http://www.who.int/nutrition/publications/malnutrition/en/
- 11.Manary MJ, Sandige HL. Management of acute moderate and severe childhood malnutrition. BMJ. 2008;337:a2180. [DOI] [PubMed] [Google Scholar]
- 12.WHO Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. : WHO child growth standards: methods and development. Geneva: WHO; 2006 [Google Scholar]
- 13.Peto R, Pike MC, Armitage P, Breslow N, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: analysis and examples. Br J Cancer. 1977;35:1–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prudhon C, Golden MHN, Briend A, Mary JY. A model to standardize mortality of severely malnourished children using nutritional status on admission to therapeutic feeding centres. Eur J Clin Nutr. 1997;51:771–7 [DOI] [PubMed] [Google Scholar]
- 15.The Sphere Project The Sphere handbook. Minimum standards in food security, nutrition and food aid. Geneva; 2004[cited 2009 Nov 17] Available from: http://www.sphereproject.org/content/view/66/84/lang,English/ [Google Scholar]
- 16.Defourny I, Drouhin E, Terzian M, Taty M, Sekkenes J, Tectonidis M. Scaling up the treatment of acute childhood malnutrition in Niger. Emergency Nutrition Network Field Exchange; 2006[cited 2009 Nov 13]. Available from: http://hdl.handle.net/10144/66014
- 17.Anderson RL, Wolf WJ. Compositional changes in Trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J Nutr. 1995;125:S581–8 [DOI] [PubMed] [Google Scholar]
- 18.Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr. 1992;56:573–8 [DOI] [PubMed] [Google Scholar]
- 19.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:S223–8 [DOI] [PubMed] [Google Scholar]
- 20.Hoppe C, Gregers AS, Jacobsen S, Molgaard C, Friis H, Sangild PT, Michaelsen KF. The use of whey or skimmed milk powder in fortified blended foods for vulnerable groups. J Nutr. 2008;138:S145–61 [DOI] [PubMed] [Google Scholar]